Abstract
Isoflavonoids are plant natural compounds predominantly found in leguminous plant. They play important functions in both nitrogen fixation and stress resistance. Many clinical studies have linked dietary intake of isoflavonoids to human health benefits. Binding of 14-3-3 proteins to GmMYB176, an isoflavonoid regulator, modulates expression of key isoflavonoids gene expression and its biosynthesis. We have recently demonstrated that the interaction of 14-3-3 proteins with GmMYB176 regulates nuclear-cytoplasmic localization of GmMYB176 thereby affecting target gene expression. Here, we report GmMYB62 as a new R1 MYB client protein of soybean 14-3-3s that may function together with GmMYB176 for gene regulation in soybean.
Keywords: 14-3-3, R1 MYB, protein-protein interaction, soybean, transcription factors
Plant 14–3-3 proteins have been shown to play important roles in several regulatory processes, such as metabolic pathways, gene expression and signal transduction.1 Although the involvement of 14–3-3 proteins in plant primary metabolism and signaling pathways is well established, studies to delineate their role in plant secondary metabolism has just begun. Recently, we found that soybean 14–3-3 proteins regulate intracellular localization of GmMYB176 and affect isoflavonoid biosynthesis.2 GmMYB176 is an R1 MYB transcription factor (TF) that regulates expression of CHS8 gene and isoflavonoid biosynthesis in soybean.3 Many phenylpropanoid genes are regulated by R2R3 MYB TFs in plants.4,5 GmMYB176 is the first R1 MYB TF identified to have a role in plant secondary metabolism. Additionally, GmMYB176 is the first R1 MYB client of 14–3-3 proteins.6 Here, we have identified a new R1 MYB TF, GmMYB62, as 14–3-3 client in soybean. We also demonstrate that GmMYB62 can interact with GmMYB176 and speculate that GmMYB62 may act together with GmMYB176 for gene regulation.
GmMYB62 Interacts with Soybean 14–3-3 Proteins
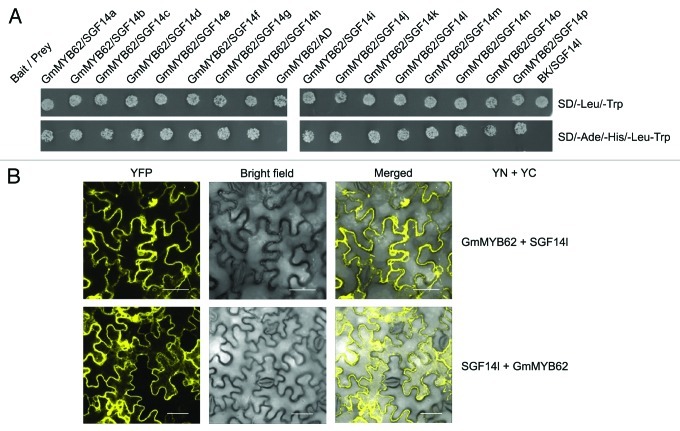
Previously, we conducted a global gene expression analysis during the embryo development in soybean and identified CHS7 and CHS8 as the genes crucial for increased isoflavonoid accumulation in soybean seeds.7 Out of several MYB TFs present on the array, 39 MYBs were differentially expressed during embryo development. This data set was used as a source to identify GmMYB176 as CHS8 gene regulator.3 In this study, we used the same data set and identified two other R1 MYB TFs, GmMYB62 and GmMYB75, whose expression co-relates with the expression of GmMYB176. To investigate if soybean 14–3-3 can interact with other R1 MYB TFs or not, we used a targeted yeast two hybrid (Y2H) assay where GmMYB62 and GmMYB75 were used as bait and SGF14s as prey proteins. As shown in Figure 1A, GmMYB62 interacts with all 16 SGF14s. The interaction between 14–3-3 proteins and GmMYB62 was further validated in planta by using bi-molecular fluorescence complementation (BiFC) assay where translational fusion of SGF14l and GmMYB62 were created with N-terminal (YN) and C-terminal (YC) half of YFP separately under the control of CaMV35S promoter. Both SGF14l-YN and GmMYB62-YC constructs were co-expressed transiently in tobacco epidermal cells by infiltration and protein expression was monitored by confocal laser scanning microscopy. The results confirmed that GmMYB62 and 14–3-3 proteins interact in planta and the interaction is mainly localized in the cytoplasm (Fig. 1B). Negative controls for BiFC assay included co-expression of GmMYB62-YN or YC with the non-fusion half of YFP and co-expression of SGF14l-YN or YC with the non-fusion half of YFP. No YFP signals were observed in the negative controls. Similar experiment was conducted to study interaction between GmMYB75 and SGF14s. No interaction between GmMYB75 and SGF14 was observed in (data not shown).
