Abstract
We analyzed the process of inflorescence formation in Impatiens balsamina by studying the architecture of the plant under different photoperiod treatments. Floral reversion under noninductive conditions in this species is caused by the lack of persistence of the induced state in the leaf. This can be used to control the amount of inductive signal and to examine its quantitative influence on morphological changes in the plant. The floral transition was characterized by a continuum of variation at the level of meristem identity, primordium initiation, and floral organ identity. This continuum was enhanced during reversion, suggesting that the establishment of a continuum partly reflects limiting amounts of inductive signal exported from the leaf to the meristem. The transcription patterns of two homologs of genes involved in the control of floral meristem identity, Imp-FLO and Imp-FIM, were similar in terminal and axillary flowers and may be associated with the continuum exhibited by I. balsamina. By analyzing the fate of axillary meristem primordia initiated before and after the beginning of the inductive period, we showed that de novo initiation of axillary meristem primordia by the evoked meristem is not required and that primordia initiated before evocation can adopt different fates, depending on the amount of inductive signal. The influence of age and/or position on primordium responsiveness to the inductive signal is discussed.
The transition to flowering is characterized by dramatic changes in plant morphology. These modifications commonly include changes in leaf morphology and phyllotaxis, shortening of internodes, and flower formation. Depending on the mode of growth and inflorescence formation, flowering can occur at axillary positions on shoots or inflorescences or as solitary flowers and may culminate in a terminal flower. Although the subjects of inflorescence morphology and flowering physiology have received much attention (Bernier, 1988; Weberling, 1989; Bernier et al., 1993), there have been few attempts to link these disciplines.
Flowering can be triggered by a number of environmental stimuli, including photoperiod and temperature. Photoperiod induction occurs in the leaf and results in the formation of a mobile inductive signal. Despite the broad variety of flowering responses to different stimuli, the mechanisms that underlie the flowering process seem to be conserved in different species. Graft transmission of flowering between species with different photoperiod requirements suggests that the inductive signal is universal, but its molecular nature has remained elusive. Studies have revealed that the signal may be multifactorial (Bernier, 1988; Bernier et al., 1993).
The classical view of flowering physiology is that the inductive signal is rapidly exported via the phloem sap to the shoot apical meristem, which undergoes evocation (Evans, 1969; Zeevaart, 1976; Bernier, 1988; McDaniel, 1992). The changes in the activity of evoked meristems cause de novo initiation of flower primordia, but it is unclear how often previously formed axillary meristem primordia are modified and whether such modifications are mediated by the inductive signal from the leaf directly or indirectly via the apical meristem. In plants with an absolute photoperiod requirement it is possible to identify axillary meristem primordia that are initiated before and after the beginning of the inductive treatment and to analyze their fate in mature plants. Furthermore, the manipulation of the level of inductive signal in the plant during photoperiod treatments should provide information on the mechanisms that control the progression to flowering and inflorescence formation.
Impatiens balsamina is a very attractive model for the analysis of the flowering process because it has an absolute requirement for SD conditions for flowering, and flower reversion can be obtained in a predictable way after transfer to LD conditions (Battey and Lyndon, 1984, 1986, 1988, 1990; Pouteau et al., 1995, 1997, 1998). Both flower formation and reversion are characterized by a continuum of changes in organ identity, and a large range of mosaic organs is produced (Battey and Lyndon, 1988; Pouteau et al., 1998). Following increasing amounts of induction in SD conditions, reversion takes place at progressively later stages of flower development. Reversion of the terminal flower correlates with the lack of persistence of an induced state in the leaf (Pouteau et al., 1997). Partial progression to flowering exhibited before return to leaf formation can thus be considered to reflect the amount of inductive signal exported from leaves before transfer to LD conditions.
In addition to the failure of leaves to become a permanent source of inductive signal, flower reversion also implies that the terminal meristem does not become committed to flowering in I. balsamina. Among the regulatory genes involved in flower morphogenesis in snapdragon and Arabidopsis, a number are involved in the specification of floral meristem identity. These include the meristem identity genes FLO and LFY and their mediators or coregulators, FIM and UFO, in snapdragon and Arabidopsis, respectively (Coen et al., 1990; Weigel et al., 1992; Simon et al., 1994; Ingram et al., 1995; Blázquez et al., 1997; Lee et al., 1997). Analysis of the regulation of I. balsamina homologs of these genes (Imp-FLO and Imp-FIM, respectively) in the apical meristem shows a number of similarities and differences (Pouteau et al., 1997, 1998). Imp-FLO and Imp-FIM are transcribed during vegetative growth, flowering, and reversion. However, Imp-FIM specifically exhibits a new transcription pattern during petal initiation and is not transcribed during the initiation of reproductive organs, whereas Imp-FLO transcription is apparently constitutive. However, it is unclear whether the new transcription patterns of Imp-FLO and Imp-FIM are specific to the apical meristem or whether the same transcription patterns as those observed in snapdragon and Arabidopsis occur in axillary meristems of I. balsamina.
We have analyzed the process of inflorescence formation in I. balsamina by characterizing plant architecture under continuous SD conditions and during reversion experiments carried out after increasing periods of induction. Flowering over the whole plant was characterized by a form continuum at three levels, which was emphasized through the removal of the inductive signal by transferring plants to noninductive, LD conditions.
The analysis of Imp-FLO and Imp-FIM transcription in axillary flowers showed essentially no difference compared with terminal flowers; the possible association between the regulation of these two genes in I. balsamina and the gradual progression to flowering is discussed. De novo initiation of axillary meristem primordia by the evoked apical meristem is not required for flower formation, and primordia initiated before apical meristem evocation adopted different fates, depending on the amount of inductive signal received. The degree of inflorescence development decreased basipetally in response to decreasing amounts of inductive signal. The youngest, uppermost axillary meristem primordia were most strongly induced in response to SD conditions and their fate was least affected by transfer to LD conditions. The influence of age and/or position on axillary meristem responsiveness and the possible role of the apical meristem in controlling flowering and inflorescence formation are discussed.
MATERIALS AND METHODS
Plant Material
We used an Impatiens balsamina cultivar (Dwarf Bush Flowered) that is red-flowered and determinate and one that gives the most uniform reversion response. Plants were grown as previously described (Pouteau et al., 1997, 1998). Plant growth after sowing was in LD conditions of 24 h at 21°C ± 1°C. At the top of the plants on d 0, the total photon flux density was 260 to 280 μmol m−2 s−1 during the day (8 h) and 5 μmol m−2 s−1 during the night (16 h). The compost was kept moist by the application of 200 mL of tap water per tray every day.
