Abstract
Androgens are intricately involved in reproductive and aggressive behaviours, but the role of the androgen receptor in mediating these behaviours is less defined. Further, activity of the hypothalamic-pituitary-gonadal (HPG) axis and hypothalamic-pituitary-adrenal (HPA) axis can influence each other at the level of the androgen receptor. Knowledge of the mechanisms for androgens’ effects on behaviours through the androgen receptor will guide future studies in elucidating male reproductive and aggressive behaviour repertoires.
Keywords: Testosterone, HPG, HPA, oestrogen
1. Androgen Receptors in Brain
Androgen receptors (AR’s) are highly conserved and are present in organisms ranging from yeast to humans (1). A vital role of AR’s is the neuromodulation of sexual and possibly aggressive behaviours (2). In this review we will discuss current knowledge of AR’s effects on sexual and aggressive behaviours. Further, we will examine the role of the hypothalamic-pituitary-adrenal axis (HPA) on hypothalamic-pituitary-gonadal (HPG) function at the level of the AR.
1.1 Androgen Mode of Action
Androgens are members of the steroid receptor superfamily of transcription factors (3). Androgens, such as testosterone, act at receptor sites in specific brain areas (4). The primary mode of action is regulation of gene transcription. Androgens enter the cell, bind to the cytosolic androgen receptor (AR), induce a conformational change that causes the dissociation of heat shock proteins and translocation of the receptor from the cytosol into the nucleus, and finally dimerization of the receptor (5). The AR dimer binds to a specific DNA sequence, known as the hormone response element, resulting in up- or down- regulation of gene transcription (4). The AR is generally expressed as a single AR, and consists of N-terminal regulatory domain, DNA-binding domain, a small hinge region, and ligand binding domain (6). The N-terminal regulatory domain mediates most of AR’s transcriptional activity (7).
Androgens can have a second mode of action, which is similar to oestrogens having actions that are independent of genomic DNA interactions (8). Based on rapid actions of testosterone, non-genomic effects of androgens were proposed over 30 years ago (9). Further, AR’s have been localized at extranuclear sites, such as hippocampal dendritic spines (10) and axons of the cerebral cortex (11). These extranuclear sites and rapid effects of testosterone indicate the presence of a putative membrane-associated AR (mAR) (12). Further indicative that mAR may be different from AR, mAR is sensitive to g-protein coupled receptor antagonists but not AR antagonists (flutamide) (13, 14). Functionally, mAR has been associated with increased intracellular calcium (14). These data show that AR action may occur in a broader spectrum than previously believed.
1.2 Sites of Androgen Action
Distribution of AR mRNA containing cells has been identified in the forebrain, midbrain, brain stem, and spinal cord (15, 16). Further, high concentrations of AR’s were present in the medial preoptic area (MPOA), ventromedial hypothalamus (VMN), medial amygdala (AMY), nucleus accumbens, bed nucleus of stria terminalis (BNST), and septum (septum) as demonstrated by AR binding, immunohistochemistry, and in situ hybridization (16–24). It is notable that the MPOA and the medial AMY have been implicated in the neural control of male sexual behaviour (25), whereas the septum, BNST, and nucleus accumbens are associated with aggression regulation (26–28).
1.3. Androgen Receptor Blockade
The most popular method employed to investigate AR’s effects on behaviour is via antiandrogens. Although many antiandrogens are available, studies on behaviour have focused on those that a) have no detectable androgenic activity, b) no anti-gonadotropic activity, and c) can cross the blood-brain barrier (unless they are implanted intracranially). The most specific and widely used androgen receptor blockers have been flutamide (FLU), flutamide’s active metabolite, hydroxyflutamide (OHF), and cyproterone acetate (CA) (29). CA (1,2α-methylene-6-chloro-17α-acetoxypregna-4,6-diene-3,20-dione) is a steroidal androgen receptor antagonist with weak progestational and glucocorticoid activity (30). Both flutamide and CA do not interfere with 5α-dihydrotesterone formation but rather block androgen binding to the AR (31–33). In contrast to CA, flutamide (a,a,a-trifluoro-2-methyl-4’-nitro-m-propionotoluidide [or 4'-nitro-3'-trifluoro-methyl-isobutyranilide]) is considered a pure antiandrogen, as it is devoid of anti-gonadotropic (34), progestogenic, estrogenic and androgenic activities (34, 35), but recent reports showed that flutamide and CA can exert some agonist activity at the level of the AR in neuronal and non-neuronal cells (36–39). Flutamide’s active metabolite, hydroxyflutamide (OHF; formerly SCH16423: a,a,a-trifluoro-2-methyl-4’-nitro-m-lactotoluidide) (35, 40), has been widely used as an antiandrogen as well. OHF has been shown to inhibit cell nuclear AR binding in pooled tissue from the hypothalamus, preoptic area, medial amygdala, and septum (32, 41).
2. Sexual Behaviour and Androgen Receptors
Virtually all reproductive-related behaviours, including copulation, aggression, scent marking and ultrasonic vocalizations are androgen-dependent (42). Thus, they are facilitated in the presence of androgens (both endogenous and exogenous) and decline following castration (21, 32). However, it appears that separate and perhaps overlapping brain regions mediate different androgen-dependent behaviours, and even different components of these behaviours.
2.1 Studies Using Systemic Antiandrogen Administration
Antiandrogens have been given systemically to determine if blocking AR’s in the entire brain would suppress male sexual behaviour. Timing of antiandrogen exposure, with respect to hormonal status, has been shown to be important. For example in some studies, antiandrogens and testosterone replacement were instituted at the time of castration, while in other studies administration occurred 3 weeks following castration, in which male rats were in an androgen-deficient condition. In testosterone-treated castrated males, administration of both FLU and OHF at the time of castration was only marginally effective in suppressing male sexual behaviour (32). However, when antiandrogens and testosterone replacement were instituted 3 weeks following castration in androgen-deficient castrated male rats, male sexual behaviour was inhibited (32, 41, 43).
