Abstract
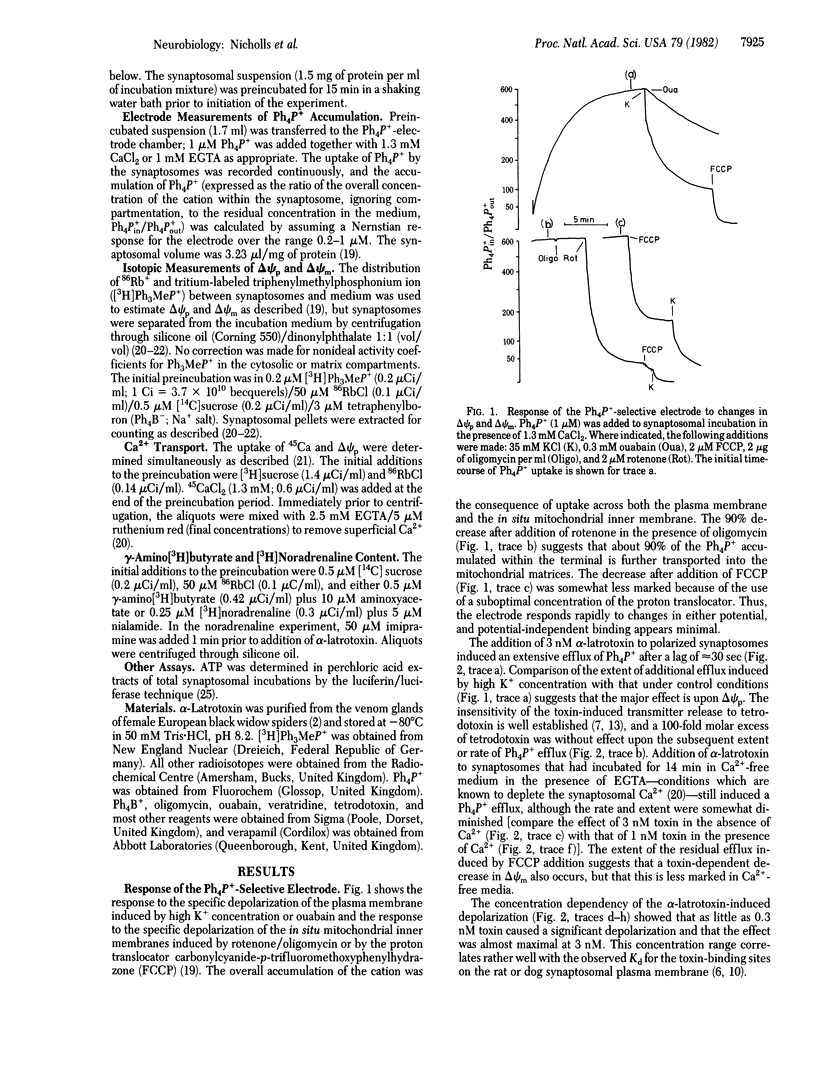
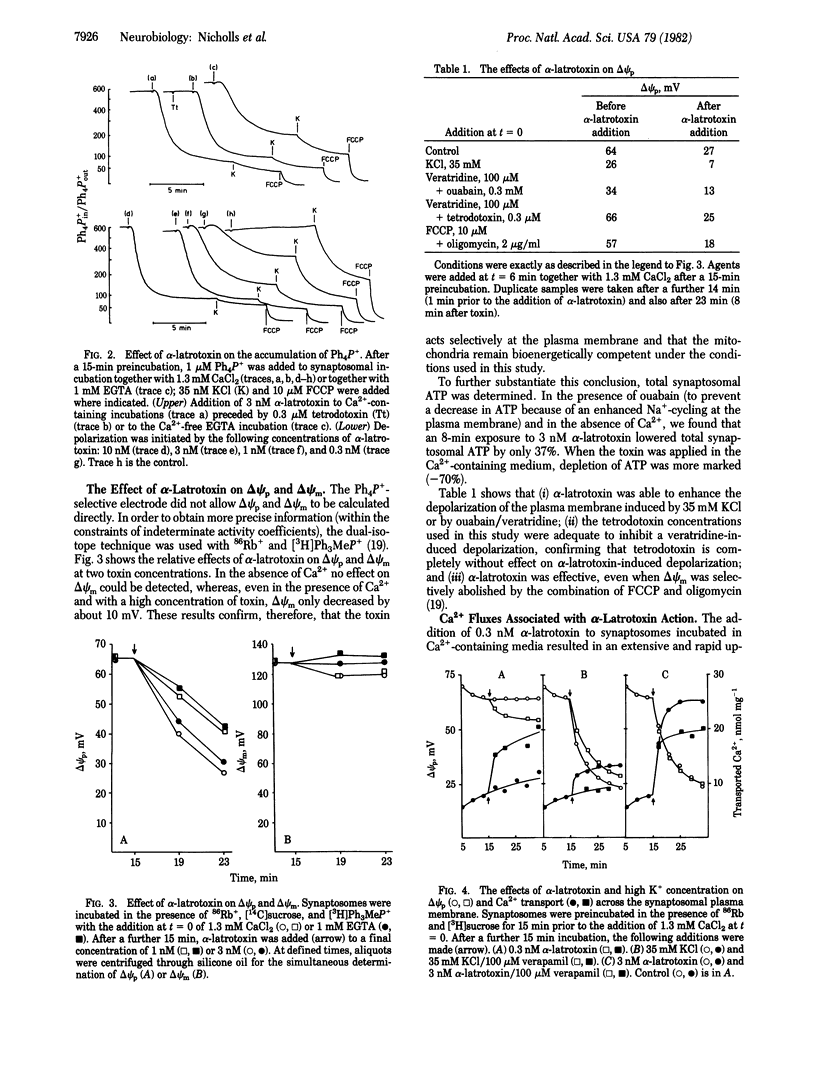
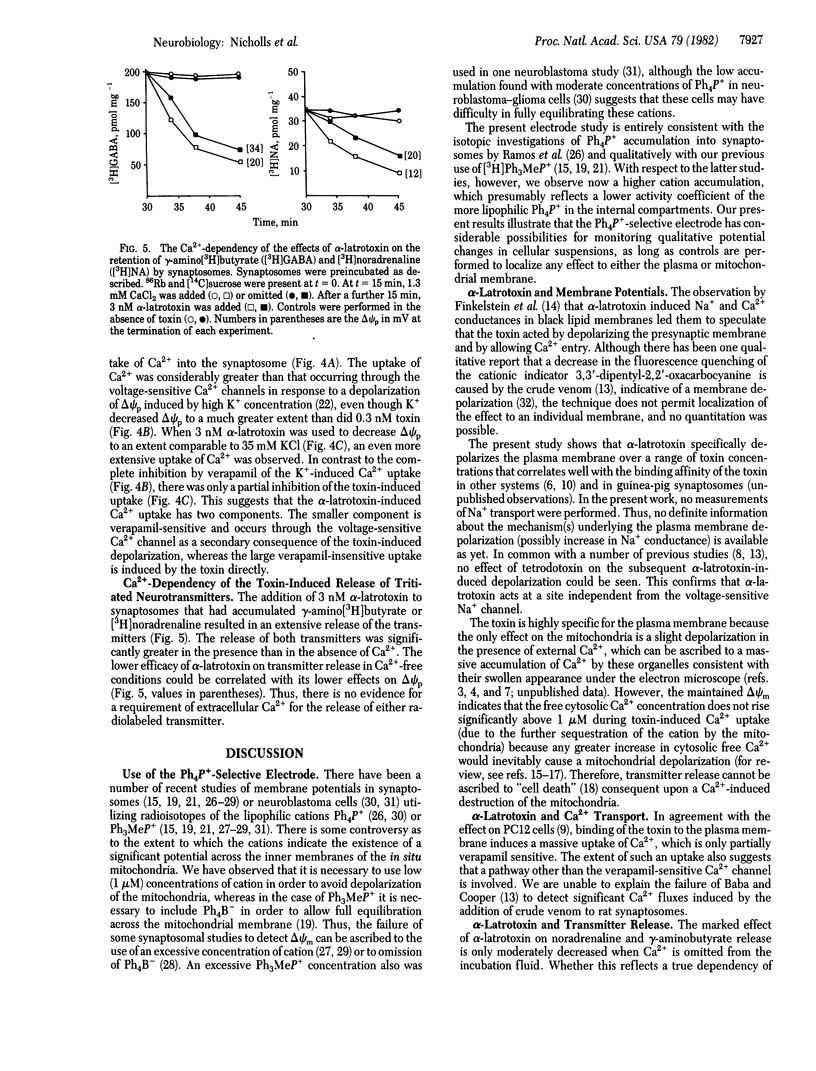
The effect of alpha-latrotoxin from black widow spider venom upon guinea pig cerebral cortical synaptosomes is described. Plasma membrane potential (delta psi p), in situ mitochondrial membrane potential (delta psi m), Ca2+ transport, gamma-amino[3H]butyrate release, [3H]noradrenaline release, and synaptosomal ATP were monitored under parallel conditions. Potentials were determined both isotopically and with a tetraphenylphosphonium-selective electrode. alpha-Latrotoxin depolarizes delta psi p selectively, both in the presence and absence of Ca2+. A slight toxin-induced depolarization of delta psi m is a consequence of a massive Ca2+ uptake across the plasma membrane. Depolarization of delta psi p is insensitive to tetrodotoxin, and Ca2+ entry is only partially inhibited by verapamil. Release of [3H]noradrenaline and gamma-amino[3H]butyrate is markedly stimulated by the toxin in the presence of Ca2+, and this effect is only slightly reduced in Ca2+-free conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Nicholls D. G. Ca2+ transport by intact synaptosomes: the voltage-dependent Ca2+ channel and a re-evaluation of the role of sodium/calcium exchange. Eur J Biochem. 1981 Jul;117(3):491–497. doi: 10.1111/j.1432-1033.1981.tb06364.x. [DOI] [PubMed] [Google Scholar]
- Akerman K. E., Nicholls D. G. Calcium transport by intact synaptosomes. Influence of ionophore A23187 on plasma-membrane potential, plasma-membrane calcium transport, mitochondrial membrane potential, respiration, cytosolic free-calcium concentration and noradrenaline release. Eur J Biochem. 1981 Mar 16;115(1):67–73. [PubMed] [Google Scholar]