Photoperiod Treatments
Developmentally uniform plants with an average of nine primordia were selected on d 0 (7 to 8 d after sowing and 10–11 d after imbibition). After d 0, flowering in SD conditions and flower reversion after various periods of induction in SD conditions were obtained as previously described (Pouteau et al., 1997). SD conditions consisted of an 8-h period of illumination identical to that applied for LD conditions, but complete darkness was maintained during the 16-h night. No plant grown in continuous LD conditions developed any floral features for at least 3 months.
Plants under different photoperiod treatments were randomly sampled at different times for the preparation of material for in situ hybridization assays. The number of nodes and primordia initiated by the shoot apical meristem was determined in 10 plants at each sampling time. Approximately 10 plants were grown until maturity to record the characteristics at each node of organ identity, axillary shoot identity, and internode elongation.
In experiments designed to analyze the influence of plant age on flowering, plants were induced in SD conditions after seedling emergence (6 d before d 0), after d 0 (control), and 15 d after d 0. One-half of the plants was left under continuous SD conditions, and the other half was transferred to LD conditions after 5 d of SD conditions.
In Situ Hybridization
The methods for digoxigenin labeling of RNA probes, tissue preparation, and in situ hybridization were as described by Bradley et al. (1993). psep1–9 cut with HindIII and psep3–1 cut with EcoRI were used as the templates for T7 RNA polymerase to generate antisense and sense RNA probes of an Imp-FIM fragment, respectively (Pouteau et al., 1998). pflo1 cut with EcoRI and pflo7 cut with BamHI were used as templates for T7 RNA polymerase to generate antisense and sense RNA probes of an Imp-FLO fragment, respectively (Pouteau et al., 1997). No signal was detected with sense RNA probes of Imp-FIM and Imp-FLO.
RESULTS
Continuum in Plant Architecture
Plant Architecture under Continuous SD Conditions
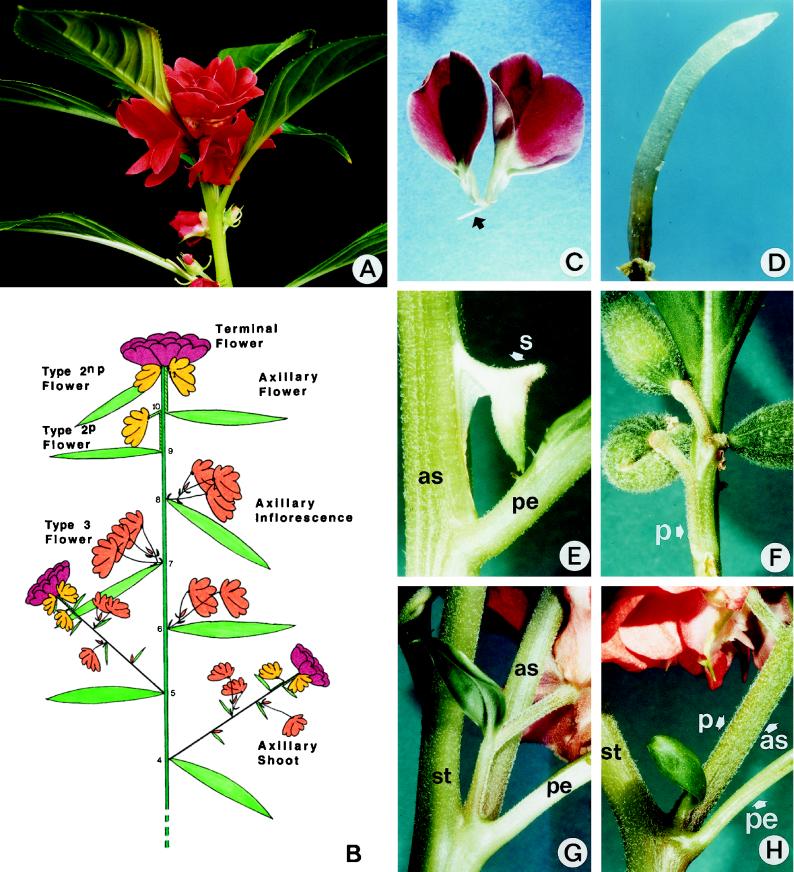
To identify the different axillary structures formed by the apical meristem, plant architecture was analyzed under continuous inductive SD conditions (Figs. 1 and 2). Plants formed a terminal inflorescence (Fig. 1, A and B) consisting of a large terminal flower and two or three solitary axillary flowers, each subtended by a leaf (referred to as type-2 flowers). The organization of the terminal flower was described previously (Battey and Lyndon, 1984; Pouteau et al., 1998). The lower type-2 flowers had a pedicel that was often partly or completely adnate to the main stem and were subtended by normal leaves separated by internodes (type-2p flowers). The upper type-2 flowers lacked a pedicel and were borne in the axils of leaves that were not separated by internodes (type-2np flowers). The three nodes below the terminal inflorescence bore a leaf subtending an axillary inflorescence. These structures were contracted inflorescences consisting of a small number of flowers (two to five): these type-3 flowers were subtended by true bracts (i.e. leaves extremely reduced to the size of small scales) and were not separated by internodes. The five nodes below the lowermost axillary inflorescence bore a leaf that subtended a flowering axillary shoot. The organization of these structures recapitulated that of the main stem (Fig. 1B).
Figure 1.
Plant architecture under SD conditions. A, Terminal inflorescence showing the terminal flower and two type-2 axillary flowers below. B, Diagram summarizing the main features of plant architecture and the different types of axillary structures and flowers. C, Rudimentary flower composed of only two unexpanded petals and one filament (arrow). D, Rudimentary flower consisting of one single filament. E, Rudimentary flower borne on an axillary shoot, composed of one single sepal. F, Mosaic between a type-2 flower and an axillary inflorescence showing a fasciated pedicel bearing three pods but no bract. G, Mosaic between an axillary inflorescence and a flowering axillary shoot showing a flower subtended by a leaf-bract mosaic fused to the base of a shoot grown in the axil of a main stem leaf. H, Same as G, but the pedicel of the flower at the base of the shoot is adnate to the shoot stem. as, Stem of an axillary shoot; pe, petiole of a main stem leaf; p, pedicel; st, main stem; s, sepal.