The vast majority of studies have focused on the performance aspect of male sexual behaviour. However, two important factors to consider is the motivational component of male sexual behaviour and reproductive-associated behaviours. In the absence of sexual motivation, copulation is greatly diminished (41). Restoration of sexual motivation with androgens, as measured by partner preference, can be prevented by FLU (41). With respect to reproductive-associated behaviours, systemic administration of OHF decreases ultrasonic vocalizations and scent marking (41), but the neural sites involved have yet to be identified. Thus, AR’s appear to play a critical role in the expression of sexual behaviour, sexual motivation, and reproductive-associated behaviours in rats.
2.2 Studies Using Antiandrogen Implants to Localize AR Effects
The benefit to using intracranial antiandrogen implants is that the entire brain is exposed to testosterone, whereas AR’s are blocked only at the site of interest by the intracranial implant. This has proven to be a valuable method for identifying sites of AR action in brain with regard to specific behaviours, as implanted animals can be tested for their behavioural response in the presence of the blocking agent.
a. The medial preoptic area (MPOA)
The MPOA has long been associated with male sexual behaviour and has high concentrations of AR’s (20–24). A role for AR activation in the MPOA in mediating male sexual behaviour has been examined using intracranial implants. Testosterone implants into the MPOA reinstate male sexual behaviour in castrates (44). Intracranial implants of OHF into the MPOA results in a substantial reduction in mounts, intromissions, and ejaculations in a testosterone-treated castrated male rats (45–47). Notably, implants of OHF into the MPOA decrease sexual motivation only when placed in the posterior aspect of this brain site (46). Although far fewer studies have examined sexual motivation, it appears that both sexual performance and sexual motivation are under the control of AR’s, though their site specificity may differ. It should be noted that AR is not the only steroid receptor in the MPOA that mediates male sex behaviours, but that the oestrogen receptor (ER) also mediates male sex behaviours, possibly relying on ER activation owing to oestrogens synthesized locally by aromatase. Interestingly, it has been shown that testosterone can increase aromatase activity, while castration decreases aromatase activity in the MPOA (48). Further, AR inhibition with flutamide can decrease aromatase activity in the MPOA, and thus indicating that aromatase activity, and by extension ER activation, may be AR dependent in the MPOA (48).
Inhibition of testosterone conversion to oestrogen by infusion of the aromatase inhibitor, fadrozole, into the MPOA of gonadally intact male rats decreased sex behaviours (49), and sex behaviours were restored with MPOA implants of oestrogen and oestrogen-BSA conjugate, suggesting that non-genomic mechanisms mediated oestrogen action (50). Thus, both AR and ER in the MPOA are critical for the expression of male sexual behaviour.
b. Amygdala and Olfactory Bulbs
The olfactory bulbs and the amygdala are intimately connected and both contain AR’s (51). Removal of the olfactory bulbs drastically decreases male sexual behaviour (51). Moreover, olfactory bulb ablation significantly decreases AR binding in the amygdala 1–2 days after surgery (51). This decrease in AR binding was correlated with a decline in sexual behaviour (51). Implants of OHF into the medial amygdala were moderately effective in suppressing male sexual behaviour (45), suggesting that AR activation in the AMY may play a secondary role in facilitating male sexual behaviour. Alternatively, the AMY may be important in some other, yet to be identified, component of male reproductive behaviour.
c. Septum
In spite of the relatively high concentration of AR’s in the septum (45, 46), blocking AR’s in this brain area had no effect on either performance or motivational components of male sexual behaviour. This suggests that other androgen-dependent behaviours may be mediated by the septum.
d. Ventromedial Hypothalamus (VMN
Few studies have examined the role of the VMN in male sexual behaviour despite its high concentration of AR’s. Testosterone implanted into the VMN did not restore copulation in castrated male rats (44). This is interesting because OHF placed into the VMN is very effective in blocking male sexual behaviour (45). A later study showed that OHF in the anterodorsal VMN, but not the posteroventral VMN, decreased male sexual behaviour (47). Notably, VMN implants of testosterone were effective in restoring sexual motivation, and OHF in the VMN decreased sexual motivation (44, 45). These results suggest a role for the VMN in modulating sexual motivation.
2.3 Testosterone and Oestrogen
A number of years ago, a large body of evidence emerged suggesting that the male sex hormone was oestrogen and not testosterone. This was referred to as the aromatization hypothesis, as testosterone must be metabolically converted to estradiol via the P450 aromatase enzyme in the brain to activate male sexual behaviours (52, 53). The basis for this hypothesis was 1) very large doses of oestrogen restored male sexual behaviour (54) and 2) the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) blocked the effects of androgens on male sexual behaviour (55). Later studies demonstrated that physiological doses of estradiol did not restore male sexual behaviour, whereas physiological doses of testosterone were very effective (21). Only testosterone restored ejaculation and increased AR occupation (21). The non-aromatizable metabolite of testosterone, dihydrotestosterone (DHT), increased AR occupation, but had no effect on male sexual behaviour (21). In one study, the aromatase inhibitor, fadrozole, was given alone or in combination with testosterone, or with the addition of a very small dose of estradiol (56). This experiment showed that blocking aromatization did indeed prevent the restoration of male sexual behaviour, but not sexual motivation. The combination of testosterone with fadrozole and a very small dose of estradiol restored sexual behaviour. However, estradiol alone had no effect on either sexual behaviour or sexual motivation (56). It was concluded that both testosterone and a small amount of estradiol through aromatase are needed to facilitate male sexual behaviour.
Animals lacking in functional AR’s have been used to study the role of androgen receptor activation in mediating male sexual behaviour. The results have been somewhat equivocal, but this may be related to the fact that the defect is present from birth, rather than adulthood. Therefore, one cannot rule out the possibility that the changes in behaviour are influenced by early development. In a study on male mice lacking a functional AR gene (57), both male sexual behaviour and sexual motivation were low. Testicular feminized (Tfm) mice and rats, which are genetic males with defective AR’s, have been used to study the effect of loss of AR function on male sexual behaviour. Tfm mice lack functional ARs due to a mutation of the AR gene that results in a shortened AR transcript and no AR protein (58, 59). Although these mice lack sensitivity to androgens (60), they still have circulating androgens, such as testosterone (61). Further, Tfm mice have a greater AR defect than Tfm rats and also exhibit less male sexual behaviour than Tfm rats (1). Tfm rats show reduced sexual behaviour, but not motivation (62). Both Tfm mice and rats have normal levels of oestrogen receptor (ER) binding (1), but also have decreased aromatase activity that is unaffected by testosterone administration (63). These results suggest that Tfm males have normal oestrogen receptor capacity but not oestrogen receptor activation due to decreased aromatase activity. This is significant because oestrogens have been shown to play a role in male sexual behaviour. Consistent with these studies, male sex behaviours were decreased and ERα expression was unaffected in a conditional AR inactivation mouse model that is unaffected by development (64). The forgoing data are fairly convincing in demonstrating a role for AR’s and possibly ER in mediating male reproductive responses. The mechanism by which oestrogens contribute to AR action is unknown.
