Abstract
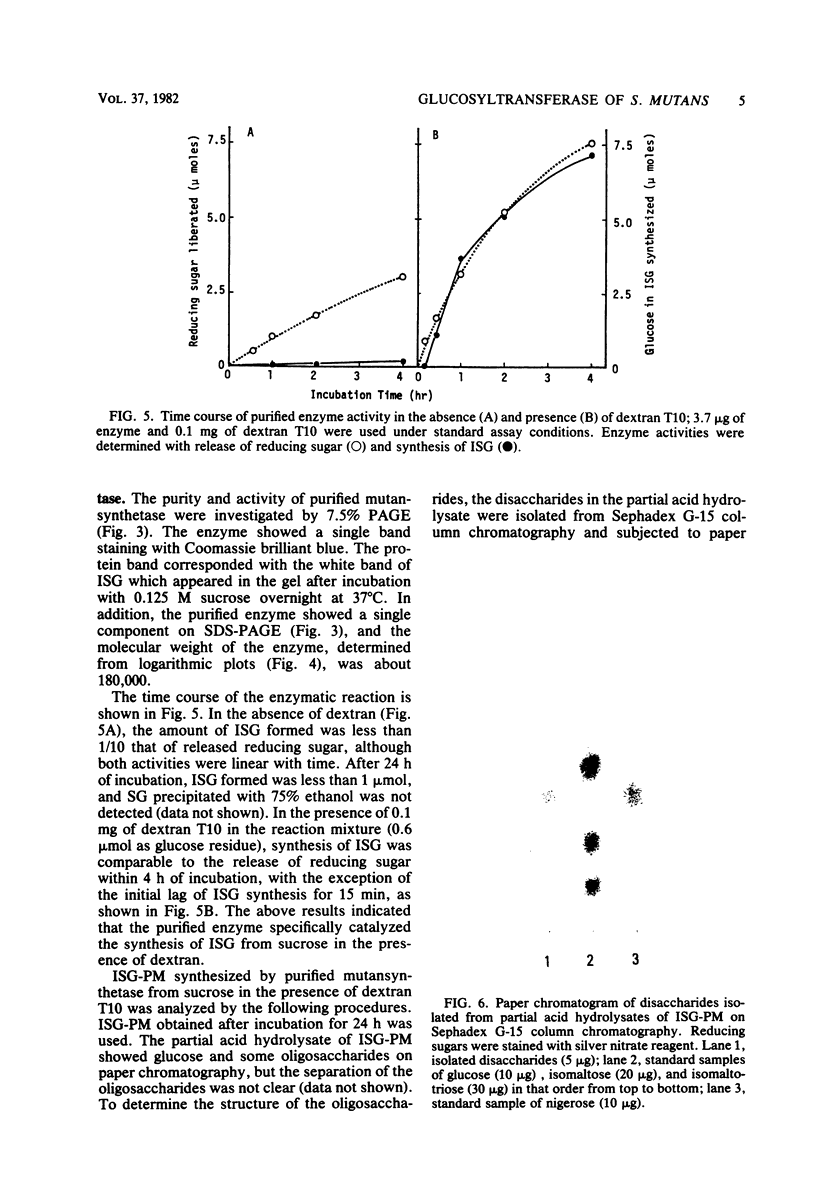
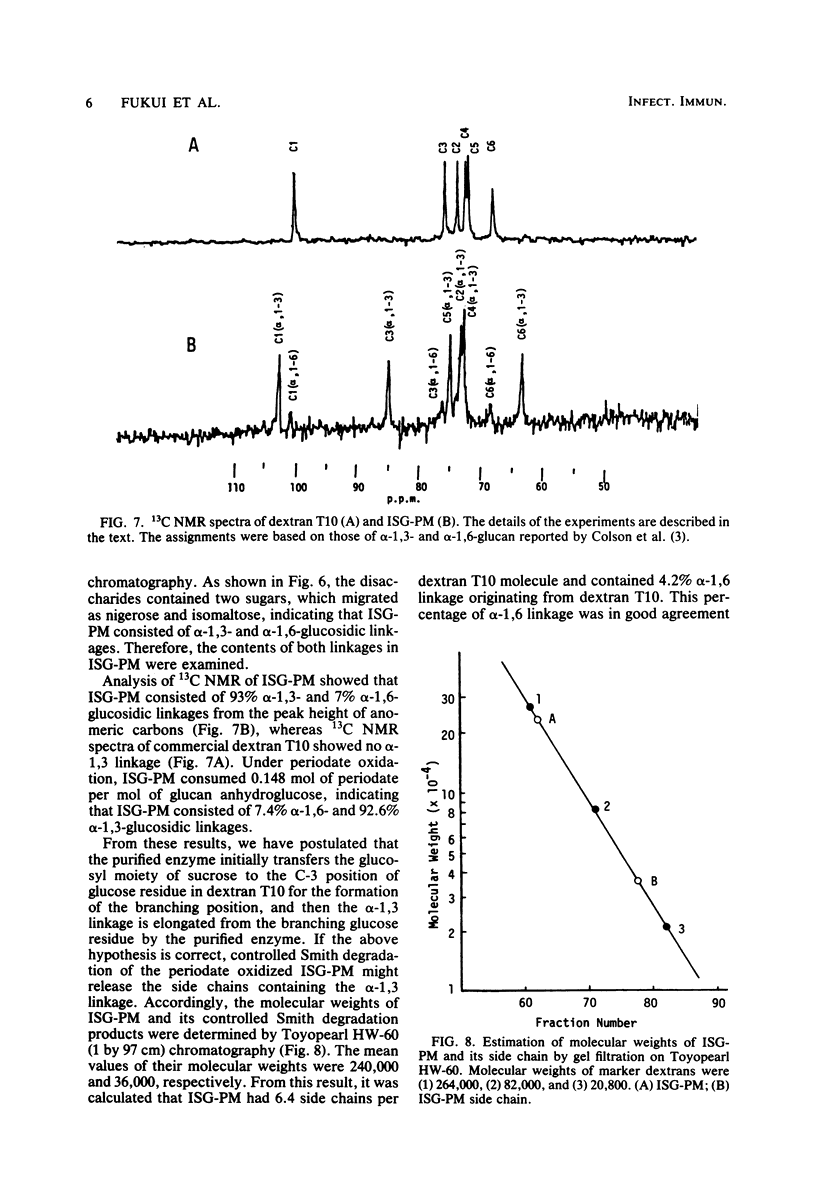
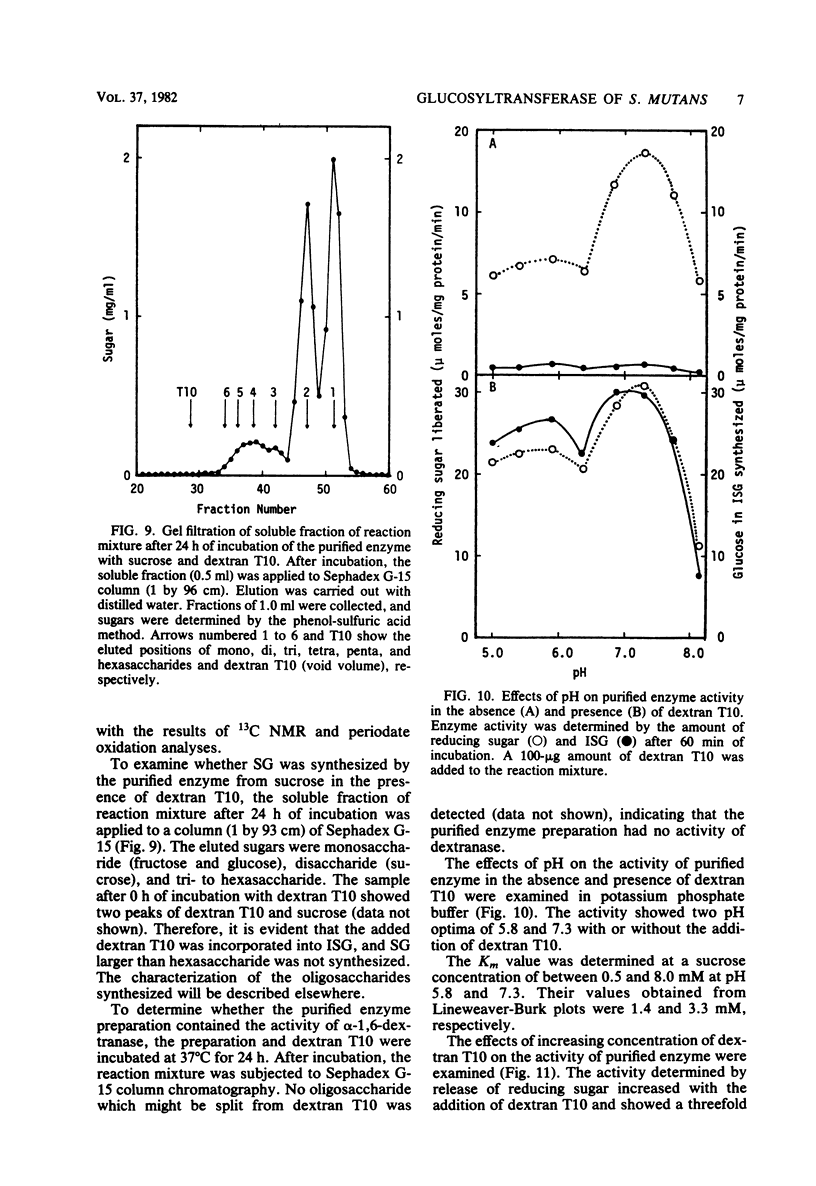
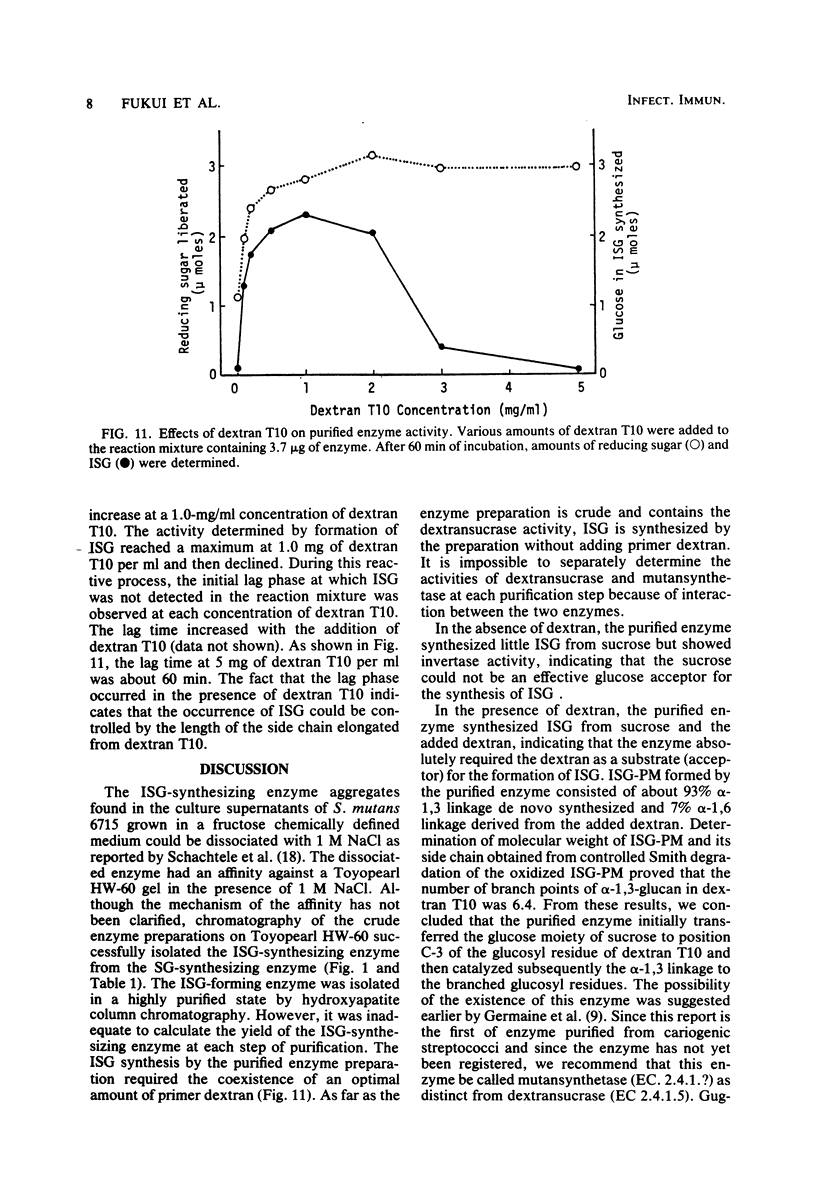
A glucosyltransferase responsible for water-insoluble glucan synthesis was purified from the culture fluids of Streptococcus mutans 6715-15 strain by column chromatography on Toyopearl HW-60 and subsequently on hydroxyapatite. The enzyme preparation gave a single band on analysis by polyacrylamide gel electrophoresis. The pH dependency of the activity showed two optimal peaks at 5.8 and 7.3 and the Km values for sucrose were 1.4 and 3.3 mM at the respective optimal pHs. The molecular weight determined by sodium dodecyl sulfate gel electrophoresis was 180,000. Although the enzyme scarcely synthesized water-insoluble and water-soluble glucans from sucrose, water-insoluble glucan formed from sucrose in the presence of dextran T10 consisted of over 93% alpha-1, 3-glucosidic linkage. Analysis of the structure of water-insoluble glucan indicated that the enzyme catalyzed the formation of branch points in alpha-1,6-glucan (dextran) and transferred the glucosyl moiety of sucrose to the C-3 position of the branching glucose residue of dextran. Since this enzyme has not yet been registered, we named it mutansynthetase (EC 2.4.1.?).