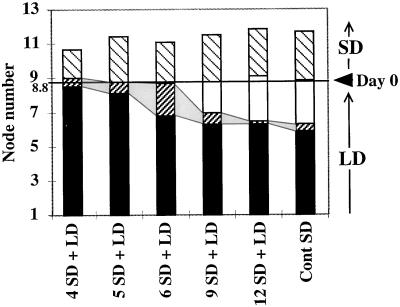
Figure 2.
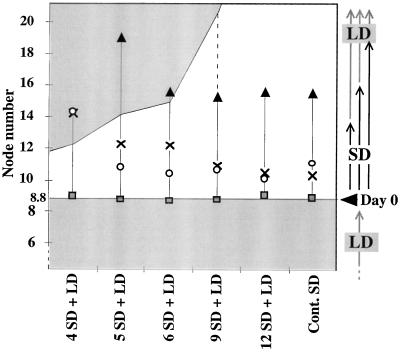
Plant architecture during reversion. After initial growth in LD conditions until d 0, plants were induced in SD conditions for different times (4, 5, 6, 9, and 12 short days) and transferred back to LD conditions. Control plants were grown after d 0 under continuous (Cont) SD conditions until maturity. Ten plants for each treatment were analyzed and the number and identity of axillary structures along the main stem were recorded: flowering axillary shoots (▪), axillary inflorescence/shoot mosaic structures (▨), axillary inflorescences of type-3 flowers (□), and type-2 flowers (▧). ses varied between 0.13 and 0.50. The nodes initiated in LD conditions before transfer to SD conditions are indicated below the d-0 mark, and those initiated after transfer to SD conditions are indicated above the d-0 mark (Day 0).
A continuum of changes in plant architecture could be observed at three levels: (a) the formation of mosaic axillary structures that were intermediate between the different classes of flowers, inflorescences, and flowering shoots, as described above; (b) the progressive change of axillary flower architecture along the main stem; and (c) the gradual change in organ identity in the flowers.
Mosaic Axillary Structures
During flowering under continuous SD conditions and reversion after transfer to LD conditions, mosaic axillary structures were occasionally observed at the junctions between the zones giving rise to axillary inflorescences and type-2 flowers. They usually consisted of two or three flowers that were not subtended by bracts and had partially fused pedicels (Fig. 1F). Mosaic axillary structures were even more frequently observed at the junctions between the zones corresponding to axillary inflorescences and flowering axillary shoots. These structures, called mosaic shoots, corresponded to flowering axillary shoots fused to the pedicel of a solitary flower subtended by a bract or bract-like leaf (Fig. 1, G and H). Mosaic shoots were found in 40% of the plants grown under continuous SD conditions. Their frequency increased in response to reversion treatments: on average, up to 1.9 nodes per plant exhibited mosaic shoots during reversion after 5 d of SD conditions (Fig. 2; see below).
Gradual Change in Axillary Flower Architecture
Type-3 flower architecture and the gradual change in type-2 flower architecture were analyzed in plants grown under continuous SD conditions (Table I). Typical type-3 flowers were pentamerous and had 3 sepals, 10 petals, 5 stamens, and a central pod comprised of 5 carpels. They were zygomorphic and usually displayed 3 or 4 asymmetrical lateral petals, 5 symmetrical ventral petals, and 1 large dorsal petal with a green tip and a green rib on the abaxial side between the two lobes. The 3 sepals (2 lateral and 1 ventral) were spurred and similar in shape. Although most flowers had a total of 18 floral organs (excluding carpels), variations from one flower to another were observed (extreme variants had 14 and 24 organs, respectively) but were less pronounced than in the terminal flower (Table I; Pouteau et al., 1998).
Table I.
Flower architecture in the terminal inflorescence
| Plant Part | Type 3 | Type 2p | Type 2np | Terminal |
|---|---|---|---|---|
| n | ||||
| Sepals | 3.3 ± 0.10 | 2.0 ± 0.18 | 1.1 ± 0.30 | 2.1 ± 0.18 |
| Petals | 10.0 ± 0.21 | 10.0 ± 0.84 | 6.4 ± 0.37 | 18.5 ± 1.15 |
| True | 5.6 ± 0.18 | 5.6 ± 0.40 | 4.3 ± 0.43 | 6.3 ± 0.62 |
| Staminate | 0.3 ± 0.09 | 1.7 ± 0.51 | 0.3 ± 0.18 | 1.6 ± 0.96 |
| Asymmetrical | 3.5 ± 0.13 | 2.8 ± 0.34 | 1.9 ± 0.33 | — |
| Stamens | 5.2 ± 0.17 | 5.3 ± 0.80 | 4.5 ± 0.25 | 13.9 ± 0.90 |
Plants were grown under continuous SD conditions until maturity and were dissected. Terminal and axillary flowers in 10 plants were analyzed. This corresponded to 10 terminal flowers and 46 type 3 flowers (from axillary inflorescences), 12 type 2p flowers, and 12 type 2np flowers. Sepals include regular and modified sepals. True petals are fully expanded and anthocyanin-pigmented petals. Staminate petals showed various degrees of transformation into stamens. Asymmetrical petals were found mostly in lateral position in the flowers. Data are ±se.
Type-2 flowers at gradually higher nodes showed a progressive reduction in organ number and a decrease in sepal identity. Type-2p flowers had 1 sepal less but the same number of petals and stamens compared with type-3 flowers. Type-2np flowers had two sepals less, three or four petals less, and one stamen less. Variation in organ numbers was markedly higher than in type-3 flowers. In about 10% to 20% of the plants, the most acropetal axillary structure corresponded to a rudimentary structure that was often composed of one or two solitary petals or a filament of unknown identity (Fig. 1, C and D).
Gradual Change in Floral Organ Identity
Mosaic or incomplete organs were commonly observed in type-3 and type-2 flowers. In type-3 flowers an average of 0.4 of the 3.3 sepals were modified and often had some petal features. An average of only 5.6 of the 10.0 petals were true petals (i.e. had 100% petal-pigmented tissue; Pouteau et al. [1998]), and 4.4 petals displayed staminate features. Approximately 30% of the pods had staminate features. Mosaic organs were less frequent than in the terminal flower (Pouteau et al., 1998).