3. Aggression and Androgen Receptors
Male aggression is mediated by androgens, and elimination of endogenous androgens by castration abolishes aggression in a variety of species (2). In spite of the extensive literature demonstrating the critical relationship between androgens and aggression, very little is known about the role of the AR as well as the specific neural sites of AR activity that are either necessary or sufficient to elicit aggression in males. The application of antiandrogens or AR antagonists, cyproterone acetate (CA) and flutamide (FLU), has been used to assess this relationship. The effects of CA on aggression have been studied using animal models. However, the results have been equivocal. For example, the antiandrogen CA reportedly decreased intermale aggression in mice (65) and gerbils (66), whereas in other studies there was no significant effect on aggression (67–69). Others have reported that male mice given CA did show an initial increase in fight latency but that decreased intermale aggression was evident only after 4 weeks. (70). These authors suggest that extensive exposure to the anti-androgenic blocking agent is necessary to suppress aggression (70). This contrasts with one other study in which long term CA treatment was administered to mice exposed to increasing doses of testosterone where no effect on aggression was found (71). However, based on this finding it is not clear whether the sustained aggression was due to the inability of CA to suppress aggression or whether the increasing testosterone exposure was sufficient to override the inhibitory effect of CA.
The AR antagonist flutamide (FLU) also failed to decrease aggression in castrated male mice given exogenous testosterone (33). Further, FLU failed to reduce the level of aggression in male castrated mice given the anabolic steroid methyltestosterone (MT) (72). However, FLU did have an inhibitory effect on the ventral prostate, seminal vesicles, and lavator ani weights compared to males given MT alone, suggesting FLU differentially affects androgen-sensitive tissue and behaviour (72). The assessment of AR in mediating male aggression was also examined using mice lacking functional androgen receptors (Tfm). Clark et. al. (2007) found that Tfm male mice did not display enhanced aggression following administration of various anabolic steroids, indicating that the presence of a functional AR may be necessary for androgens to elicit aggressiveness in males (73). However, the ER has also been reported to mediate aggression in males. Exogenous oestrogen administration in castrated Tfm mice and wild-type mice increased aggression (74). Further, ERα activation is associated with increased aggression, while ERβ activation is associated with decreased aggression (74, 75). While these findings implicate both AR and ER in the evocation of aggressive behaviours, both the relative contribution of AR activation and the specific neural sites involved are poorly understood.
4. Hypothalamic-Pituitary-Adrenal (HPA) system Involvement
Stress can inhibit sex steroid hormone-dependent physiology and behaviour (76–78). The neuroendocrine system involved in mediating responses to stress is the hypothalamic-pituitary-adrenal axis (HPA), which consists of direct influences and feedback interactions between the hypothalamus, pituitary, and the adrenal gland (79). Upon activation of the HPA axis, the paraventricular nucleus of the hypothalamus and the pituitary secrete corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), respectively (79). These hormones act upon the adrenal gland to produce glucocorticoid hormones, such as corticosterone (79). Through a negative feedback cycle, glucocorticoids can act on receptors in the hypothalamus and pituitary to suppress ACTH and CRH secretion (79). Glucocorticoids can act at two receptors in the brain: mineralocorticoid receptors and the glucocorticoid receptors (80). These receptors are widely distributed in many brain regions that contain high concentrations of AR’s, such as hypothalamus, hippocampus, cortex, amygdala, and septum (15, 16, 80), indicating the involvement of glucocorticoids in other physiological functions.
4.1 Sex Steroid Hormone-Dependent Behaviours and Stress
It is known that HPA activation can inhibit hypothalamic-pituitary-gonadal (HPG) activation, i.e. reproductive physiology and associated behaviours (76–78, 81). However, HPG activation can also inhibit HPA activation. Brain regions, such as the hypothalamus and amygdala, which are activated by stress and express glucocorticoid receptors, can also be activated by sexual experience (80, 82–85). Studies have shown that prior HPG activation, through aggression or sexual experience can decrease stress reactions (86, 87). Interestingly, even exogenous manipulation of the HPG axis, via administration of testosterone, can block stress-induced glucocorticoid increase (88). These studies indicate that the HPA and the HPG systems interact and influence each other.
