Abstract
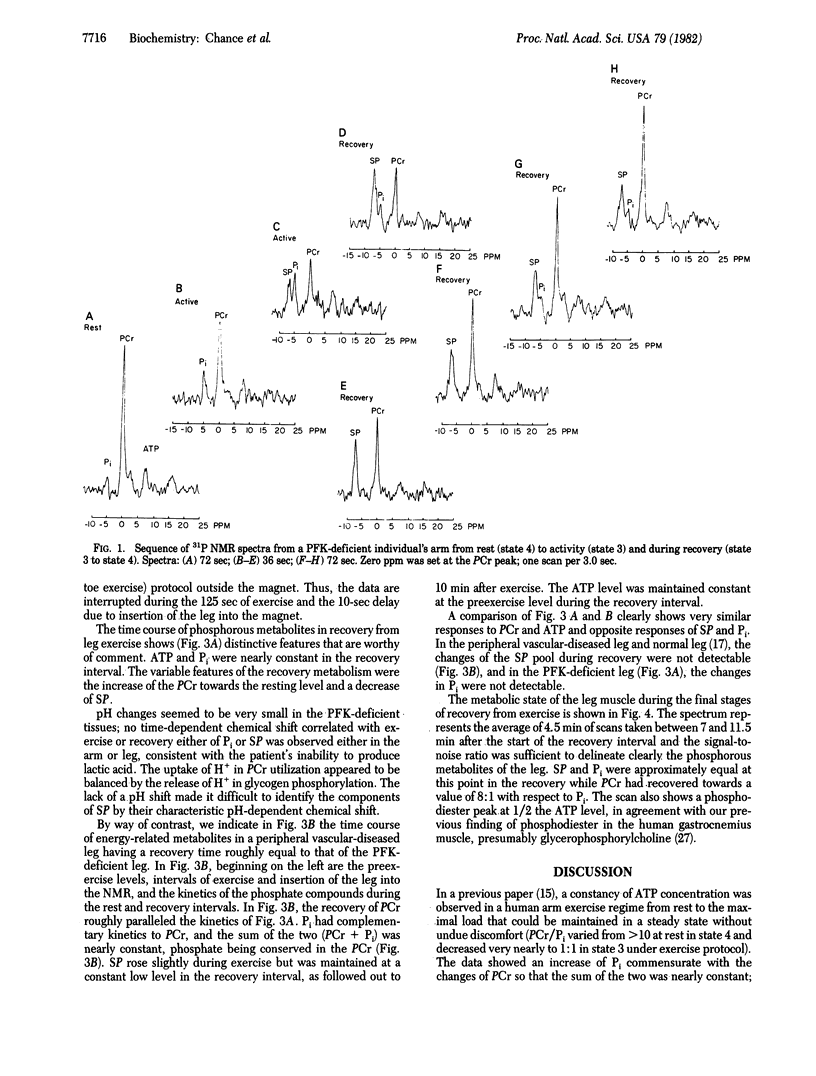
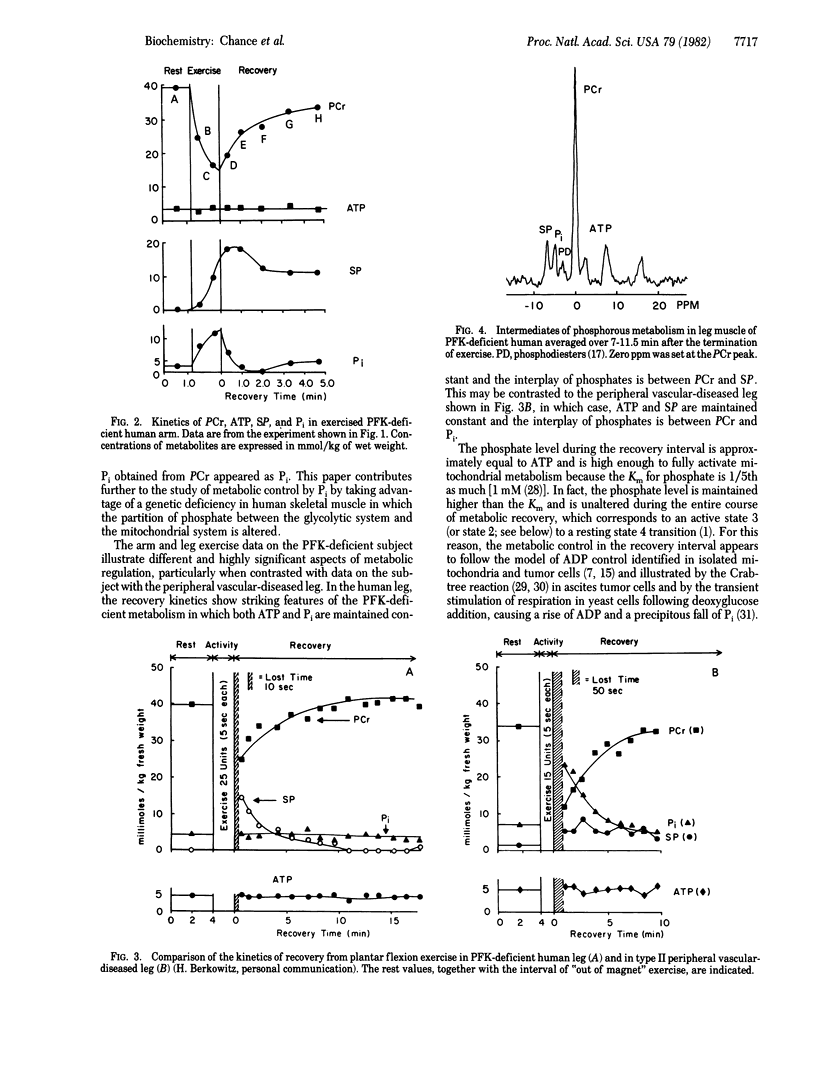
Metabolic control of mitochondrial respiratory activity by Pi and ADP has been evaluated by 31P NMR measurements of the levels of Pi in normal exercising human skeletal tissues in the resting-active-resting transition and, in this contribution, in the phosphofructokinase (PFK)-deficient leg. The latter studies show near constancy of Pi in the recovery from maximal exercise of the leg, with large changes of sugar phosphate (SP) complementary to the changes of phosphocreatine (PCr). The PFK deficiency permits observation of PCr resynthesis in postexercise recovery under conditions of nearly constant Pi and ATP--a phenomenon not evident in normal exercising muscle. The constancy of free Pi is inconsistent with its role in control of mitochondrial activity, leaving ADP as a key metabolic control element. These results help clarify previous controversies on the nature of control of metabolic activity of mitochondria and extend the idea of ADP control of mitochondrial metabolic states in vivo and, in addition, provide an appropriate exercise protocol for the evaluation of a genetic deficiency affecting mitochondrial metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- CHANCE B. Enzymes in action in living cells: the steady state of reduced pyridine nucleotides. Harvey Lect. 1953;49:145–175. [PubMed] [Google Scholar]
- CHANCE B., HESS B. Metabolic control mechanisms. II. Crossover phenomena in mitochondria of ascites tumor cells. J Biol Chem. 1959 Sep;234:2413–2415. [PubMed] [Google Scholar]
- CHANCE B., HESS B. On the control of metabolism in ascites tumor cell suspensions. Ann N Y Acad Sci. 1956 Mar 14;63(5):1008–1016. doi: 10.1111/j.1749-6632.1956.tb50908.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. VI. The efficiency of the reaction. J Biol Chem. 1961 May;236:1577–1584. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J Biol Chem. 1956 Jul;221(1):477–489. [PubMed] [Google Scholar]