Transcription of Imp-FIM and Imp-FLO in Axillary Flowers
To determine whether the novel transcription pattern of Imp-FIM during petal initiation and the constitutive transcription of Imp-FLO during vegetative growth, flowering, and reversion were specific to the apical meristem (Pouteau et al., 1997, 1998), Imp-FIM and Imp-FLO RNA patterns in type-2 axillary flowers were analyzed by in situ hybridization.
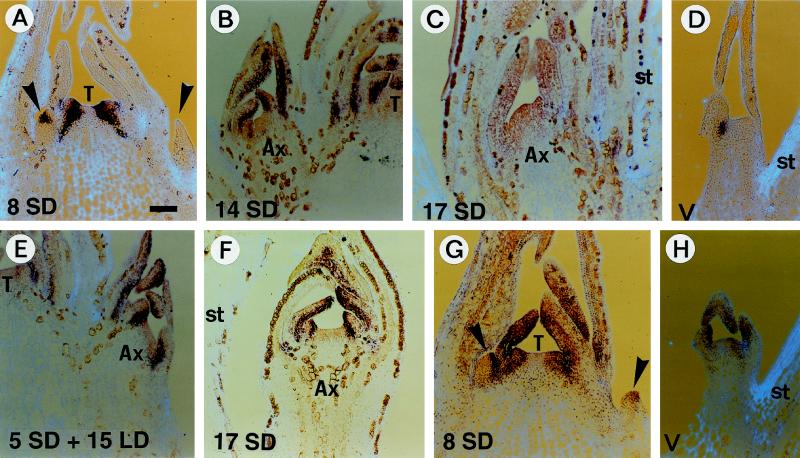
The earliest expression of Imp-FIM in type-2np flowers occurred after 8 d in SD conditions, when the first petal primordium was initiated in the terminal flower (Fig. 3A). This early transcription corresponded to one single stripe of signal in the meristem. Although no primordium was morphologically visible at this stage, it is likely that it corresponded with the position of initiation of the first sepal primordium. After this stage, type-2np flowers developed in step with the terminal flower. The Imp-FIM transcription pattern was essentially identical in both types of flowers: it accumulated within petal primordia but was absent from stamen primordia (Fig. 3, B and C). Development of type-2p flowers was slightly behind, but a similar pattern of Imp-FIM transcription was observed within petal primordia (Fig. 3F).
Figure 3.
In situ hybridization analysis of Imp-FIM and Imp-FLO transcription in type-2 flowers. A to F, Imp-FIM transcription in a terminal inflorescence after 8 d in SD conditions (A); in type-2np flowers after 8 d in SD conditions (B), 17 d in SD conditions (C), and 5 SD + 15 LD (E); in a vegetative axillary shoot after 8 d in LD conditions (D); and in type-2p flowers fixed after 17 d in SD conditions (F). G and H, Imp-FLO transcription in a terminal inflorescence after 8 d in SD conditions (G) and in a vegetative axillary shoot after 8d in LD conditions (H). Apical sections were probed with digoxigenin-labeled Imp-FIM or Imp-FLO antisense RNA and viewed under light-field microscopy (the RNA signal is purple on a light-blue tissue background). Leaf tissue and, more obviously, floral tissues remained strongly pigmented after fixation and embedding due to the accumulation of brown-stained granules. All photos were taken under a light-field microscope with the same magnification factor. Scale bars = 100 μm. The terminal meristem (T) or stem tissue (st) are indicated when visible to orient the sections. Arrowheads point to young axillary floral meristems, and developing axillary flowers (Ax) are labeled.
Plants transferred to LD conditions after 5 d in SD conditions had the greatest axillary flower reversion; return to leaf formation occurred after the production of a number of petals (see below; Fig. 5). In plants grown for 5 d in SD conditions and then 15 d in LD conditions, Imp-FIM was transcribed mostly at the base of the primordia in type-2np flowers (Fig. 3E). This was similar to the pattern observed at the same stage in the terminal meristem, which was initiating whorls of leaves at this time (Pouteau et al., 1998). Therefore, transcription of Imp-FIM was essentially identical in terminal and axillary flowers during flowering and reversion. Transcription in vegetative meristems of axillary shoots was as in the vegetative apical meristem (Fig. 3D).
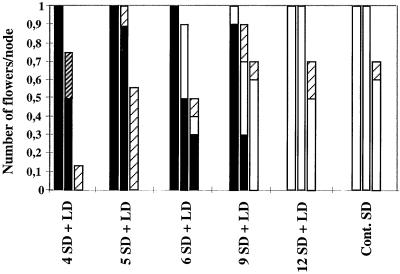
Figure 5.
Reversion of type-2 axillary flowers. Reversion treatments and the SD controls were as in Figures 2 and 4. Reversion of type-2 flowers was analyzed in 10 plants for each treatment. The frequencies of reverting (▪) and nonreverting (□) flowers and of rudimentary flowers with vegetative (▨) or floral (▨) features were recorded from the lowermost to the uppermost node (left to right). ses varied from 0.10 to 0.35.
Imp-FLO was transcribed in vegetative, flowering (Fig. 3, G and H), and reverting axillary meristems, similar to the terminal meristem. After 8 d of SD conditions, a slight increase in Imp-FLO transcript was observed in young axillary flower primordia and in the terminal meristem (Fig. 3G; Pouteau et al., 1997).
Reversion Analysis of the Progression to Flowering
The progression to flowering under SD conditions can be described by analyzing reversion in plants transferred to LD conditions after different periods of induction (4–18 d) in SD conditions. The progression to flowering in the terminal flower of I. balsamina during reversion was described previously (Pouteau et al., 1997). We analyzed reversion in the remainder of the plant, below the terminal flower.
Progression to Flowering in Axillary Meristems Produced after Transfer to SD Conditions
In all reversion treatments and in the SD (flowering) control, the first type-2 axillary flower was initiated in the axil of the youngest primordium visible on d 0 (ninth leaf primordium; see Methods; Figs. 2 and 4). Therefore, the position of the first node bearing a type-2 flower was not affected, even after inductive SD treatments as short as 4 d. Therefore, only primordia initiated on or after transfer to SD conditions on d 0 were recruited to form the terminal inflorescence.