- Akerman K. E., Nicholls D. G. Intrasynaptosomal compartmentation of calcium during depolarization-induced calcium uptake across the plasma membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):41–48. doi: 10.1016/0005-2736(81)90509-5. [DOI] [PubMed] [Google Scholar]
- Baba A., Cooper J. R. The action of black widow spider venom on cholinergic mechanisms in synaptosomes. J Neurochem. 1980 Jun;34(6):1369–1379. doi: 10.1111/j.1471-4159.1980.tb11217.x. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980 Oct;87(1):297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Drown C., Rafalowska U., Silver I. A. Synaptosomes from rat brain: morphology, compartmentation, and transmembrane pH and electrical gradients. J Neurochem. 1981 Jun;36(6):2063–2072. doi: 10.1111/j.1471-4159.1981.tb10835.x. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Erecińska M., Werrlein R., Silver I. A. Cellular energy metabolism, trans-plasma and trans-mitochondrial membrane potentials, and pH gradients in mouse neuroblastoma. Proc Natl Acad Sci U S A. 1979 May;76(5):2175–2179. doi: 10.1073/pnas.76.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C., Rafalowska U. Transmembrane electrical potential measurements in rat brain synaptosomes. FEBS Lett. 1979 Dec 1;108(1):274–278. doi: 10.1016/0014-5793(79)81227-2. [DOI] [PubMed] [Google Scholar]
- FINKELSTEIN A., Rubin L. L., Tzeng M. C. Black widow spider venom: effect of purified toxin on lipid bilayer membranes. Science. 1976 Sep 10;193(4257):1009–1011. doi: 10.1126/science.948756. [DOI] [PubMed] [Google Scholar]
- Frontali N., Ceccarelli B., Gorio A., Mauro A., Siekevitz P., Tzeng M. C., Hurlbut W. P. Purification from black widow spider venom of a protein factor causing the depletion of synaptic vesicles at neuromuscular junctions. J Cell Biol. 1976 Mar;68(3):462–479. doi: 10.1083/jcb.68.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Rubin L. L., Mauro A. Double mode of action of black widow spider venom on frog neuromuscular junction. J Neurocytol. 1978 Apr;7(2):193–202. doi: 10.1007/BF01217918. [DOI] [PubMed] [Google Scholar]