4.2 Androgen Receptors and Stress
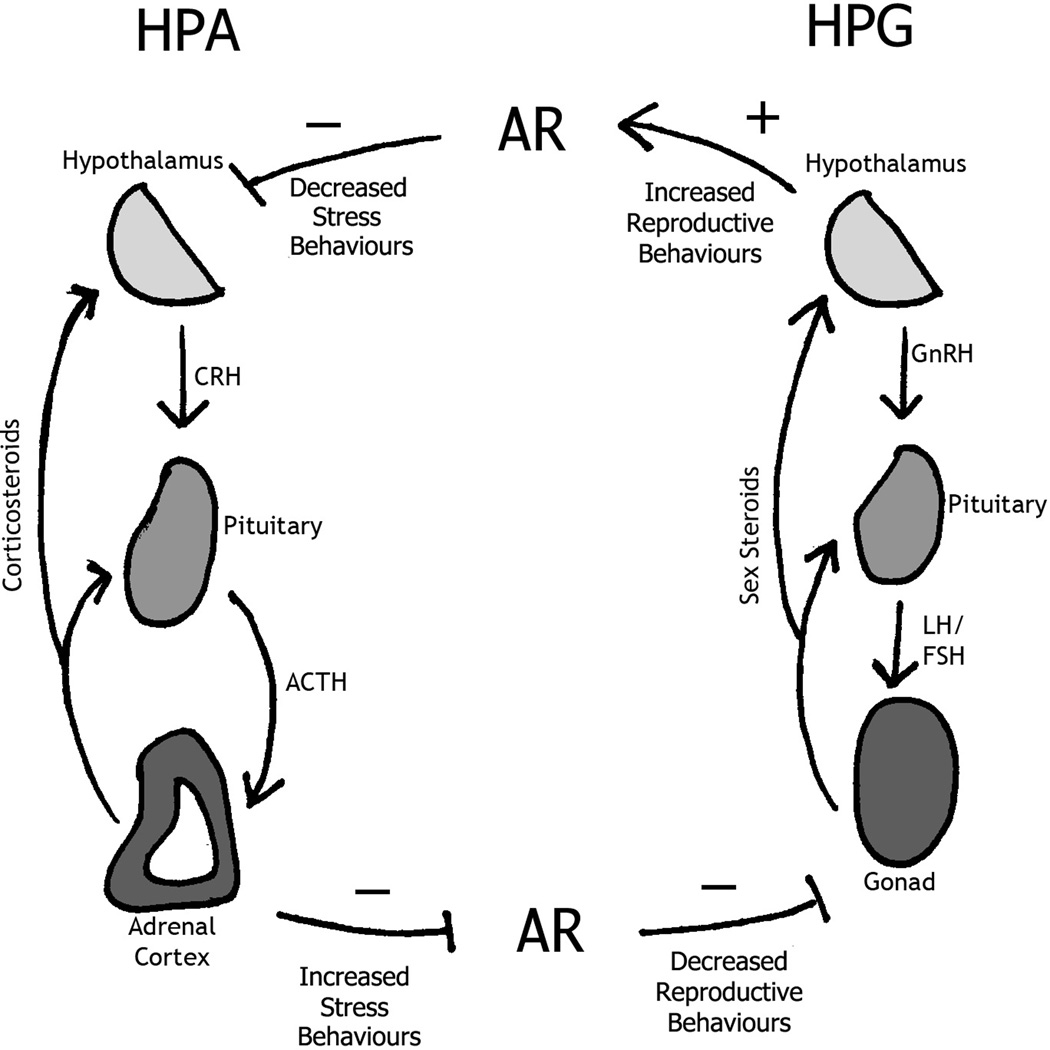
This interaction between the HPA and HPG systems could be mediated by androgen receptor (AR) activation (Figure 1). AR has been shown to be involved in anxiety related behaviours in rodents (89, 90). Studies have shown that AR activation inhibits stress response, while AR inhibition can increase stress response (89, 90). Consistent with behavioural studies, mice lacking androgen receptors (ARKO mice) have increased HPA activation, as evidenced by increased corticosterone and ACTH release (91). Even modulation of the HPG system, via AR inhibition, during the perinatal critical period can alter HPA function in adulthood (92, 93), indicating that HPG-associated AR activation during the perinatal period is essential for adult HPA function (94).
Figure 1.
Androgen receptor (AR) control of hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axis. Activation of the HPG axis results in increased AR activation, resulting in decreased activation of the HPA axis. Conversely, HPA axis activation decreased AR activation and subsequent HPG activation. HPA and HPG axis can modulate each other at the level of the AR. Abbreviations: GnRH-gonadotropin-releasing hormone, LH-luteinizing hormone, FSH-follicle-stimulating hormone, ACTH-adrenocorticotropic hormone, CRH-corticotropin-releasing hormone.
4.3 Testicular Feminization Mutation (Tfm
The Tfm (testicular feminization mutant) mice are a useful model to study HPG and HPA interactions. Consistent with previous reports that found increased HPA activation in response to pharmacological AR inhibition (89, 90), Tfm mice also display increased HPA activation (95, 96), such as increased anxiety-associated behaviours and corticosterone levels (95). Interestingly, the locus coeruleus, a brain region implicated in the stress response, contains more neurons and has a larger volume in a Tfm rat model compared to wild type (97), indicating that the lack of HPG-associated AR activation results in an over-activation of the HPA system. Results from studies using either pharmacological or genetic inhibition of the AR have consistently shown that an AR-mediated mechanism underlies HPA and HPG interactions.
5. Looking Forward
In this section of the review we will discuss three unresolved issues related to AR action and sociosexual behaviours: 1) possible non-genomic actions of AR’s, 2) a potential role for the oestrogen receptor in activating male sexual behaviour, and 3) the reason for the long lag time for the cessation and restoration of male sexual behaviour.
5.1 Effects of Oestrogen Receptor Inhibition
The permissive role of oestrogen in facilitating the display of male sexual behaviour is poorly understood. The implication is that oestrogen acts via the oestrogen receptor (ER). Further, studies have shown that oestrogens, possibly through the ER, can up-regulate AR expression both centrally (hypothalamus and amygdala) and peripherally (prostate) (98, 99). The most powerful evidence for a role of ER in mediating male sexual behaviour comes from ER knockout mice (100–102). In ERα knockout mice, male sexual behaviour and aggression were dramatically reduced (100, 101). However, sexual motivation remained normal (100–102). Interestingly, ERβ knockout mice display normal male sexual behaviours, thus suggesting an ERα rather than ERβ mediated mechanism (103). The problem is that it is impossible to determine if the ER-mediated effects on sexual and aggressive behaviours is due to organizational or activational effects of oestrogen acting at the ER, as ER and oestrogens are known to play an integral role in the organization of the brain with regard to adult behavioural patterns (1).
The use of ER antagonists has proven to be beneficial to parse out the role of oestrogen. Commonly used ER antagonists include: ICI 182,780, CI-628, RU-58668, PHTPP, and MPP. RU-58668 and ICI 182,780 are both pure ERα and ERβ antagonists with no partial agonist activity (104, 105). RU-58668 is able to cross the blood brain barrier (106), unlike ICI 182,770 (107). The ER antagonist CI-628 can inhibit ERα and ERβ, but has partial agonist activity (108). Lastly, MPP (methyl-pipericlino-pyrazole) is a specific ERα antagonist (109), whereas PHTPP is a selective ERβ antagonist (110). In Japanese quails, the ER antagonist CI-628 decreased male sex behaviours and aggression (111–113), but the results are equivocal in rodents with regard to CI-628 (114–116) and RU-58668 (41). No studies have examined ER antagonism on specific brain nuclei or the role of ERα and ERβ on male sex behaviours and aggression. Therefore, the question of a role for the ER in facilitating the expression of male sexual behaviour remains unanswered.