Figure 4.
Progression to flowering in nodes initiated after transfer to SD conditions. Control plants grown under continuous SD conditions and plants transferred to LD conditions after different periods of SD induction after initial growth in LD conditions until d 0 were as in Figure 2. Ten plants for each treatment were analyzed and the lowermost nodes exhibiting different inflorescence traits were recorded: Shaded box, type-2 flower; ○, modified leaf; ×, absence of internode above; ▴, leaf having petal pigmented sectors. ses varied between 0.18 and 0.52 and were higher for the measures of the lowermost node not followed by an internode or with a modified leaf after 4 SD + LD and the lowermost nodes with a leaf containing petal pigment after 5 SD + LD and continuous SD (0.70, 1.10, 1.47, and 0.8, respectively). The areas corresponding to nodes initiated under LD conditions before transfer to SD conditions (below the d-0 mark [Day 0]) or after transfer from SD conditions are shaded. The area corresponding to nodes initiated under SD conditions is left blank.
With an inductive SD treatment of 5 d or more, the total number of type-2 flowers was the same as in the SD control (Fig. 2). However, some or all of the axillary flowers reverted after an inductive SD treatment of less than 12 d (Fig. 5). After 4 d in SD conditions followed by LD conditions, about two-thirds of the type-2 structures were virescent, with few floral features. Reversion treatments also resulted in increased frequencies of rudimentary structures. These were highest in treatments resulting in the highest reversion responses (SD treatments of 4 and 5 d followed by LD treatment), suggesting a link between them. In all SD treatments of less than 12 d, reversion of type-2 flowers was consistently observed in the lowermost type-2 flower (Fig. 5). Therefore, the lowermost axillary meristem of type-2 flowers either received a lower amount of inductive signal or was less responsive to the inductive signal.
The transition from inflorescence features to terminal flower features was gradual, and terminal flower features responded differently to the amount of induction provided (Fig. 4). The repression of internode elongation, the production of petal pigment in the appendages, and the modification in shape and/or venation of the appendages required a minimum SD treatment of 9, 6, and 5 d, respectively, to occur at the same node level as in the SD control. The treatment of 5 d in SD conditions followed by LD conditions was characterized by the most severe uncoupling in the development of terminal flower features compared with the SD control. After 4 d in SD conditions followed by transfer to LD conditions, most floral features were repressed and little morphological modification of the appendages occurred.
Progression to Flowering in Axillary Meristem Primordia Initiated before Transfer to SD Conditions
Figure 2 shows how the fate of axillary meristems initiated before transfer of plants to inductive SD conditions was strongly influenced by the duration of the inductive treatment. After a SD treatment period of 4 d, only a small number of axillary inflorescence/shoot mosaic structures and no axillary inflorescences were formed. Only after inductive SD treatments of 9 d or more did axillary inflorescences develop, and these replaced the mosaic structures. Increasing the duration of the inductive treatment therefore increased the extent of inflorescence formation on the main stem in a basipetal direction.
Axillary shoots borne on the lowest five nodes of the main stem under continuous SD conditions were identically organized. They formed a terminal inflorescence consisting of a terminal flower and one solitary type-2 flower below it (Fig. 1B). The three nodes below this terminal inflorescence bore a leaf subtending an axillary inflorescence or an axillary shoot. Approximately 10% of the main stem axillary shoots displayed rudimentary structures in their terminal inflorescence. In contrast to those in the main stem terminal inflorescence, these rudimentary structures usually comprised one or two sepals and, less frequently, a filament (Fig. 1E; Table II).
Table II.
Organization of flowering axillary shoots
| Treatment | No. of Axillary
Structures
|
Reversion in Terminal Flowers | |||
|---|---|---|---|---|---|
| Total | Inflorescence or shoot | Type-2 flower | Rudimentary flower | ||
| % | |||||
| 13 SD + LD | 4.9 ± 0.36 | 2.6 ± 0.12 | 2.1 ± 0.34 | 0.6 ± 0.72 | 89 |
| 15 SD + LD | 5.2 ± 0.30 | 2.8 ± 0.09 | 2.2 ± 0.25 | 0.9 ± 0.96 | 90 |
| 18 SD + LD | 5.0 ± 0.31 | 3.0 ± 0.11 | 1.8 ± 0.27 | 0.4 ± 0.55 | 65 |
| Control | 4.1 ± 0.08 | 2.9 ± 0.06 | 1.2 ± 0.08 | 0.1 ± 0.39 | 0 |
Plants were grown under SD conditions for different periods (13, 15, and 18 d) and transferred to LD conditions. Control plants were grown under continuous SD conditions until maturity. Flowering axillary shoots (38, 39, and 41, respectively) from 10 plants were dissected for the 13 SD + LD, 15 SD + LD, and 18 SD + LD treatments. Sixty-nine flowering axillary shoots from 14 control SD plants were analyzed. The number and type of axillary structures borne on each shoot were recorded (axillary shoots and inflorescences and type-2 flowers). Rudimentary type-2 flowers corresponded in most cases to one or two sepals and less frequently to a filament. The percentage of reversion in the terminal flowers of axillary shoots was recorded in 36, 30, 31, and 52 axillary shoots in the 13 SD + LD, 15 SD + LD, 18 SD + LD, and control treatments, respectively. Data are ±se.
Analysis of axillary shoot architecture during reversion experiments showed an increase in the number of type-2 flowers compared with those treated with continuous SD conditions, even after SD inductions as long as 18 d (Table II). This increase was more pronounced in the uppermost axillary shoots, where up to two more type-2 flowers were formed in the plants given 15 d of SD treatment followed by LD treatment than in plants given continuous SD treatment. There was no detectable increase in the number of type-2 flowers on axillary shoots at the two lowest nodes. A large part of this increase resulted from an increase in the number of rudimentary structures, up to about one rudimentary structure per branch after 15 d of SD treatment followed by LD treatment (Table II). Reversion of terminal flowers on axillary shoots was high after 18 d of SD treatment followed by LD treatment (65%) and increased to 90% after 15 d of SD treatment followed by LD treatment (Table II). In contrast, no terminal flower on the main stem reverted after 18 d of SD treatment followed by LD treatment, and only 10% of them reverted after 15 d of SD treatment followed by LD treatment (Pouteau et al., 1997).