- Grasso A., Alemà S., Rufini S., Senni M. I. Black widow spider toxin-induced calcium fluxes and transmitter release in a neurosecretory cell line. Nature. 1980 Feb 21;283(5749):774–776. doi: 10.1038/283774a0. [DOI] [PubMed] [Google Scholar]
- Grasso A., Senni M. I. A toxin purified from the venom of black widow spider affects the uptake and release of radioactive gamma-amino butyrate and N-epinephrine from rat brain synaptosomes. Eur J Biochem. 1979 Dec 17;102(2):337–344. doi: 10.1111/j.1432-1033.1979.tb04248.x. [DOI] [PubMed] [Google Scholar]
- Hansson E., Jacobson I., Venema R., Sellström A. Measurement of the membrane potential of isolated nerve terminals by the lipophilic cation [3H]triphenylmethylphosphonium bromide. J Neurochem. 1980 Mar;34(3):569–573. doi: 10.1111/j.1471-4159.1980.tb11182.x. [DOI] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V., Raiteri M. A reevaluation of veratridine as a tool for studying the depolarization-induced release of neurotransmitters from nerve endings. Neurochem Res. 1980 Mar;5(3):281–295. doi: 10.1007/BF00964616. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- Meldolesi J. Studies on alpha-latrotoxin receptors in rat brain synaptosomes: correlation between toxin binding and stimulation of transmitter release. J Neurochem. 1982 Jun;38(6):1559–1569. doi: 10.1111/j.1471-4159.1982.tb06633.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Akerman K. E. Biochemical approaches to the study of cytosolic calcium regulation in nerve endings. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):115–122. doi: 10.1098/rstb.1981.0176. [DOI] [PubMed] [Google Scholar]
