Abstract
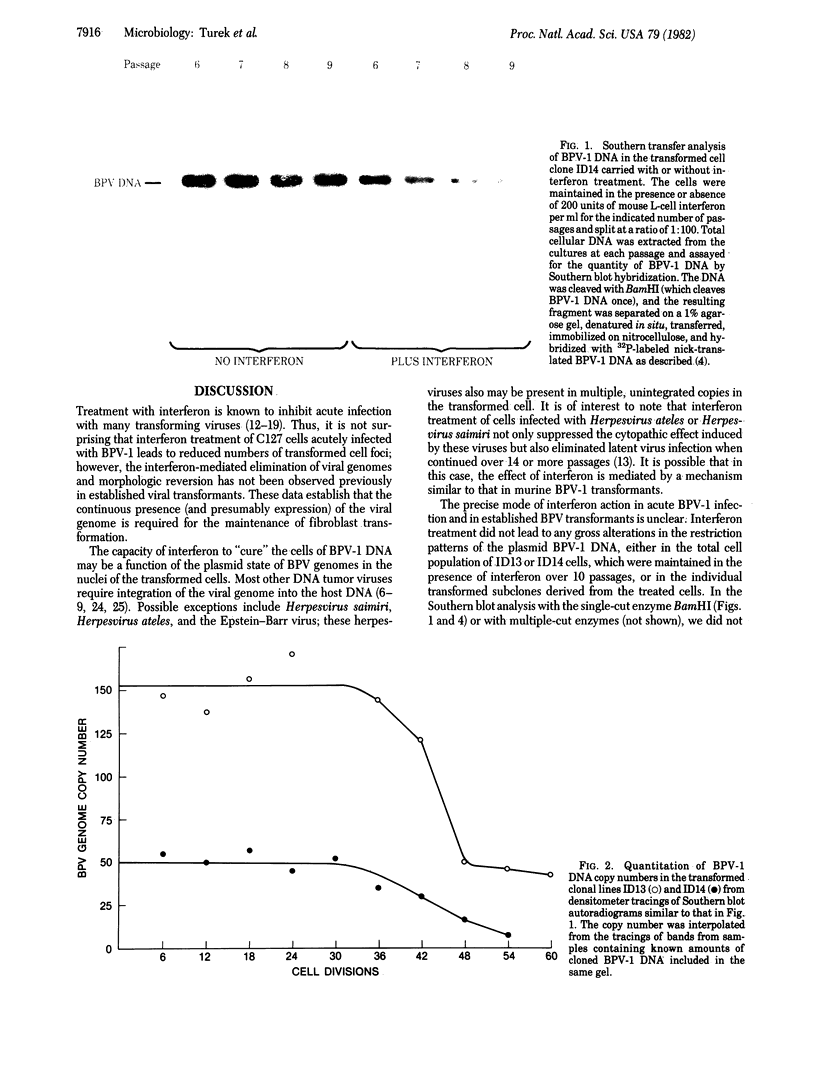
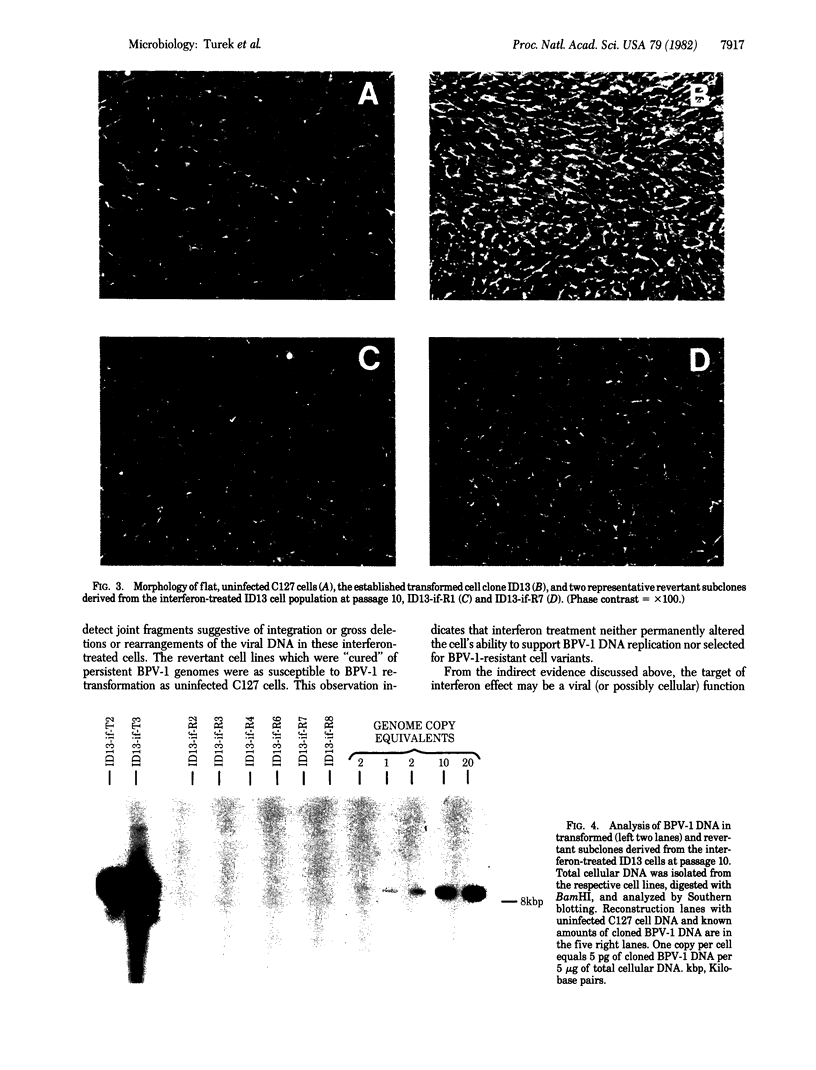
The effect of mouse L-cell interferon on bovine papillomavirus type 1 (BPV-1) transformation of murine cells was examined. Mouse interferon reduced the level of BPV-1-induced transformation of mouse C127 cells by 95%. Long-term treatment of established BPV-1-transformed mouse cell clones with mouse L-cell interferon led to a decrease in the average number of the plasmid viral genomes present in these cells to 1/3 to 1/8. Although revertant lines could not be isolated from these lines in the absence of treatment with interferon, flat revertants were easily selected from two independent clonal transformed lines carried for 60 generations in the continued presence of 200 units of interferon per ml. These flat revertants had the biological characteristics of nontransformed C127 cells and could be retransformed by BPV-1. Southern blot hybridization failed to detect BPV-1 DNA in any of eight independent revertant lines examined under conditions that could detect 0.2 copies per cell. We conclude that interferon treatment has resulted in a selective reduction of the amount of extrachromosomal BPV-1 DNA in transformed cells and has cured some treated cells completely of their viral DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery R. J., Norton J. D., Jones J. S., Burke D. C., Morris A. G. Interferon inhibits transformation by murine sarcoma viruses before integration of provirus. Nature. 1980 Nov 6;288(5786):93–95. doi: 10.1038/288093a0. [DOI] [PubMed] [Google Scholar]
- Blanchet-Bardon C., Puissant A., Lutzner M., Orth G., Nutini M. T., Guesry P. Interferon treatment of skin cancer in patients with epidermodysplasia verruciformis. Lancet. 1981 Jan 31;1(8214):274–274. doi: 10.1016/s0140-6736(81)92110-3. [DOI] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Breitburd F., Favre M., Zoorob R., Fortin D., Orth G. Detection and characterization of viral genomes and search for tumoral antigens in two hamster cell lines derived from tumors induced by bovine papillomavirus type 1. Int J Cancer. 1981 May 15;27(5):693–702. doi: 10.1002/ijc.2910270517. [DOI] [PubMed] [Google Scholar]
- Chia W., Rigby P. W. Fate of viral DNA in nonpermissive cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6638–6642. doi: 10.1073/pnas.78.11.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Tamulevich R., Bekesi J. G., King N. W., Falk L. A., Silva D., Holland J. F. Selective antiviral activity of human interferons on primate oncogenic and neurotropic herpesviruses. Int J Cancer. 1981 Jan 15;27(1):113–121. doi: 10.1002/ijc.2910270118. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN J. C., LEVY J. P., LASNERET J., THOMAS M., BOIRON M., BERNARD J. INDUCTION DE FIBROMES SOUS-CUTAN'ES CHEZ LE HAMSTER DOR'E PAR INOCULATION D'EXTRAITS ACELLULAIRES DE PAPILLOMES BOVINS. C R Hebd Seances Acad Sci. 1963 Oct 14;257:2328–2331. [PubMed] [Google Scholar]
