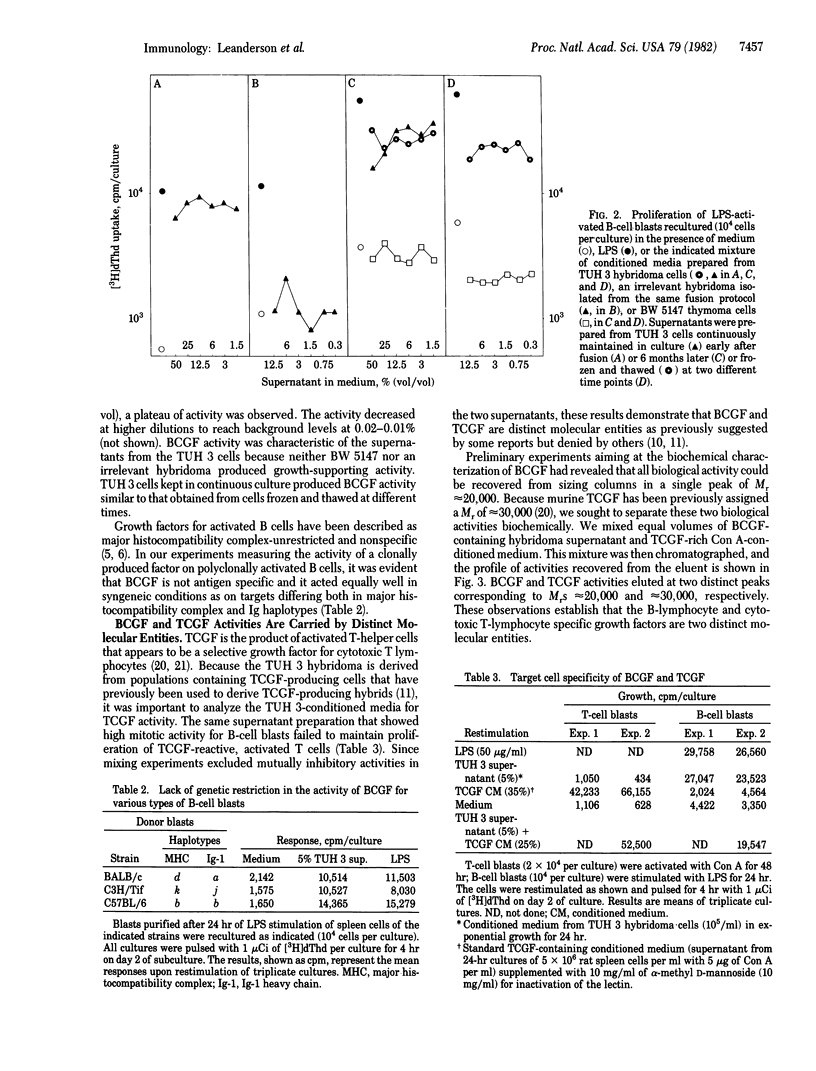
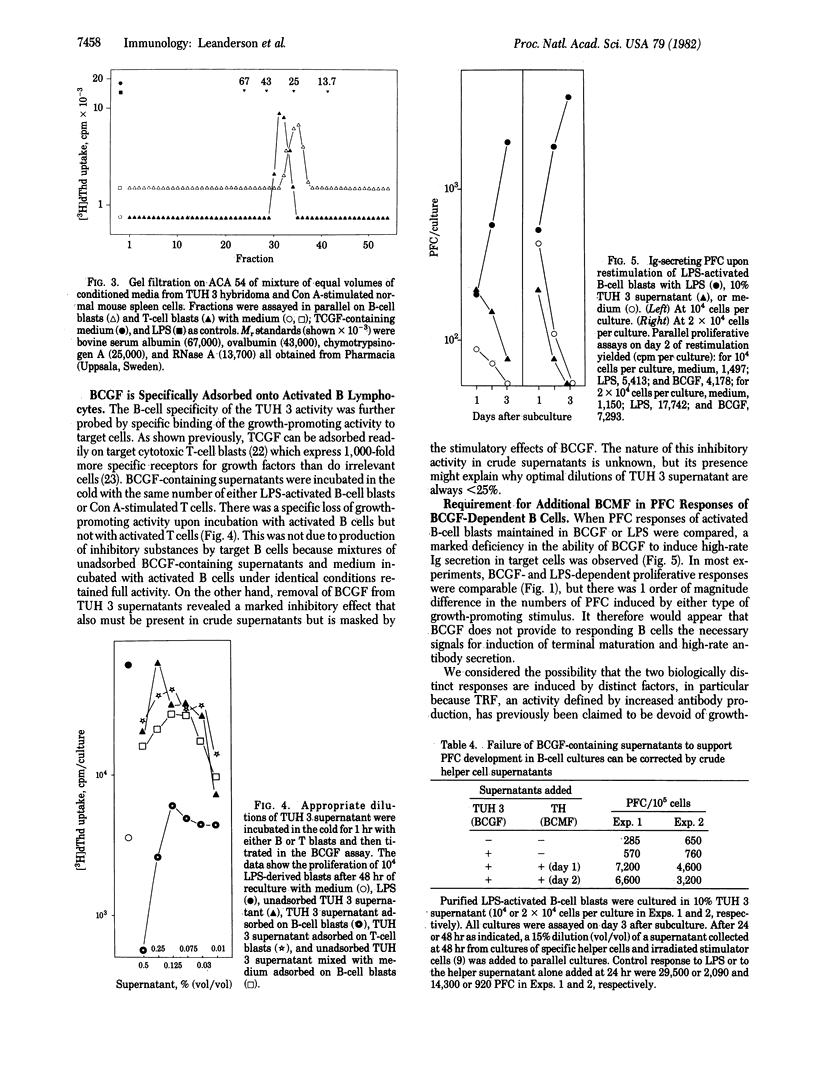
Abstract
A T-cell hybridoma was derived by somatic cell hybridization between concanavalin A-activated BALB/c spleen cells and the AKR thymoma BW 5147. Media conditioned by hybridoma cells, even at high dilutions (1:1,000) support the growth of lipopolysaccharide-stimulated B-cell blasts but not that of T-cell growth factor (TCGF)-reactive T-cells. This activity, herein designated B-cell growth factor (BCGF), has a Mr of approximately equal to 20,000 and it can readily be separated from TCGF (Mr approximately equal to 30,000) by gel filtration. BCGF is constitutively produced by the hybridoma cells, it is removed from conditioned media by incubation with target cells at +4 degrees C, and it is equally effective on B-cell blasts carrying different major histocompatibility complex and Ig haplotypes. BCGF shows no T-cell replacing factor (TRF) activity, and it is poor in supporting the development of Ig-secreting plaque-forming cells in B-cell blast cultures. Terminal maturation, however, can be induced in BCGF-dependent blasts by addition of conditioned media from normal helper T cell cultures, suggesting that two distinct factors are involved in the helper cell-dependent growth and maturation of B lymphocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Melchers F. T cell-dependent activation of resting B cells: requirement for both nonspecific unrestricted and antigen-specific Ia-restricted soluble factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2497–2501. doi: 10.1073/pnas.78.4.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Mitogen-activated B-cell blasts reactive to more than one mitogen. J Exp Med. 1979 Mar 1;149(3):553–564. doi: 10.1084/jem.149.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabe R. R., Martinez-Alonso C., Coutinho A. The specificity of nonspecific concanavalin A-induced helper factors. Eur J Immunol. 1979 Jul;9(7):546–552. doi: 10.1002/eji.1830090710. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Larsson E. L., Grönvik K. O., Andersson J. Studies on T lymphocyte activation II. The target cells for concanavalin A-induced growth factors. Eur J Immunol. 1979 Aug;9(8):587–592. doi: 10.1002/eji.1830090803. [DOI] [PubMed] [Google Scholar]