5.2 Proteins
One of the most intriguing, but elusive aspects of male sexual behaviour is the reason for the long lag time for the decline of sexual behaviour following castration, and the restoration of sexual behaviour after hormone replacement therapy. In rats, testosterone levels fall to undetectable levels in less than 24 h (117). Thus, the gradual decline of sexual behaviour over 2–3 weeks is not due to the presence of testosterone. Following the reinstatement of daily testosterone therapy, the restoration of the full copulatory behaviour pattern requires 7–14 days (32, 55, 118). This is a long time considering that female receptivity can be reinstated within 48 hours (119). With regard to male sexual behaviour, a “critical exposure” time of approximately 21 hours per day for 7–10 days is needed to restore ejaculation (118). Neural AR’s were significantly decreased within 3 hours of testosterone-capsule removal and was at castration levels by 6 hours. This suggests that high levels of AR must be maintained over time to activate male sexual behaviour. It is known that in many species, including humans, that prior sexual experience plays a crucial role in maintaining sexual behaviour following castration. The more experience, the longer sexual behaviour is maintained (120). One possibility is that proteins induced by androgens are essential for the maintenance of the behavioural response. While this is a plausible mechanism, it has proven extremely difficult to demonstrate the presence of androgen-regulated proteins in brain. In our laboratory, we used various hybridization and PCR techniques to identify four proteins that were up-regulated and two proteins that were down-regulated by testosterone in the hypothalamus (unpublished observations). However, we were unable to conclusively demonstrate that these were androgen-regulated gene products. Thus far, no one has been able to definitively link brain proteins to behaviour. This problem has plagued researchers for decades and is still unresolved.
6. Conclusion
Localization and pharmacological inhibition of AR’s have provided overwhelming evidence of AR’s involvement in male reproductive function and associated behaviours, as well as knowledge of a feedback system involving both the HPA and HPG circuits. However, there is much to be learned and many unknowns of AR action, such as 1) which specific neural sites mediate what specific aspects of reproductive behaviour, 3) what is the mechanism of action for oestrogen’s effects on AR activation, 3) the role of the AR in aggressive behaviours, and 4) the impact on aging on AR action, since testosterone levels decrease with age (121). The strength of the previous findings on AR’s effects on reproductive and aggressive behaviours would greatly benefit by addressing these questions.
Acknowledgements
This work was supported by the following grants: NIH grants 27727 to M.Y.M., NIH F32 NS061417, AHA BGIA4180116, and UNTHSC Seed Grant to R.L.C. The authors would like to thank Ms. Kathryn M. Cunningham for her artwork.
References
- 1.McGinnis MY, Marcelli M, Lamb DJ. Consequences of mutations in androgen receptor genes, molecular biology and behavior. In: Pfaff DW, editor. Hormones, Brain and Behavior. New York: Academic Press; 2002. pp. 347–380. [Google Scholar]
- 2.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior, What we've learned from the testicular feminization mutation. Hormones and Behavior. 2008;53(5):613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenbaum S, Baniahmad A. Nuclear receptors, Structure, function and involvement in disease. The International Journal of Biochemistry &, Cell Biology. 1997;29(12):1325–1341. doi: 10.1016/s1357-2725(97)00087-3. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 5.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators, a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28(7):778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann AO, Faber PW, van Rooij HC, Kuiper GG, Ris C, Klaassen P, van der Korput JA, Voorhorst MM, van Laar JH, Mulder E. The human androgen receptor, domain structure, genomic organization and regulation of expression. J Steroid Biochem. 1989;34(1–6):307–310. doi: 10.1016/0022-4731(89)90098-8. al. e. [DOI] [PubMed] [Google Scholar]
- 7.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266(1):510–518. [PubMed] [Google Scholar]
- 8.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 9.Diez A, Sancho MJ, Egana M, Trueba M, Marino A, Macarulla JM. An interaction of testosterone with cell membranes. Horm Metab Res. 1984;16(9):475–477. doi: 10.1055/s-2007-1014823. [DOI] [PubMed] [Google Scholar]
- 10.Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130(1):151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 11.DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138(3):801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Su C, Rybalchenko N, Schreihofer DA, Singh M, Abbassi B, Cunningham RL. Cell models for the study of sex steroid hormone neurobiology. J Steroids Hormon Sci. 2011 doi: 10.4172/2157-7536.s2-003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148(5):2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- 14.Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16(11):1429–1431. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- 15.Yu WHA, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001;46:1–10. doi: 10.1002/1097-4695(200101)46:1<1::aid-neu1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain, an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 17.Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999;39(3):359–370. doi: 10.1002/(sici)1097-4695(19990605)39:3<359::aid-neu3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain, sex differences and similarities in autoregulation. Endocrinology. 1998;139(4):1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Guasti A, Swaab D, Rodriguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology. 2003;28(4):501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis MY, Davis PG, Meaney MJ, Singer M, McEwen BS. In vitro measurement of cytosol and cell nuclear androgen receptors in male rat brain and pituitary. Brain Res. 1983;275:75–82. doi: 10.1016/0006-8993(83)90418-3. [DOI] [PubMed] [Google Scholar]
- 21.McGinnis MY, Dreifuss RM. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinol. 1989;124:618–626. doi: 10.1210/endo-124-2-618. [DOI] [PubMed] [Google Scholar]