Figure 1. GmMYB62 interacts with all soybean SGF14s. (A) Yeast two-hybrid assay showing interaction between the GmMYB62 and all 16 SGF14 proteins in soybean. Yeast cells were co-transformed with combination of DNA-binding domain (BK, bait) and activation domain (AD, prey) fused constructs as indicated. SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp correspond to dropout medium lacking Trp and Leu and Ade, Trp, Leu and His, respectively. The co-transformed yeast cultures after 100X dilution were spotted on SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp plate and incubated at 30°C for 5 d. Positive interactions result in yeast growth on the SD/-Ade/-His/-Leu/-Trp plate. (B) BiFC assay to demonstrate the interaction between GmMYB62 and SGF14l. Nicotiana benthamiana leaves co-transformed with constructs of GmMYB62 or SGF14l fused to N-terminal (YN) and C-terminal (YC) half of YFP, respectively (as indicated) were imaged 48 h after infiltration, using confocal microscope. (i) GmMYB62-YN+SGF14l-YC; (ii) SGF14l-YN+GmMYB62-YC. Images are shown as YFP, bright field only and merged YFP and bright-field of epidermal N. benthamiana leaf cells. Scale bars indicate 40 µm.
GmMYB62 Interacts with GmMYB176 in planta
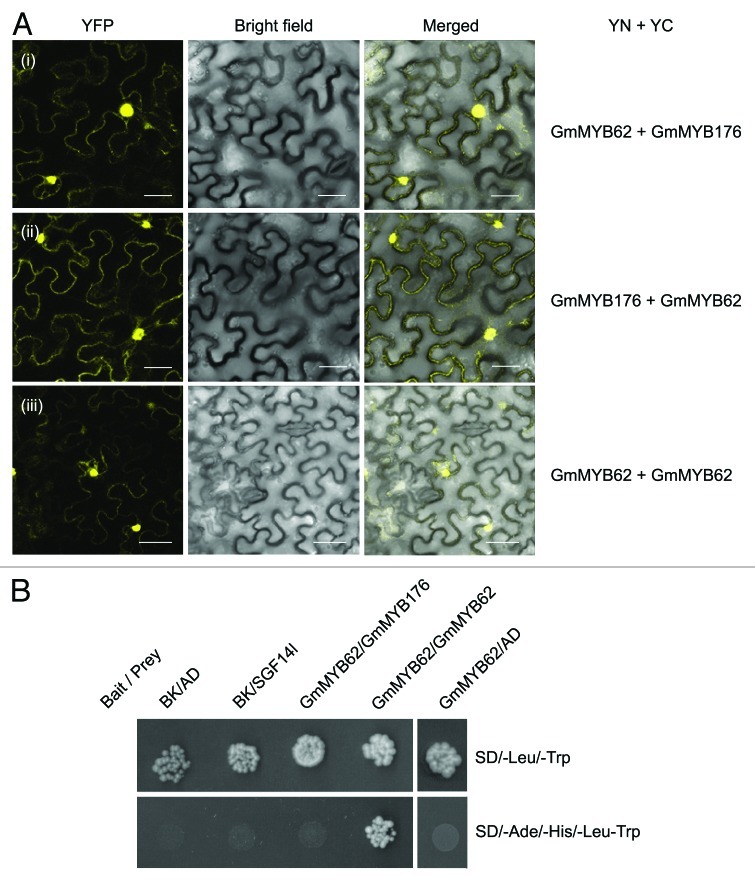
14–3-3s are dimeric proteins which allow them to serve as scaffolds by bringing two different regions of the same phosphoprotein into proximity or two different proteins together. Our previous work and present findings demonstrated that soybean 14–3-3 proteins interact with both GmMYB176 and GmMYB62. To investigate whether GmMYB62 interacts with GmMYB176, we performed BiFC and targeted Y2H assays. The GmMYB62-YN and GmMYB176-YC constructs were coexpressed transiently in tobacco epidermal cells and YFP expression was monitored by confocal laser scanning microscopy. Reciprocal BiFC assay were also performed. Control experiments for BiFC assay were conducted as explained before. As shown in Figure 2A, GmMYB62 and GmMYB176 interact in planta and the interaction is mainly restricted to the nucleus. Some weak interaction between GmMYB62 and GmMYB176 was also observed in the cytoplasm. Interestingly, no positive interaction between GmMYB176 and GmMYB62 was detected in the targeted Y2H assay (Fig. 2B). 14–3-3s are highly conserved eukaryotic proteins. Therefore, binding of tobacco 14–3-3s with GmMYB62 and GmMYB176 cannot be ruled out. Our results suggest that tobacco 14–3-3 proteins may act as scaffolds to bring GmMYB176 and GmMYB62 together.
Figure 2. GmMYB62 forms a homodimer and interacts with GmMYB176 in planta. (A) BiFC assay to demonstrate the interaction between GmMYB62 and GmMYB176 and GmMYB62 monomers. N. benthamiana leaves co-transformed with constructs of genes fused to N-terminal (YN) or C-terminal (YC) half of YFP, (as indicated) were imaged 48 h after infiltration, using confocal microscope. (i) GmMYB62-YN+ GmMYB176-YC; (ii) GmMYB176-YN+GmMYB62-YC; (iii). GmMYB62-YN+ GmMYB62-YC. Images are shown as YFP, bright field only and merged YFP and bright-field of epidermal N. benthamiana leaf cells. Scale bars indicate 40 µm. (B) Y2H assay showing interaction between the GmMYB62 monomers. Yeast cells were co-transformed with combination of DNA-binding domain (BK, bait) and activation domain (AD, prey) fused constructs as indicated. SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp correspond to dropout medium lacking Trp and Leu and Ade, Trp, Leu and His, respectively. A 100 fold diluted co-transformed yeast cultures were spotted on SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp plate and incubated at 30°C for 5 d. Positive interactions result in yeast growth on the SD/-Ade/-His/-Leu/-Trp plate.