- Ramos S., Grollman E. F., Lazo P. S., Dyer S. A., Habig W. H., Hardegree M. C., Kaback H. R., Kohn L. D. Effect of tetanus toxin on the accumulation of the permeant lipophilic cation tetraphenylphosphonium by guinea pig brain synaptosomes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4783–4787. doi: 10.1073/pnas.76.10.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval M. E. Sodium-dependent efflux of [3H]GABA from synaptosomes probably related to mitochondrial calcium mobilization. J Neurochem. 1980 Oct;35(4):915–921. doi: 10.1111/j.1471-4159.1980.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Akerman K. E., Nicholls D. G. Calcium-ion transport by intact synaptosomes. Intrasynaptosomal compartmentation and the role of the mitochondrial membrane potential. Biochem J. 1980 Dec 15;192(3):873–880. doi: 10.1042/bj1920873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng M. C., Cohen R. S., Siekevitz P. Release of neurotransmitters and depletion of synaptic vesicles in cerebral cortex slices by alpha-latrotoxin from black widow spider venom. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4016–4020. doi: 10.1073/pnas.75.8.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng M. C., Siekevitz P. The binding interaction between alpha-latrotoxin from black widow spider venom and a dog cerebral cortex synaptosomal membrane preparation. J Neurochem. 1979 Jul;33(1):263–274. doi: 10.1111/j.1471-4159.1979.tb11728.x. [DOI] [PubMed] [Google Scholar]
- Tzeng M. C., Siekevitz P. The effect of the purified major protein factor (alpha-latrotoxin) of black widow spider venom on the release of acetylcholine and norepinephrine from mouse cerebral cortex slices. Brain Res. 1978 Jan 6;139(1):190–196. doi: 10.1016/0006-8993(78)90073-2. [DOI] [PubMed] [Google Scholar]