- 22.McGinnis MY, Katz SE. Sex differences in cytosolic androgen receptors in gonadectomized male and female rats. J Neuroendocrinol. 1996;8:193–197. doi: 10.1046/j.1365-2826.1996.04494.x. [DOI] [PubMed] [Google Scholar]
- 23.Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinol. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- 24.Roselli CE, Handa RJ, Resko JA. Quantitative distribution of nuclear androgen receptors in microdissected areas of the rat brain. Neuroendocrinol. 1989;49(5):449–453. doi: 10.1159/000125151. [DOI] [PubMed] [Google Scholar]
- 25.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen K, Peters PJ, Bronson FH. Effects of intracranial implants of testosterone propionate on intermale aggression in the castrated male mouse. Horm Behav. 1974;5(1):83–92. doi: 10.1016/0018-506x(74)90009-9. [DOI] [PubMed] [Google Scholar]
- 27.Albert DJ, Petrovic DM, Walsh ML, Jonik RH. Medial accumbens lesions attenuate testosterone-dependent aggression in male rats. Physiol Behav. 1989;46(4):625–631. doi: 10.1016/0031-9384(89)90342-9. [DOI] [PubMed] [Google Scholar]
- 28.van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior, involvement of brain opioids and dopamine. Brain Res Brain Res Rev. 1995;21(2):162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- 29.Honer C, Nam K, Fink C, Marshall P, Ksander G, Chatelain RE, Cornell W, Steele R, Schweitzer R, Schumacher C. Glucocorticoid receptor antagonism by cyproterone acetate and RU486. Mol Pharmacol. 2003;63(5):1012–1020. doi: 10.1124/mol.63.5.1012. [DOI] [PubMed] [Google Scholar]
- 30.Cyproterone Acetate. Medicines and Healthcare products Regulatory Authority. 2006:1–42. [Google Scholar]
- 31.Fang S, Liao S. Antagonistic action of anti-androgens on the formation of a specific dihydrotestosterone-receptor protein complex in rat ventral prostate. Mol Pharmacol. 1969;5(4):428–431. [PubMed] [Google Scholar]
- 32.McGinnis MY, Mirth MC. Inhibition of cell nuclear androgen receptor binding and copulation in male rats by an antiandrogen, Sch16423. Neuroendocrinol. 1986;43:63–68. doi: 10.1159/000124510. [DOI] [PubMed] [Google Scholar]
- 33.Clark CR, Nowell NW. The effect of the non-steroidal antiandrogen flutamide on neural receptor binding of testosterone and intermale aggressive behavior in mice. Psychoneuroendocrinol. 1980;5(1):39–45. doi: 10.1016/0306-4530(80)90007-4. [DOI] [PubMed] [Google Scholar]
- 34.Neri R, Florance K, Koziol P, Van Cleave S. A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4'-nitro-3'-trifluoromethylisobutyranilide) Endocrinol. 1972;91:427–437. doi: 10.1210/endo-91-2-427. [DOI] [PubMed] [Google Scholar]
- 35.Neri RO. Studies on the biological mechanism of action of non-steroidal antiandrogens. In: Martini L, Motta M, editors. Androgens and Antiandrogens. New York: Raven Press; 1977. pp. 179–190. [Google Scholar]
- 36.Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects, induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148(6):2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 37.Kemppainen JA, Wilson EM. Agonist and antagonist activities of hydroxyflutamide and Casodex relate to androgen receptor stabilization. Urology. 1996;48(1):157–163. doi: 10.1016/s0090-4295(96)00117-3. [DOI] [PubMed] [Google Scholar]
- 38.Lu S, Simon NG, Wang Y, Hu S. Neural androgen receptor regulation, effects of androgen and antiandrogen. J Neurobiol. 1999;41(4):505–512. doi: 10.1002/(sici)1097-4695(199912)41:4<505::aid-neu6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919(1):160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 40.Tucker H, Crook JW. Nonsteroidal antiandrogens. Snythesis and structure-activity relationships of 3 substituted derivatives of 2-hdroxypropionanilides. J Med Chem. 1988;31:954–957. doi: 10.1021/jm00400a011. [DOI] [PubMed] [Google Scholar]
- 41.Vagell ME, McGinnis MY. The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm Behav. 1998;33:163–179. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]
- 42.Rubinow D, Schmidt P. Androgens, brain, and behavior. Am J Psychiatry. 1996;153(8):974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 43.Gladue BA, Clemens LG. Flutamide inhibits testosterone-induced masculine sexual behavior in male and female rats. Endocrinol. 1980;106:1917–1922. doi: 10.1210/endo-106-6-1917. [DOI] [PubMed] [Google Scholar]
- 44.Harding SM, McGinnis MY. Effects of testosterone in the VMN on copulation, partner preference, and vocaliztions in male rats. Horm Behav. 2003;43:327–335. doi: 10.1016/s0018-506x(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 45.McGinnis MY, Williams GW, Lumia AR. Inhibition of male sex behavior by androgen receptor blockade in preoptic area or hypothalamus, but not amygdala or septum. Physiol Behav. 1996;60(3):783–789. doi: 10.1016/0031-9384(96)00088-1. [DOI] [PubMed] [Google Scholar]
- 46.McGinnis MY, Montana RC, Lumia AR. Effects of hydroxyflutamide in the medial preoptic area or lateral septum on reproductive behaviors in male rats. Brain Res Bull. 2002;59(3):227–234. doi: 10.1016/s0361-9230(02)00869-9. [DOI] [PubMed] [Google Scholar]
- 47.Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiology &, Behavior. 2004;81(4):671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Roselli CE, Resko JA. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology. 1984;114(6):2183–2189. doi: 10.1210/endo-114-6-2183. [DOI] [PubMed] [Google Scholar]
- 49.Clancy AN, Zumpe D, Michael RP. Intracerebral infusion of an aromatase inhibitor, sexual behavior and brain estrogen receptor-like immunoreactivity in intact male rats. Neuroendocrinology. 1995;61(2):98–111. doi: 10.1159/000126830. [DOI] [PubMed] [Google Scholar]
- 50.Huddleston GG, Paisley JC, Graham S, Grober MS, Clancy AN. Implants of estradiol conjugated to bovine serum albumin in the male rat medial preoptic area promote copulatory behavior. Neuroendocrinology. 2007;86(4):249–259. doi: 10.1159/000107695. [DOI] [PubMed] [Google Scholar]
- 51.Lumia AR, Zebrowski AF, McGinnis MY. Olfactory bulb removal decreases androgen receptor binding in amygdala and hypothalamus and disrupts masculine sexual behavior. Brain Res. 1987;404(1–2):121–126. doi: 10.1016/0006-8993(87)91362-x. [DOI] [PubMed] [Google Scholar]
- 52.Feder H. Perinatal hormones and their role in development of sexually dimorphic behaviors. In: Adler N, editor. Neuroendocrinology of Reproduction. NY: Plenum Press; 1981. pp. 127–157. [Google Scholar]
- 53.Naftolin F, MacLusky N. Aromatization hypothesis revisited. In: Serio M, editor. Sexual differentiation, basic and clinical aspects. Raven Press: NY; 1984. pp. 79–91. [Google Scholar]