GmMYB62 Forms a Homodimer
To elucidate if GmMYB62 monomers can interact with each other, we conducted BiFC assay where translational fusion of GmMYB62 with YN and YC half of YFP were used. Both GmMYB62-YN and GmMYB62-YC were infiltrated in tobacco leaf epidermal cells and transient expression was monitored by confocal microscopy. As shown by the YFP signals, GmMYB62 monomers interact together and the interaction was localized in the nucleus (Fig. 2Aiii). We also performed targeted Y2H assay to confirm if GmMYB62 forms a homodimer. As shown in Figure 2B, the interaction between GmMYB62 monomers was determined by colony growth on selective media.
14–3-3 proteins bind with a wide array of target proteins and regulate the activities of target proteins via protein-protein interaction.8,9 Binding of a 14–3-3 protein to its target can affect localization of the target protein within the cell. Upon binding to a 14–3-3 protein, a telomerase protein (TERT) is transported to the nucleus,10 while histone deacetylases (HDAC4 and HDAC5) are sequestered in the cytoplasm upon 14–3-3 interaction.11 Recently we discovered that 14–3-3s regulates the subcellular localization of GmMYB176 thereby affecting isoflavonoid biosynthesis in soybean.2 GmMYB62 is a new R1 MYB client of soybean 14–3-3 proteins that forms a homodimer in the nucleus. However, upon interaction with SGF14l, GmMYB62 was restricted to the cytoplasm (compare Figure 1B and 2Aiii). Our results demonstrate the dual implications of SGF14s binding to GmMYB62 and GmMYB176: (a) bringing GmMYB62 and GmMYB176 together, and (b) modulating their intracellular localization. Even though the significance of these interactions is not fully understood yet, the involvement of SGF14 and GmMYB176 and their interaction with GmMYB62 suggests the possibility that GmMYB62 may play a role in isoflavonoid biosynthesis in soybean.
Taken together, GmMYB62 is a new R1 MYB client protein of soybean 14–3-3 proteins. Binding of GmMYB62 to 14–3-3 helps its association with GmMYB176 in the nucleus that possibly leads into gene regulation. Identification of target gene (s) and processes regulated by these interactions are currently being studied.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada’s Discovery Grant awarded to SD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20940
References
- 1.Chevalier D, Morris ER, Walker JC. 14-3-3 and FHA domains mediate phosphoprotein interaction. Annu Rev Plant Physiol Plant Mol Biol. 2009;60:67–91. doi: 10.1146/annurev.arplant.59.032607.092844. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Chen L, Dhaubhadel S. 14-3-3 proteins regulate the intracellular localization of the transcriptional activator GmMYB176 and affect isoflavonoid synthesis in soybean. Plant J. 2012 doi: 10.1111/j.1365-313X.2012.04986.x. In press. [DOI] [PubMed] [Google Scholar]
- 3.Yi J, Derynck MR, Li X, Telmer P, Marsolais F, Dhaubhadel S. A single-repeat MYB transcription factor, GmMYB176, regulates CHS8 gene expression and affects isoflavonoid biosynthesis in soybean. Plant J. 2010;62:1019–34. doi: 10.1111/j.1365-313X.2010.04214.x. [DOI] [PubMed] [Google Scholar]
- 4.Sablowski RWM, Moyano E, Culianez-Macia FA, Schuch W, Martin C, Bevan M. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994;13:128–37. doi: 10.1002/j.1460-2075.1994.tb06242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solano R, Nieto C, Avila J, Cañas L, Diaz I, Paz-Ares J. Dual DNA binding specificity of a petal epidermis-specific MYB transcription factor (MYB.Ph3) from Petunia hybrida. EMBO J. 1995;14:1773–84. doi: 10.1002/j.1460-2075.1995.tb07166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhaubhadel S, Li X. A new client for 14-3-3 proteins: GmMYB176, an R1 MYB transcription factor. Plant Signal Behav. 2010;5:1–3. doi: 10.4161/psb.5.7.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhaubhadel S, Gijzen M, Moy P, Farhangkhoee M. Transcriptome analysis reveals a critical role of CHS7 and CHS8 genes for isoflavonoid synthesis in soybean seeds. Plant Physiol. 2007;143:326–38. doi: 10.1104/pp.106.086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–97. doi: 10.1016/S0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, et al. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91:961–71. doi: 10.1016/S0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 10.Seimiya H, Sawada H, Muramatsu Y, Shimizu M, Ohko K, Yamane K, et al. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J. 2000;19:2652–61. doi: 10.1093/emboj/19.11.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A. 2000;97:7835–40. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]