- Dennert G., Weiss S., Warner J. F. T cells may express multiple activities: specific allohelp, cytolysis, and delayed-type hypersensitivity are expressed by a cloned T-cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4540–4543. doi: 10.1073/pnas.78.7.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Ivars F., Coutinho A., Larsson E. L. Regulation of T cell growth factor production: arrest of TCGF production after 18 hours in normal lectin-stimulated mouse spleen cell cultures. J Immunol. 1981 Aug;127(2):407–411. [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Schimpl A., Wecker E. Autoradiographic studies on the proliferation of antibody-producing cells in vitro. J Exp Med. 1974 Mar 1;139(3):754–760. doi: 10.1084/jem.139.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Lefkovits I., Elliott B., Coutinho A. Derivation of hybrids between a thymoma line and spleen cells activated in a mixed leukocyte reaction. Eur J Immunol. 1977 Nov;7(11):758–761. doi: 10.1002/eji.1830071103. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Lindahl K. F., Langhorne J., Coutinho A. Quantitative studies on concanavalin A-induced, TCGF-reactive T cells. I. Correlation between proliferation and lectin-dependent cytolytic activity. J Immunol. 1981 Sep;127(3):1081–1085. [PubMed] [Google Scholar]
- Melchers F., Andersson J., Lernhardt W., Schreier M. H. H-2-unrestricted polyclonal maturation without replication of small B cells induced by antigen-activated T cell help factors. Eur J Immunol. 1980 Sep;10(9):679–685. doi: 10.1002/eji.1830100905. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Andersson J., Lernhardt W., Melchers F. Antigen-specific T-helper cells stimulate H-2-compatible and H-2-incompatible B-cell blasts polyclonally. J Exp Med. 1980 Jan 1;151(1):194–203. doi: 10.1084/jem.151.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Restricted helper function of F1 hybrid T cells positively selected to heterologous erythrocytes in irradiated parental strain mice. II. Evidence for restrictions affecting helper cell induction and T-B collaboration, both mapping to the K-end of the H-2 complex. J Exp Med. 1978 Apr 1;147(4):1159–1174. doi: 10.1084/jem.147.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]