Influence of Plant Age on the Progression to Flowering
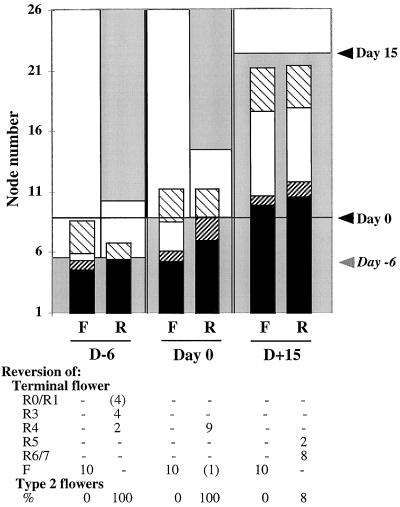
To determine the effects of plant age on the progression to flowering, induction and reversion after 5 d of SD treatment were carried out at emergence of the seedlings (d 6) and 15 d after d 0 and compared with d-0 controls (Fig. 6).
Figure 6.
Influence of plant age on the progression to flowering. Flowering under continuous SD conditions (F) and reversion after 5 SD + LD (R) were carried out at three stages after initial growth in LD conditions: at emergence of the seedlings 6 d before d 0 (D-6), on d 0 (Day 0), and on d 15 (D+15). The areas corresponding to nodes initiated under LD conditions are shaded, and the areas corresponding to nodes initiated under SD conditions are left blank. The number of nodes initiated in LD conditions before transfer into SD conditions fall below the “Day −6,” “Day 0,” and “Day 15” marks, respectively, in the three treatments. The number of nodes initiated at emergence of the seedling was not recorded; therefore, an estimate is given. Ten plants for each treatment were analyzed, and the number and identity of axillary structures along the main stem were recorded. Symbols are as in Figure 2. ses varied between 0.16 and 0.40. Reversion in the terminal flower was recorded using the reversion scale described in previous work (Battey and Lyndon, 1984; Pouteau et al., 1997). This scale describes the degree of flower development before return to leaf initiation. It ranges from R0 (no flower development) to R8 (carpels). Intermediate reversion types include R1 (virescent axillary structures), R3 (repression of internode elongation), R4 (modified venation, petal pigment), R5 (petals), and R6/7 (stamens). F, Nonreverting flower. The frequency of reversion in type-2 flowers was recorded.
In the d-6 experiments, the number of type-2 flowers initiated under continuous SD conditions was similar to the d-0 control, but fewer axillary inflorescences and mosaic shoots were formed. The amount of induction during the 5 d of SD treatment after emergence was less compared with the d-0 control, since transfer to LD conditions after this time resulted in drastic reduction of floral features in the whole plant. Reversion in the terminal flower occurred significantly earlier than in the d-0 controls, and 40% of the plants had few or no flowering features. No axillary inflorescence or mosaic shoot was formed, and half as many type-2 flowers were formed, all of which reverted.
In contrast, the inductive effect of 5 d of SD treatment when given 15 d after d 0 was markedly higher than in the d-0 control: reversion in the terminal flower occurred later, mostly after stamen formation, the different classes of axillary structures were mostly unaffected, and only 9% of type-2 flowers reverted. Under continuous SD conditions from d 15, type-2 flowers and axillary inflorescences and mosaic shoots were all derived from primordia initiated before transfer to SD conditions. The total number of type-2 flowers was slightly increased, and there were about 3 times more axillary inflorescences than in the d-0 controls. These observations suggest that the plant becomes more responsive to SD induction as it ages. Also, the fate of axillary meristems remains uncommitted until late and can be altered if a sufficient amount of induction is provided, possibly through an increased number of receptive leaves or through increased competence of the meristem.
DISCUSSION
Form Continuum in I. balsamina
The analysis of inflorescence architecture in I. balsamina shows that flowering progresses as a continuum at three levels: (a) meristem identity, in which the formation of mosaic structures is observed at the junctions between the zones marked by axillary flowering shoots, axillary inflorescences, and flowers of the terminal inflorescence; (b) primordium initiation, in which successive axillary flowers exhibit a gradual reduction in the total number of floral organs, with extreme reduction to one or two organs in some of the uppermost flowers and a reduction in sepal identity; and (c) organ identity, which changes gradually in successive organs produced in terminal and axillary flowers and is accompanied by the formation of mosaic organs (Pouteau et al., 1998; Tooke et al., 1998; this work).
Progressive changes in plant morphology are also observed in other species during the transition from vegetative to floral development. This is often characterized by heteroblasty in successive leaves, which show gradual changes in morphology (Poethig, 1997), or by gradual changes in organ identity adopted by successive primordia, such as in the Nymphaeaceae family (Sporne, 1974). Furthermore, mosaic flowering shoots have been described in a number of mustard species including Arabidopsis (Hempel and Feldman, 1995; Hempel, 1996), in which flowering mutants often exhibit a progressively weaker mutant phenotype in successive flowers along the main axis (Haughn et al., 1995).
The concept of continuum morphology can be applied to interpret the form continuum exhibited by I. balsamina and other species during the transition from vegetative to floral development. According to continuum morphology, as opposed to classical morphology, plant organs and structures are not sharply delimited from each other but instead form a continuum. The plant itself constitutes a morphological unit in which various morphological subunits are successively integrated and are continuously modified throughout the life of the plant (Sattler, 1996; Sattler and Rutishauser, 1997). The form continuum exhibited during flowering in I. balsamina suggests the quantitative nature of underlying developmental changes.
Influence of the Inductive Signal on the Form Continuum
The evidence suggests that this continuous variation in form reflects the amount and/or translocation rate of the inductive signal from the leaf. Removal of the inductive signal in I. balsamina results in reversion and can cause an increase in the form continuum at all three levels mentioned above (Pouteau et al., 1998; Tooke et al., 1998; this work). Increased developmental plasticity during reversion is also illustrated by the uncoupling of terminal inflorescence traits such as the formation of axillary flowers, the suppression of internode elongation, and modifications in leaf morphology.