- Frenkeĺ N., Locker H., Batterson W., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. VI. Defective DNA originates from the S component. J Virol. 1976 Nov;20(2):527–531. doi: 10.1128/jvi.20.2.527-531.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund S., Lundquist P. G., Cantell K., Strander H. Interferon therapy in juvenile laryngeal papillomatosis. Arch Otolaryngol. 1981 Jun;107(6):327–332. doi: 10.1001/archotol.1981.00790420001001. [DOI] [PubMed] [Google Scholar]
- Howley P. M. The human papillomaviruses. Arch Pathol Lab Med. 1982 Sep;106(9):429–432. [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Demonstration of two distinct classes of bovine papilloma virus. Virology. 1978 Sep;89(2):372–379. doi: 10.1016/0042-6822(78)90179-4. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lvovsky E., Levine P. H., Fuccillo D., Ablashi D. V., Bengali Z. H., Armstrong G. R., Levy H. B. Epstein-Barr virus and Herpesvirus saimiri: sensitivity to interferons and interferon inducers. J Natl Cancer Inst. 1981 Jun;66(6):1013–1019. doi: 10.1093/jnci/66.6.1013. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Clegg C. The effect of mouse interferon on the transformation of NIH/3T3 mouse cells by murine sarcoma virus. Virology. 1978 Jul 15;88(2):400–402. doi: 10.1016/0042-6822(78)90298-2. [DOI] [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Ostrow R. S., Bender M., Niimura M., Seki T., Kawashima M., Pass F., Faras A. J. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. N., Baron S., Black P. H., Takemoto K. K., Habel K., Rowe W. P. The effect of interferon on SV-40 T antigen production in SV-40-transformed cells. Virology. 1967 May;32(1):122–127. doi: 10.1016/0042-6822(67)90260-7. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Black P. H. Inhibition of SV40 T antigen formation by interferon. Proc Natl Acad Sci U S A. 1966 May;55(5):1133–1140. doi: 10.1073/pnas.55.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Baron S. The role of interferon in the inhibition of SV40 transformation of mouse cell line 3T3. Proc Natl Acad Sci U S A. 1965 Sep;54(3):752–756. doi: 10.1073/pnas.54.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Prasad I., Basilico C. State of the viral DNA in rat cells transformed by polyma virus. II. Identification of the cells containing nonintegrated viral DNA and the effect of viral mutations. J Virol. 1977 Oct;24(1):142–150. doi: 10.1128/jvi.24.1.142-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]