- Chance B., Eleff S., Leigh J. S., Jr Noninvasive, nondestructive approaches to cell bioenergetics. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Eleff S., Leigh J. S., Jr, Sokolow D., Sapega A. Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: a gated 31P NMR study. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6714–6718. doi: 10.1073/pnas.78.11.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Nakase Y., Bond M., Leigh J. S., Jr, McDonald G. Detection of 31P nuclear magnetic resonance signals in brain by in vivo and freeze-trapped assays. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4925–4929. doi: 10.1073/pnas.75.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree H. G. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23(3):536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978 Aug 31;274(5674):861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- IBSEN K. H., COE E. L., McKEE R. W. Interrelationships of metabolic pathways in the Ehrlich ascites carcinoma cells. I. Glycolysis and respiration (Crabtree effect). Biochim Biophys Acta. 1958 Nov;30(2):384–400. doi: 10.1016/0006-3002(58)90064-7. [DOI] [PubMed] [Google Scholar]
- Johnson M. J. THE ROLE OF AEROBIC PHOSPHORYLATION IN THE PASTEUR EFFECT. Science. 1941 Aug 29;94(2435):200–202. doi: 10.1126/science.94.2435.200. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–224. [PubMed] [Google Scholar]
- Ross B. D., Radda G. K., Gadian D. G., Rocker G., Esiri M., Falconer-Smith J. Examination of a case of suspected McArdle's syndrome by 31P nuclear magnetic resonance. N Engl J Med. 1981 May 28;304(22):1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A., GUILLORY R. J. An estimation of the true inorganic phosphate content of frog sartorius muscle. J Biol Chem. 1961 Jul;236:2071–2075. [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J Biol Chem. 1959 May;234(5):1036–1041. [PubMed] [Google Scholar]