Reversion in a number of other species under suboptimal or noninductive conditions can also reveal more progressive changes than those observed under continuous inductive conditions (Battey and Lyndon, 1990). Noninductive conditions can also cause a more pronounced form continuum in nonreverting species such as Arabidopsis and snapdragon. Arabidopsis plants grown under noninductive conditions exhibit a more gradual transition from rosette leaves to cauline leaves and to leaf suppression and increased severity in flowering mutant phenotypes (Haughn et al., 1995; Okamuro et al., 1996, 1997; Mizukami and Ma, 1997). In snapdragon transfer experiments from inductive to noninductive conditions resulted in more gradual changes and uncoupling of inflorescence features (Bradley et al., 1996). Therefore, a sufficient quantity of inductive signal may be required in most species to allow rapid progression to flowering and to mask the gradual nature of developmental changes that underlie this transition.
Role of Meristem Identity Genes in the Progression to Flowering
The activation of genes involved in the control of floral meristem identity has been shown to participate in rapid progression to flowering in Arabidopsis (Haughn et al., 1995; Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995). The I. balsamina homologs of two meristem identity genes, Imp-FLO and Imp-FIM, exhibit a number of differences in their transcription patterns in the apical meristem of I. balsamina compared with the patterns of their orthologs observed in Arabidopsis and snapdragon axillary meristems (Pouteau et al., 1997, 1998). Here we show that Imp-FLO and Imp-FIM transcription patterns are essentially the same in terminal and axillary flowers of I. balsamina. Therefore, the differences observed from other species are not specific to the terminal flower. These differences are also observed in the apical meristem of a nonreverting line of I. balsamina in which the progression to flowering is gradual, as in the reverting line used in this work (Pouteau et al., 1998; F. Tooke and N.H. Battey, unpublished data). Nonreversion in this line results from the persistence of an induced state in the leaf, and reversion can be obtained by removing the induced leaves (Tooke et al., 1998). It is therefore possible that the specific pattern of Imp-FLO and Imp-FIM transcription in I. balsamina is associated with the lack of commitment of the meristem in this species.
Quantitative Control of Inflorescence Formation
The apical meristem is usually considered to be the main recipient of the inductive signal exported from the leaf. As a result of evocation, the apical meristem is expected to act as the mediator of flowering in the plant (Bernier, 1988, 1997). The prevailing sequential interpretation of flowering has led to the postulate that floral axillary structures are initiated de novo by the apical meristem after the onset of evocation. For example, in white mustard the initiation of the first flowers occurs 60 h after the beginning of the inductive LD conditions (Bernier, 1997). In Arabidopsis the existence of three different phases of development has been suggested previously based on the observation of three distinct types of plant morphological units marked, respectively, by rosette leaves, axillary flowering shoot/cauline leaves, and flowers (Schultz and Haughn, 1991; Huala and Sussex, 1992; Shannon and Meeks-Wagner, 1993). However, by determining when primordia are initiated relative to the beginning of the inductive treatment, it has been shown that the shoot apical meristem can cease producing leaf primordia and begin to produce flowers during the first inductive photoperiod cycle (Hempel and Feldman, 1994). This led to the conclusion that there are only two phases of development in Arabidopsis, a vegetative phase and a reproductive phase, the latter being characterized by de novo initiation of flower primordia.
We show that axillary flower production in I. balsamina does not require de novo initiation of primordia by the apical meristem. Although during the standard inductive treatment from d 0, the lowermost axillary flower was produced in the axil of the youngest primordium morphologically detectable at the time of transfer to SD conditions, after induction at a later stage (i.e. from d 15) all axillary flowers were derived from primordia initiated before the beginning of the inductive treatment, and transfer to LD conditions after 5 d of SD conditions was less effective in promoting reversion. This stronger induction response could reflect increased competence of the plant to respond to the inductive signal, but, because more leaves are present, a more likely explanation is that this reflects a higher amount of inductive signal.
Because it results from the lack of persistence of the induced state of the leaf (Pouteau et al., 1997; Tooke et al., 1998), reversion in I. balsamina provides a means to analyze the quantitative influence of the inductive signal on inflorescence development. The gradual increase in floral features exhibited by mature plants after progressively longer periods of induction in SD conditions is expected to reflect the increase in the number of induced leaves and in the amount of inductive signal exported by them. Analysis of fate changes in axillary meristem primordia initiated before transfer to inductive SD conditions on d 0 shows that these primordia can generate axillary shoots, mosaic axillary shoots, or axillary inflorescences, depending on the amount of inductive signal in the plant. We conclude that the progression of flowering in the plant depends on the amount of inductive signal, which is influenced by external inductive conditions and plant age. Therefore, the apparent requirement for de novo initiation of axillary meristem primordia in other plants, such as white mustard and Arabidopsis (Hempel and Feldman, 1994; Bernier, 1997), may be fortuitous and result from insufficient inductive signal under the experimental conditions.
Basipetal Progression of Inflorescence Formation
Although its specific cell-partitioning function is not required for flower initiation, it is possible that the evoked apical meristem acts as the controller of flowering by being the main recipient of the inductive signal exported from the leaf. However, it is unclear whether the inductive signal can be directly exported into developing axillary meristems. Hempel and Feldman (1995) found in Arabidopsis that the sides of mosaic flowering shoots farthest from the apical meristem are specified as “flowers” and concluded from this observation that the inductive signal coming from the leaf can directly induce primordia to develop as flowers. However, it is possible that the side farthest from the apical meristem is the most responsive to the inductive signal coming from the leaf, either directly or via the apical meristem.
Analysis of fate changes after progressively longer periods of induction in axillary meristem primordia initiated before and during the inductive treatment shows that the development of floral traits is greatest in uppermost primordia and decreases in progressively lower primordia. One interpretation could be that the position of primordia relative to the apical meristem is important. Floral conversion may be more efficient in the uppermost primordia than in lower primordia because they are nearest the apical meristem. This would imply that a direct influence of the inductive signal from the leaf is not essential and that this influence is mostly mediated by the apical meristem. According to this interpretation, the apical meristem would act as the main recipient of the inductive signal from the leaf and would therefore control the specification of axillary flowers and inflorescences, possibly through the production of a secondary signal.
