Abstract
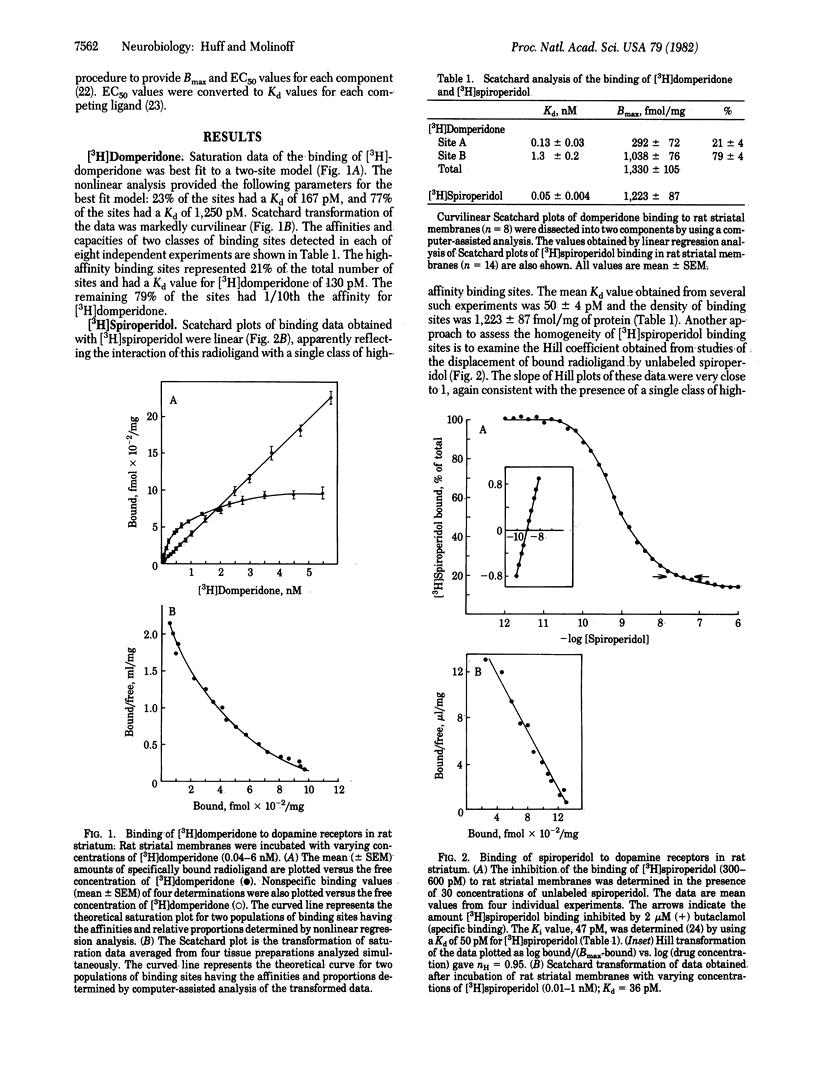
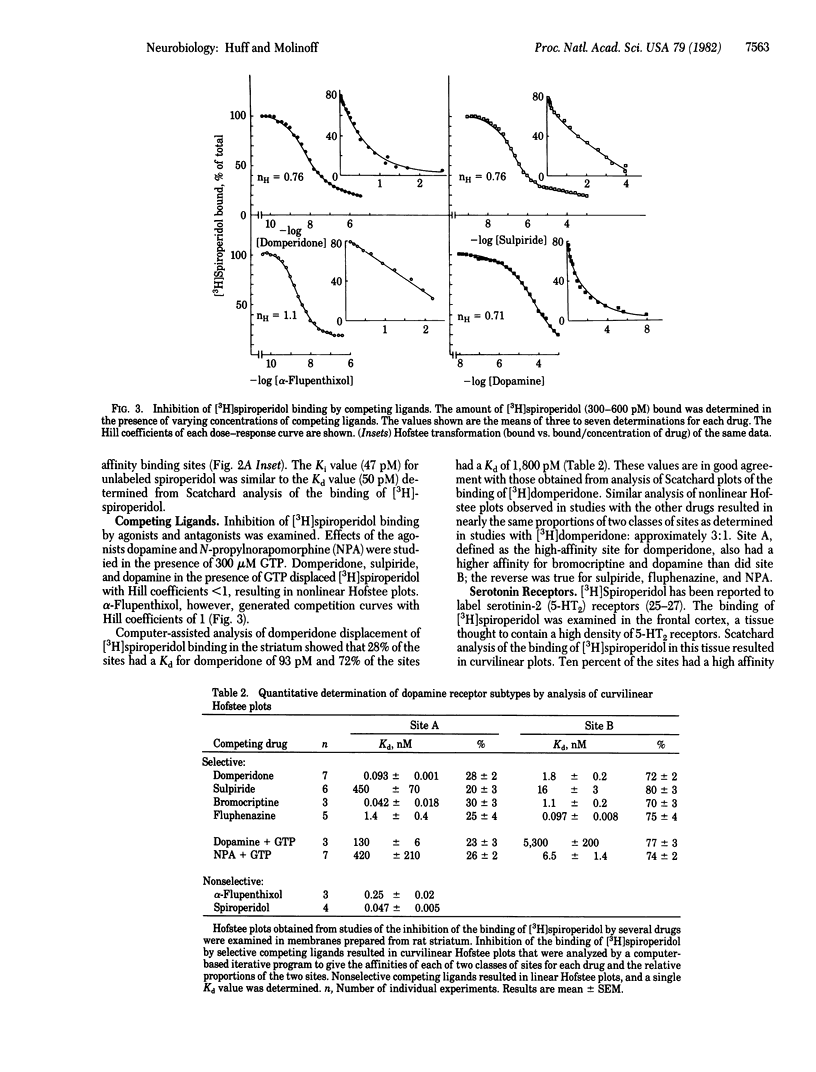
The binding of [3H]domperidone and [3H]spiroperidol was examined in membranes prepared from rat striatum. Scatchard analysis of the binding of [3H]domperidone resulted in curvilinear plots consistent with the presence of multiple classes of binding sites. Nonlinear regression analysis of untransformed data showed that the curvature was best explained by the presence of two populations of binding sites. Scatchard plots of the binding of [3H]spiroperidol were linear, suggesting that this radioligand binds to a single class of receptors. However, results obtained in studies of the inhibition of [3H]spiroperidol binding by a number of competing ligands were not consistent with the interaction of these agents with a single class of binding sites. Computer-assisted analysis of the Hofstee plots of six competing ligands gave the same relative proportion for two classes of sites as determined by analysis of the binding of [3H]domperidone. The two classes of receptors labeled with [3H]spiroperidol had affinities for domperidone that were similar to those of the two populations of binding sites for [3H]domperidone. Furthermore, the number of binding sites for [3H]spiroperidol was equal to the total number of binding sites for [3H]domperidone. These findings suggest that the two radioligands bind to the same two classes of binding sites. It is unlikely that either of the two classes of striatal sites are receptors for serotonin. The approach described will make it possible to assess the effects of physiological or pharmacological manipulations on the densities or properties of subtypes of dopamine receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry M., Martres M. P., Schwartz J. C. 3H-Domperidone: a selective ligand for dopamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1979 Sep;308(3):231–237. doi: 10.1007/BF00501387. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Creese I., Snyder S. H. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol. 1976 Sep;12(5):800–812. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clement-Cormier Y. C., George R. J. Multiple dopamine binding sites: subcellular localization and biochemical characterization. J Neurochem. 1979 Mar;32(3):1061–1069. doi: 10.1111/j.1471-4159.1979.tb04594.x. [DOI] [PubMed] [Google Scholar]
- Creese I., Stewart K., Snyder S. H. Species variation in dopamine receptor binding. Eur J Pharmacol. 1979 Nov 23;60(1):55–66. doi: 10.1016/0014-2999(79)90052-9. [DOI] [PubMed] [Google Scholar]
