Abstract
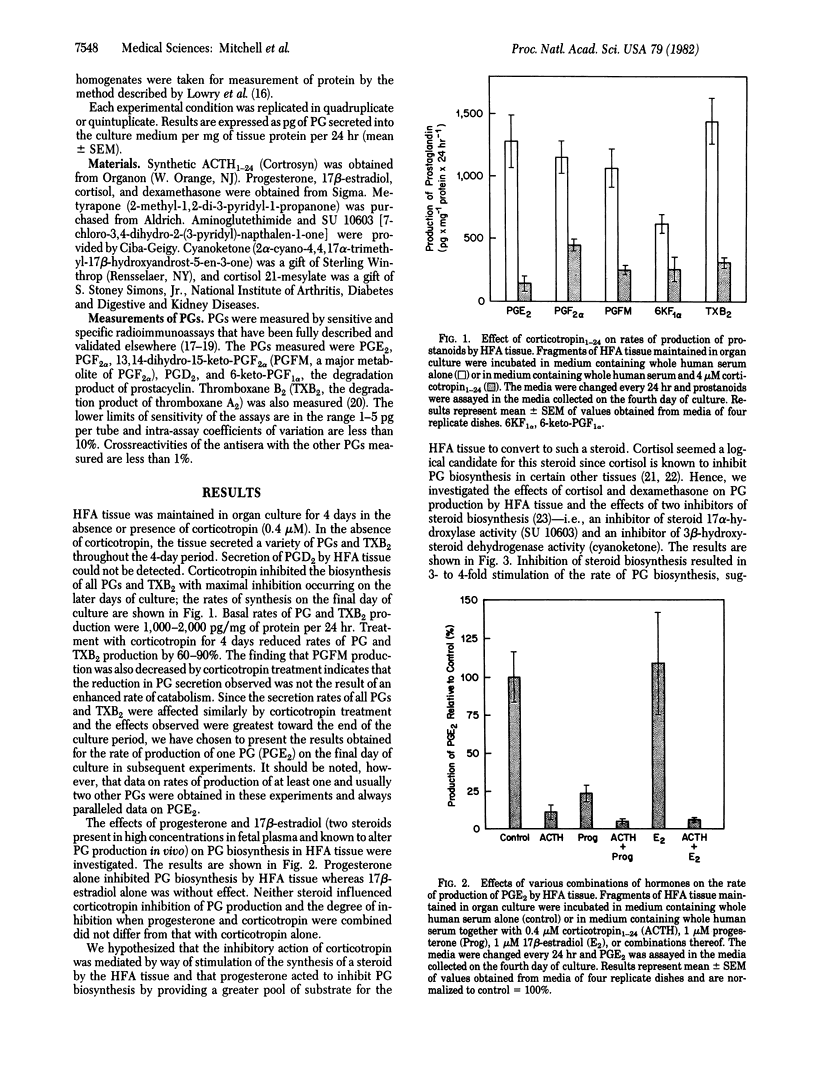
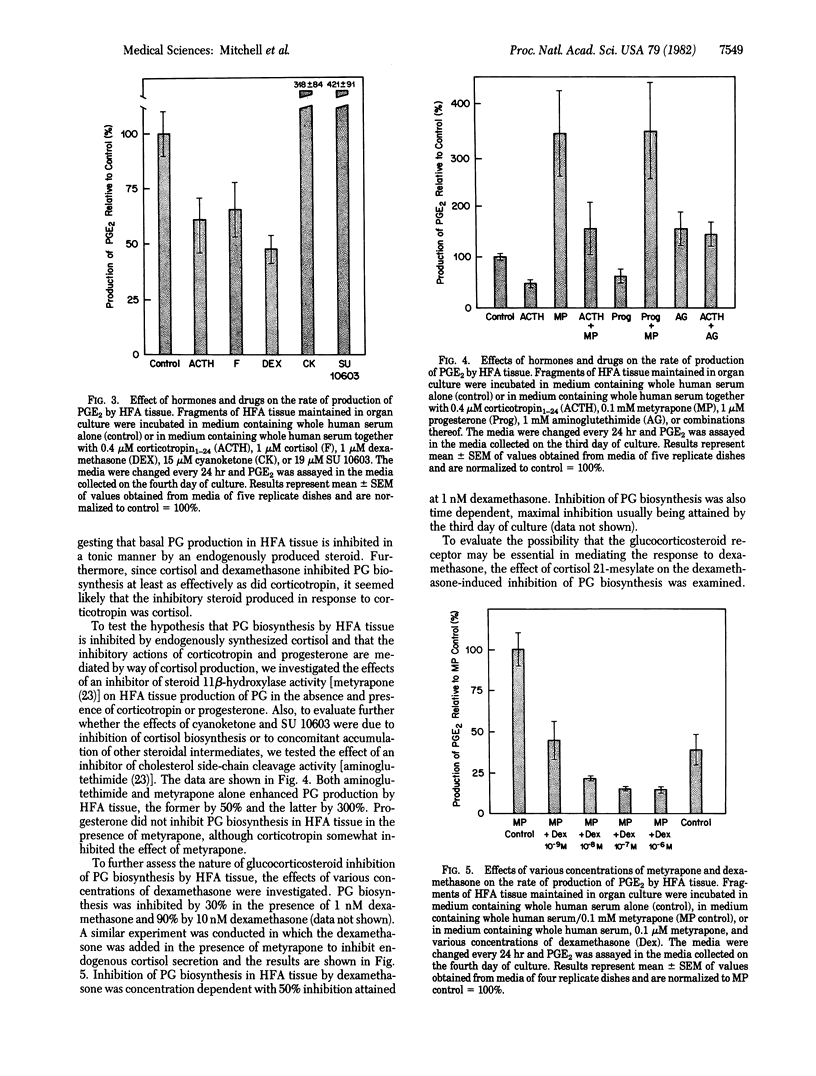
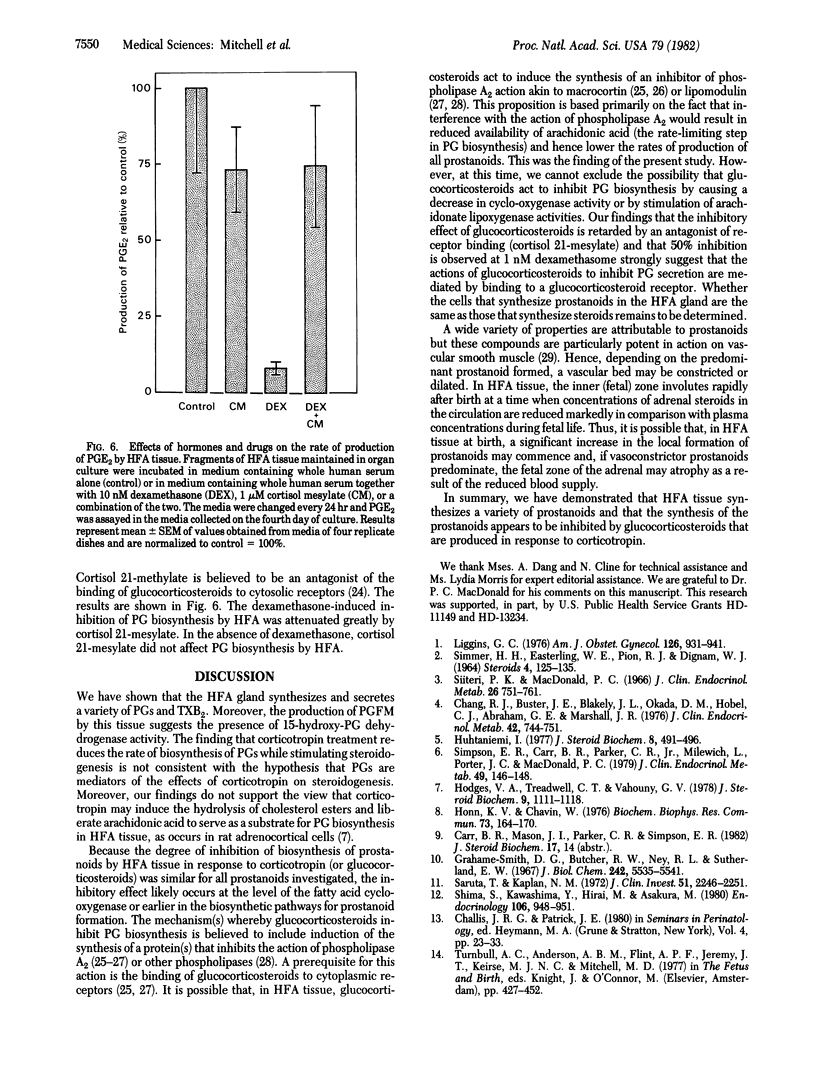
Human fetal adrenal (HFA) tissue was maintained in organ culture to evaluate the biosynthesis of prostaglandins and hormonal regulation of prostaglandin formation by this tissue. The HFA tissue secreted substantial amounts of prostaglandin E2, prostaglandin F2α, 13,14-dihydro-15-ketoprostaglandin F2α, 6-ketoprostaglandin F1α, and thromboxane B2; secretion of prostaglandin D2 could not be demonstrated. Prostaglandin biosynthesis in HFA tissue was inhibited in a time-dependent manner by corticotropin (ACTH; 0.4 μM); by the fourth day of culture, the extent of inhibition of biosynthesis of each prostaglandin was 60-90%. Progesterone (1 μM), cortisol (1 μM), and dexamethasone (1 μM) inhibited prostaglandin biosynthesis whereas estradiol (1 μM) did not. Of the compounds tested for inhibitory activity, dexamethasone was the most potent. An inhibitor of 11β-hydroxylase activity (metyrapone; 0.1 mM) effectively eliminated the inhibition of prostaglandin biosynthesis caused by corticotropin and progesterone. Metyrapone treatment alone caused a 3-fold increase in prostaglandin biosynthesis by fetal adrenal tissues. Similar stimulatory effects resulted from treatment with inhibitors of (i) 3β-hydroxysteroid dehydrogenase (cyanoketone; 15 μM), (ii) steroid 17α-hydroxylase (SU 10603; 19 μM), and (iii) cholesterol side-chain cleavage (aminoglutethimide; 1 mM). Inhibition of prostaglandin biosynthesis by dexamethasone in the presence or absence of metyrapone was concentration dependent and 50% inhibition could be demonstrated at 1 nM. A competitive inhibitor of the binding of glucocorticosteroids to cytoplasmic receptors (cortisol 21-mesylate; 1 μM) significantly reduced the inhibition of prostaglandin biosynthesis effected by dexamethasone (10 nM). These findings suggest that prostaglandin biosynthesis in the HFA gland is regulated by endogenously synthesized glucocorticosteroids, the actions of which are mediated by a glucocorticosteroid receptor. Such glucocorticosteroids induce the synthesis of a substance that inhibits prostaglandin biosynthesis.