- 54.Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182:283–285. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan ME, McGinnis MY. Effects of ATD on male sexual behavior and androgen receptor binding, A reexamination of the aromatization hypothesis. Horm Behav. 1989;23:10–26. doi: 10.1016/0018-506x(89)90071-8. [DOI] [PubMed] [Google Scholar]
- 56.Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol. 1997;9:415–421. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- 57.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM. The Androgen Receptor Governs the Execution, but Not Programming, of Male Sexual and Territorial Behaviors. Neuron. 2010;66(2):260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19(9):2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci U S A. 2007;104(46):18259–18264. doi: 10.1073/pnas.0705501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drews U. Direct and mediated effects of testosterone, analysis of sex reversed mosaic mice heterozygous for testicular feminization. Cytogenet Cell Genet. 1998;80(1–4):68–74. doi: 10.1159/000014959. [DOI] [PubMed] [Google Scholar]
- 61.Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003;148(1):111–120. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- 62.Hamson DK, Csupity AS, Ali FM, Watson NV. Partner preference and mount latency are masculinized in androgen insensitive rats. Physiology & Behavior. 2009;98(1–2):25–30. doi: 10.1016/j.physbeh.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121(6):2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- 64.Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system, effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29(14):4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nowell NW, Wouters S. Effect of cyproterone acetate upon aggressive behavior in the laboratory mouse. J Endocrinol. 1973;57:R36–R37. [Google Scholar]
- 66.Sayler A. The effect of anti-androgens on aggressive behavior in the gerbil. Physiol Behav. 1970;5:667–671. doi: 10.1016/0031-9384(70)90228-3. [DOI] [PubMed] [Google Scholar]
- 67.Brain PF, Evans CM, Poole AE. Studies on the effects of cyproterone acetate administered in adulthood or in early life on subsequent endocrine function and agnostic behavior in male albino laboratory mice. J Endocrinol. 1974;61:xiv. [PubMed] [Google Scholar]
- 68.Poole AE, Brain PF. Effects of neonatal cyproterone acetate administration on isolation-induced fighting behavior and mounting behavior in male and female TO strain albino mice. Aggress Behav. 1975;1:165–176. [Google Scholar]
- 69.Clark CR, Nowell NW. Effect of the antiandrogen cyproterone acetate on neural testosterone binding and on intermale aggressive behaviour in male mice. J Endocrinol. 1979;81:137–138. [PubMed] [Google Scholar]
- 70.Matte AC, Fabian E. The effect of cyproterone acetate on motor activity, aggression, emotionality, body weight, and testes in wild mice. Andrologia. 1978;10:155–162. doi: 10.1111/j.1439-0272.1978.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 71.Edwards DA. Effects of cyproterone acetate on aggressive behaviour and the seminal vesicles of male mice. J Endocrinol. 1970;46:477–481. doi: 10.1677/joe.0.0460477. [DOI] [PubMed] [Google Scholar]
- 72.Heilman RD, Brugmans M, Greenslade FC, DaVanzo JP. Resistance of androgen-mediated aggressive behavior in mice to flutamide, an antiandrogen. Psychopharmacology. 1976;47(1):75–80. doi: 10.1007/BF00428705. [DOI] [PubMed] [Google Scholar]
- 73.Clark AS, Penatti CAA. The contribution of the androgen receptor to the display of anabolic androgenic steroid-induced aggression in male mice. San Diego, CA: Society for Neuroscience; 2007. [Google Scholar]
- 74.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor α. Genes, Brain and Behavior. 2004;3(1):20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 75.Nomura M, Durbak L, Chan J, Smithies O, Gustafsson J-Ö, Korach KS, Pfaff DW, Ogawa S. Genotype/Age Interactions on Aggressive Behavior in Gonadally Intact Estrogen Receptor Œ≤ Knockout (Œ≤ERKO) Male Mice. Hormones and Behavior. 2002;41(3):288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- 76.Menendez-Patterson A, Florez-Lozano JA, Fernandez S, Marin B. Stress and sexual behavior in male rats. Physiol Behav. 1980;24(2):403–406. doi: 10.1016/0031-9384(80)90106-7. [DOI] [PubMed] [Google Scholar]
- 77.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Martinez-Garcia R, Velazquez-Moctezuma J. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44(4):327–337. doi: 10.1016/j.yhbeh.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 79.Gore AC, Roberts JL. Neuroendocrine Systems. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. 2nd ed. New York: Academic Press; 2003. pp. 1043–1048. [Google Scholar]
- 80.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain, an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26(3):235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 81.Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67(5):769–775. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 82.Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior, a combined fos and tract-tracing study. J Comp Neurol. 1998;397(3):421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 83.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025(1–2):139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 84.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29(8):1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Pfaus JG. Neurobiology of sexual behavior. Curr Opin Neurobiol. 1999;9(6):751–758. doi: 10.1016/s0959-4388(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 86.De Boer SF, Koolhaas JM. Defensive burying in rodents, ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463(1–3):145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 87.Spritzer MD, Weinberg A, Viau V, Galea LA. Prior sexual experience increases hippocampal cell proliferation and decreases risk assessment behavior in response to acute predator odor stress in the male rat. Behav Brain Res. 2009;200(1):106–112. doi: 10.1016/j.bbr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Cunningham RL, McGinnis MY. Prepubertal social subjugation and anabolic androgenic steroid-induced aggression in male rats. J Neuroendocrinol. 2008;20(8):997–1005. doi: 10.1111/j.1365-2826.2008.01756.x. [DOI] [PubMed] [Google Scholar]