- Creese I., Usdin T., Snyder S. H. Guanine nucleotides distinguish between two dopamine receptors. Nature. 1979 Apr 5;278(5704):577–578. doi: 10.1038/278577a0. [DOI] [PubMed] [Google Scholar]
- Cross A. J., Owen F. Characteristics of 3H-cis-flupenthixol binding to calf brain membranes. Eur J Pharmacol. 1980 Aug 8;65(4):341–347. doi: 10.1016/0014-2999(80)90337-4. [DOI] [PubMed] [Google Scholar]
- Hegstrand L. R., Minneman K. P., Molinoff P. B. Multiple effects of guanosine triphosphate on beta adrenergic receptors and adenylate cyclase activity in rat heart, lung and brain. J Pharmacol Exp Ther. 1979 Aug;210(2):215–221. [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laduron P. M., Leysen J. E. Domperidone, a specific in vitro dopamine antagonist, devoid of in vivo central dopaminergic activity. Biochem Pharmacol. 1979 Jul 15;28(14):2161–2165. doi: 10.1016/0006-2952(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Gommeren W., Laduron P. M. Spiperone: a ligand of choice for neuroleptic receptors. 1. Kinetics and characteristics of in vitro binding. Biochem Pharmacol. 1978 Feb 1;27(3):307–316. doi: 10.1016/0006-2952(78)90233-2. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Tollenaere J. P., Laduron P. M. Serotonergic component of neuroleptic receptors. Nature. 1978 Mar 9;272(5649):168–171. doi: 10.1038/272168a0. [DOI] [PubMed] [Google Scholar]
- Maguire M. E., Van Arsdale P. M., Gilman A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976 Mar;12(2):335–339. [PubMed] [Google Scholar]
- Marchais D., Tassin J. P., Bockaert J. Dopaminergic component of [3H]spiroperidol binding in the rat anterior cerebral cortex. Brain Res. 1980 Feb 3;183(1):235–240. doi: 10.1016/0006-8993(80)90136-5. [DOI] [PubMed] [Google Scholar]
- Martres M. P., Baudry M., Schwartz J. C. Characterization of 3H-domperidone binding on striatal dopamine receptors. Life Sci. 1978 Oct 30;23(17-18):1781–1784. doi: 10.1016/0024-3205(78)90108-x. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. Simultaneous determination of beta-1 and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol. 1979 Jul;16(1):34–46. [PubMed] [Google Scholar]
- Molinoff P. B., Wolfe B. B., Weiland G. A. Quantitative analysis of drug-receptor interactions: II. Determination of the properties of receptor subtypes. Life Sci. 1981 Aug 3;29(5):427–443. doi: 10.1016/0024-3205(81)90208-3. [DOI] [PubMed] [Google Scholar]