Keywords: prostanoid, corticotropin, cortisol, inhibition
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Chang R. J., Buster J. E., Blakely J. L., Okada D. M., Hobel C. J., Abraham G. E., Marshall J. R. Simultaneous comparison of delta 5-3beta-hydroxysteroid levels in the fetoplacental circulation of normal pregnancy in labor and not in labor. J Clin Endocrinol Metab. 1976 Apr;42(4):744–751. doi: 10.1210/jcem-42-4-744. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979 Mar 29;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- Gower D. B. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem. 1974 Aug;5(5):501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Gryglewski R. J., Panczenko B., Korbut R., Grodzinska L., Ocetkiewicz A. Corticosteroids inhibit prostaglandin release from perfused mesenteric blood vessels of rabbit and from perfused lungs of sensitized guinea pig. Prostaglandins. 1975 Aug;10(2):343–355. doi: 10.1016/0090-6980(75)90053-2. [DOI] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hodges V. A., Treadwell C. T., Vahouny G. V. Prostaglandin E2-induced hydrolysis of cholesterol esters in rat adrenocortical cells. J Steroid Biochem. 1978 Nov;9(11):1111–1118. doi: 10.1016/0022-4731(78)90041-9. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Chavin W. Prostaglandin modulation of the mechanism of ACTH action in the human adrenal. Biochem Biophys Res Commun. 1976 Nov 8;73(1):164–170. doi: 10.1016/0006-291x(76)90511-8. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. Studies on steroidogenesis and its regulation in human fetal adrenal and testis. J Steroid Biochem. 1977 May;8(5):491–497. doi: 10.1016/0022-4731(77)90251-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis G. P., Piper P. J. Inhibition of release of prostaglandins as an explanation of some of the actions of anti-inflammatory corticosteroids. Nature. 1975 Mar 27;254(5498):308–311. doi: 10.1038/254308a0. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol. 1976 Dec 1;126(7):931–941. doi: 10.1016/0002-9378(76)90680-3. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D. A sensitive radioimmunoassay for 6-keto-prostaglandin F1alpha: preliminary observations on circulating concentrations. Prostaglandins Med. 1978 Jul;1(1):13–21. doi: 10.1016/0161-4630(78)90072-1. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Flint A. P. Prostaglandin production by intra-uterine tissues from periparturient sheep: use of a superfusion technique. J Endocrinol. 1978 Jan;76(1):111–121. doi: 10.1677/joe.0.0760111. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Jamieson D. R., Sellers S. M., Turnbull A. C. 6-Keto-PGF1 alpha: concentrations in human umbilical plasma and production by umbilical vessels. Adv Prostaglandin Thromboxane Res. 1980;7:891–896. [PubMed] [Google Scholar]
- Mitchell M. D., Kraemer D. L., Strickland D. M. The human placenta: a major source of prostaglandin D2. Prostaglandins Leukot Med. 1982 Apr;8(4):383–387. doi: 10.1016/0262-1746(82)90061-0. [DOI] [PubMed] [Google Scholar]
- Saruta T., Kaplan N. M. Adrenocortical steroidogenesis: the effects of prostaglandins. J Clin Invest. 1972 Sep;51(9):2246–2251. doi: 10.1172/JCI107033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima S., Kawashima Y., Hirai M., Asakura M. Studies on cyclic nucleotides in the adrenal gland. X. Effects of adrenocorticotropin and prostaglandin on adenylate cyclase activity in the adrenal cortex. Endocrinology. 1980 Mar;106(3):948–951. doi: 10.1210/endo-106-3-948. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., MacDonald P. C. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966 Jul;26(7):751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Johnson D. F. Unique long-acting antiglucocorticoid in whole and broken cell systems. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5167–5171. doi: 10.1073/pnas.77.9.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Carr B. R., Parker C. R., Jr, Milewich L., Porter J. C., MacDonald P. C. The role of serum lipoproteins in steroidogenesis by the human fetal adrenal cortex. J Clin Endocrinol Metab. 1979 Jul;49(1):146–148. doi: 10.1210/jcem-49-1-146. [DOI] [PubMed] [Google Scholar]