- 89.Edinger KL, Frye CA. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav. 2006;50(2):216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test, role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Miyamoto J, Matsumoto T, Shiina H, Inoue K, Takada I, Ito S, Itoh J, Minematsu T, Sato T, Yanase T, Nawata H, Osamura YR, Kato S. The pituitary function of androgen receptor constitutes a glucocorticoid production circuit. Mol Cell Biol. 2007;27(13):4807–4814. doi: 10.1128/MCB.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology. 2005;146(4):1973–1982. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]
- 93.McCormick CM, Mahoney E. Persistent effects of prenatal, neonatal, or adult treatment with flutamide on the hypothalamic-pituitary-adrenal stress response of adult male rats. Horm Behav. 1999;35(1):90–101. doi: 10.1006/hbeh.1998.1500. [DOI] [PubMed] [Google Scholar]
- 94.Bingham B, Gray M, Sun T, Viau V. Postnatal blockade of androgen receptors or aromatase impair the expression of stress hypothalamic-pituitary-adrenal axis habituation in adult male rats. Psychoneuroendocrinology. 2011;36(2):249–257. doi: 10.1016/j.psyneuen.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008;54(5):758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Rizk A, Robertson J, Raber J. Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 2005;1034(1–2):132–138. doi: 10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Jordan CL, Segovia S, Guillamon A. The role of the androgen receptor in CNS masculinization. Brain Res. 2005;1035(1):13–23. doi: 10.1016/j.brainres.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 98.Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiol Behav. 2000;69(4–5):445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 99.Fernandes SA, Gomes GR, Siu ER, Damas-Souza DM, Bruni-Cardoso A, Augusto TM, Lazari MF, Carvalho HF, Porto CS. The anti-oestrogen fulvestrant (ICI 182,780) reduces the androgen receptor expression, ERK1/2 phosphorylation and cell proliferation in the rat ventral prostate. Int J Androl. 2011;34(5 Pt 1):486–500. doi: 10.1111/j.1365-2605.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 100.Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual lbehavior is disrupted in male and female mice lacking a functional estrogen receptor a gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- 101.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. modifications of testosterone-dependent behaviors by estrogen receptor-a gene disruption in male mice. Endocrinol. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 103.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96(22):12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van de Velde P, Nique F, Bouchoux F, Bremaud J, Hameau MC, Lucas D, Moratille C, Viet S, Philibert D, Teutsch G. RU 58,668, a new pure antiestrogen inducing a regression of human mammary carcinoma implanted in nude mice. J Steroid Biochem Mol Biol. 1994;48(2–3):187–196. doi: 10.1016/0960-0760(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 105.Wakeling AE, Bowler J. ICI 182,780, a new antiestrogen with clinical potential. J Steroid Biochem Mol Biol. 1992;43(1–3):173–177. doi: 10.1016/0960-0760(92)90204-v. [DOI] [PubMed] [Google Scholar]
- 106.Vagell ME, McGinnis MY. Inhibition of brain oestrogen receptors by RU 58668. J Neuroendocrinol. 1997;9:797–800. doi: 10.1046/j.1365-2826.1997.d01-1047.x. [DOI] [PubMed] [Google Scholar]
- 107.Wade GN, Blaustein JD, Gray JM, Meredith JM. ICI 182,780: a pure antiestrogen that effects behaviors and energy balance in rats without acting on the brain. Am J Physiol (Regulatory Integrative Comp Physiol 34) 1993;265:R1392–R1398. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]
- 108.Roy EJ, Wade GN. Binding of [3-H]estradiol by brain cell nuclei and female rat sexual behavior, inhibition by antiestrogens. Brain Res. 1977;126(1):73–87. doi: 10.1016/0006-8993(77)90216-5. [DOI] [PubMed] [Google Scholar]
- 109.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143(3):941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 110.Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazolo[1,5-a]pyrimidines, estrogen receptor ligands possessing estrogen receptor beta antagonist activity. J Med Chem. 2004;47(24):5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- 111.Alexandre C, Balthazart J. Effects of metabolism inhibitors, antiestrogens and antiandrogens on the androgen and estrogen induced sexual behavior in Japanese quail. Physiol Behav. 1986;38(4):581–591. doi: 10.1016/0031-9384(86)90429-4. [DOI] [PubMed] [Google Scholar]
- 112.Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen or estrogen in quail. Horm Behav. 1976;7(4):417–429. doi: 10.1016/0018-506x(76)90013-1. [DOI] [PubMed] [Google Scholar]
- 113.Schlinger BA, Callard GV. Aromatization mediates aggressive behavior in quail. Gen Comp Endocrinol. 1990;79(1):39–53. doi: 10.1016/0016-6480(90)90086-2. [DOI] [PubMed] [Google Scholar]
- 114.Beyer C, Morali G, Naftolin F, Larsson K, Perez-Palacios Effect of some antiestrogens and aromatase inhibitors on androgen induced sexual behavior in castrated male rats. Horm Behav. 1976;7(3):353–363. doi: 10.1016/0018-506x(76)90040-4. [DOI] [PubMed] [Google Scholar]
- 115.Clark CR, Nowell NW. The effect of the antiestrogen CI-628 on androgen-induced aggressive behavior in castrated male mice. Horm Behav. 1979;12(3):205–210. doi: 10.1016/0018-506x(79)90002-3. [DOI] [PubMed] [Google Scholar]
- 116.Whalen RE, Battie C, Luttge WG. Anti-estrogen inhibition of androgen induced sexual receptivity in rats. Behav Biol. 1972;7(3):311–320. doi: 10.1016/s0091-6773(72)80103-2. [DOI] [PubMed] [Google Scholar]
- 117.Krey LC, McGinnis MY. Time courses of the appearance/disappearance of nuclear androgen+receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats, relationships with gonadotropin secretion. J Steroid Biochem. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- 118.McGinnis MY, Mirth MC, Zebrowski AF, Dreifuss RM. Critical exposure time for androgen activation of male sexual behavior in rats. Physiol Behav. 1989;46:159–165. doi: 10.1016/0031-9384(89)90249-7. [DOI] [PubMed] [Google Scholar]
- 119.McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats, an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33(3):158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- 120.Rosenblatt JS, Aronson LR. The Decline of Sexual Behavior in Male Cats after Castration with Special Reference to the Role of Prior Sexual Experience. Behaviour. 1958;12(4):285–338. [Google Scholar]
- 121.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]