- Munemura M., Cote T. E., Tsuruta K., Eskay R. L., Kebabian J. W. The dopamine receptor in the intermediate lobe of the rat pituitary gland: pharmacological characterization. Endocrinology. 1980 Dec;107(6):1676–1683. doi: 10.1210/endo-107-6-1676. [DOI] [PubMed] [Google Scholar]
- Pedigo N. W., Reisine T. D., Fields J. Z., Yamamura H. I. 3H-Spiroperidol binding to two receptor sites in both the corpus striatum and frontal cortex of rat brain. Eur J Pharmacol. 1978 Aug 15;50(4):451–453. doi: 10.1016/0014-2999(78)90154-1. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971 Mar 25;246(6):1872–1876. [PubMed] [Google Scholar]
- Rosenfeld M. R., Dvorkin B., Klein P. N., Makman M. H. Differential affinities of molindone, metoclopramide and domperidone for classes of [3H]spiroperidol binding sites in rat striatum: evidence for pharmacologically distinct classes of receptors. Brain Res. 1982 Mar 4;235(1):205–211. doi: 10.1016/0006-8993(82)90214-1. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Creese I., Coyle J. T., Snyder S. H. Dopamine receptors localised on cerebral cortical afferents to rat corpus striatum. Nature. 1978 Feb 23;271(5647):766–768. doi: 10.1038/271766a0. [DOI] [PubMed] [Google Scholar]
- Seeman P. Anti-schizophrenic drugs--membrane receptor sites of action. Biochem Pharmacol. 1977 Oct 1;26(19):1741–1748. doi: 10.1016/0006-2952(77)90340-9. [DOI] [PubMed] [Google Scholar]
- Seeman P., Chau-Wong M., Tedesco J., Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P., Martres M. P., Schwartz J. C. Three classes of dopamine receptor (D-2, D-3, D-4) identified by binding studies with 3H-apomorphine and 3H-domperidone. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(2):89–102. doi: 10.1007/BF00499251. [DOI] [PubMed] [Google Scholar]
- Staunton D. A., Wolfe B. B., Groves P. M., Molinoff P. B. Dopamine receptor changes following destruction of the nigrostriatal pathway: lack of a relationship to rotational behavior. Brain Res. 1981 May 4;211(2):315–327. doi: 10.1016/0006-8993(81)90704-6. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981 Nov 26;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]
- Zahniser N. R., Heidenreich K. A., Molinoff P. B. Binding of [3H]amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene to rat striatal membranes. Effects of purine nucleotides and ultraviolet irradiation. Mol Pharmacol. 1981 May;19(3):372–378. [PubMed] [Google Scholar]
- Zahniser N. R., Molinoff P. B. Effect of guanine nucleotides on striatal dopamine receptors. Nature. 1978 Oct 5;275(5679):453–455. doi: 10.1038/275453a0. [DOI] [PubMed] [Google Scholar]