Another interpretation could be that the age of axillary meristem primordia rather than their position relative to the apical meristem is important. Uppermost primordia could be the most responsive to the inductive signal, irrespective of its acting directly from the leaf or via the apical meristem, because these primordia are the youngest at the beginning of the inductive treatment. However, a difficulty for type-2 flower primordia that are initiated after transfer to SD conditions is that the upper ones are more responsive to the inductive signal than the primordia below, although the former must be induced for a shorter period than the latter. One explanation could be that upper type-2 flower primordia are influenced by higher amounts of inductive signal at an earlier stage. Alternatively, although they cannot be detected at a morphological level at the time of transfer to SD conditions, these primordia could be already partitioned as cell sectors. In any case, primordia initiated after the transfer to SD conditions and the youngest primordia initiated before transfer to SD conditions correspond to anlagen, which differentiate only later into leaf/internode/axillary structure units. It would be interesting to determine to what extent the fate of axillary structures is influenced by the previous history of their anlagen.
In summary, inflorescence architecture in I. balsamina can be explained by the response of axillary meristem primordia to the quantity of inductive signal, a response that is conditioned by the age and/or position of the primordia and allows undifferentiated axillary meristem primordia initiated before evocation to adopt different fates. This developmental plasticity results in various combinations of vegetative and floral characters. Our interpretation is that vegetative and reproductive phases are not separate and antagonistic but interpenetrate each other to varying extents depending on the quantity of inductive signal.
ACKNOWLEDGMENTS
We are grateful to Dr Enrico Coen and members of his laboratory and to members of the laboratory of N.B. for their support and encouragement.
Abbreviations:
- LD
long-day
- SD
short-day
Footnotes
This work was funded by the Biotechnology and Biological Science Research Council Cell Molecular Biology Initiative (grant no. AT45/559 to F.T.). S.P. was supported by the Institut National de la Recherche Agronomique, Versailles, France.
LITERATURE CITED
- Battey NH, Lyndon RF. Changes in apical growth and phyllotaxis on flowering and reversion in Impatiens balsamina L. Ann Bot. 1984;54:553–567. [Google Scholar]
- Battey NH, Lyndon RF. Ann Bot. 1986;58:333–341. [Google Scholar]
- Battey NH, Lyndon RF. Ann Bot. 1988;61:9–16. [Google Scholar]
- Battey NH, Lyndon RF. Reversion of flowering. Bot Rev. 1990;56:162–189. [Google Scholar]
- Bernier G. The control of floral evocation and morphogenesis. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:175–219. [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier G. Growth changes in the shoot apex of Sinapis alba during transition to flowering. J Exp Bot. 1997;48:1071–1077. [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen ES. Nature. 1996;379:791–797. doi: 10.1038/379791a0. [DOI] [PubMed] [Google Scholar]
- Bradley D, Vincent C, Carpenter R, Coen ES. Development. 1993;122:1535–1544. doi: 10.1242/dev.122.5.1535. [DOI] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliot R, Murphy G, Carpenter R. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Evans LT. The Induction of Flowering. Some Case Histories. Melbourne, Australia: MacMillan; 1969. [Google Scholar]
- Haughn GW, Schultz EA, Martinez-Zapater JM. The regulation of flowering in Arabidopsis thaliana: meristems, morphogenesis, and mutants. Can J Bot. 1995;73:959–981. [Google Scholar]
- Hempel FD. Morphology of the transition to flowering in mustards. Semin Cell Dev Biol. 1996;7:391–400. [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. [Google Scholar]
- Hempel FD, Feldman LJ. Plant J. 1995;8:725–731. doi: 10.1046/j.1365-313x.1995.08050725.x. [DOI] [PubMed] [Google Scholar]
- Huala E, Sussex IM. LEAFY interacts with floral homeotic genes to regulate Arabidopsis floral development. Plant Cell. 1992;4:901–913. doi: 10.1105/tpc.4.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram GC, Goodrich J, Wilkinson MD, Simon R, Haughn GW, Coen ES. Plant Cell. 1995;7:1501–1510. doi: 10.1105/tpc.7.9.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D. Curr Biol. 1997;7:95–104. doi: 10.1016/s0960-9822(06)00053-4. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- McDaniel C. Induction and determination: developmental concepts. Flowering Newslett. 1992;14:3–6. [Google Scholar]
- Mizukami Y, Ma H. Plant Cell. 1997;9:393–408. doi: 10.1105/tpc.9.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro JK, Bart G, DenBoer BGW, Lotys-Prass C, Szeto W, Jofuku KJ. Proc Natl Acad Sci USA. 1996;93:13831–13836. doi: 10.1073/pnas.93.24.13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro JK, Szeto W, Lotys-Prass C, Jofuku KJ. Plant Cell. 1997;9:37–47. doi: 10.1105/tpc.9.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS. Leaf morphogenesis in flowering plants. Plant Cell. 1997;9:1077–1087. doi: 10.1105/tpc.9.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S, Nicholls D, Tooke F, Coen E, Battey N. Flowering Newslett. 1995;19:31–35. [Google Scholar]
- Pouteau S, Nicholls D, Tooke F, Coen E, Battey N. Development. 1997;124:3343–3351. doi: 10.1242/dev.124.17.3343. [DOI] [PubMed] [Google Scholar]
- Pouteau S, Nicholls D, Tooke F, Coen E, Battey N. Transcription pattern of a FIM homologue in Impatiens during floral development and reversion. Plant J. 1998;14:235–246. doi: 10.1046/j.1365-313x.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- Sattler R. Classical morphology and continuum morphology: opposition and continuum. Ann Bot. 1996;78:577–581. [Google Scholar]
- Sattler R, Rutishauser R. The fundamental relevance of morphology and morphogenesis to plant research. Ann Bot. 1997;80:571–582. [Google Scholar]
- Schultz EA, Haughn GW. Plant Cell. 1991;3:771–781. doi: 10.1105/tpc.3.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. Plant Cell. 1993;5:639–655. doi: 10.1105/tpc.5.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Carpenter R, Doyle S, Coen E. Fimbriata controls flower development by mediating between meristem and organ identity genes. Cell. 1994;78:99–107. doi: 10.1016/0092-8674(94)90576-2. [DOI] [PubMed] [Google Scholar]
- Sporne KR (1974) The Morphology of Angiosperms—The Structure and Evolution of Flowering Plants. Hutchinson, London
- Tooke F, Pouteau S, Battey N. Non-reversion of Impatiens in the absence of meristem commitment. J Exp Bot. 1998;49:1681–1688. [Google Scholar]
- Weberling F. Morphology of Flowers and Inflorescences. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-n. [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. Physiology of flower formation. Annu Rev Plant Physiol. 1976;27:321–348. [Google Scholar]