Full text
PDF
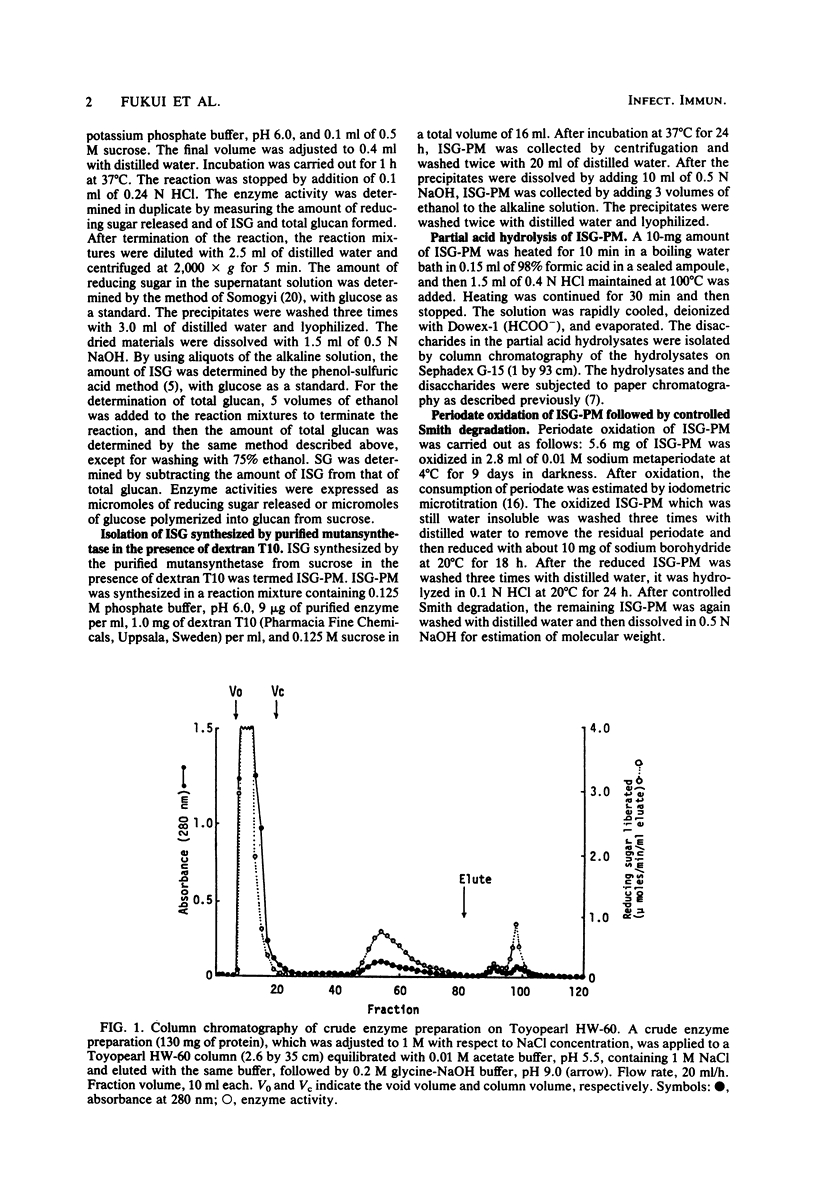

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Jennings H. J., Smith I. C. Composition, sequence, and conformation of polymers and oligomers of glucose as revealed by carbon-13 nuclear magentic resonance. J Am Chem Soc. 1974 Dec 25;96(26):8081–8087. doi: 10.1021/ja00833a038. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Figures W. R., Edwards J. R. D-Glucosyltransferase of Streptococcus mutans: isolation of two forms of the enzyme that bind to insoluble dextran. Carbohydr Res. 1981 Jan 15;88(1):107–117. doi: 10.1016/s0008-6215(00)84605-4. [DOI] [PubMed] [Google Scholar]
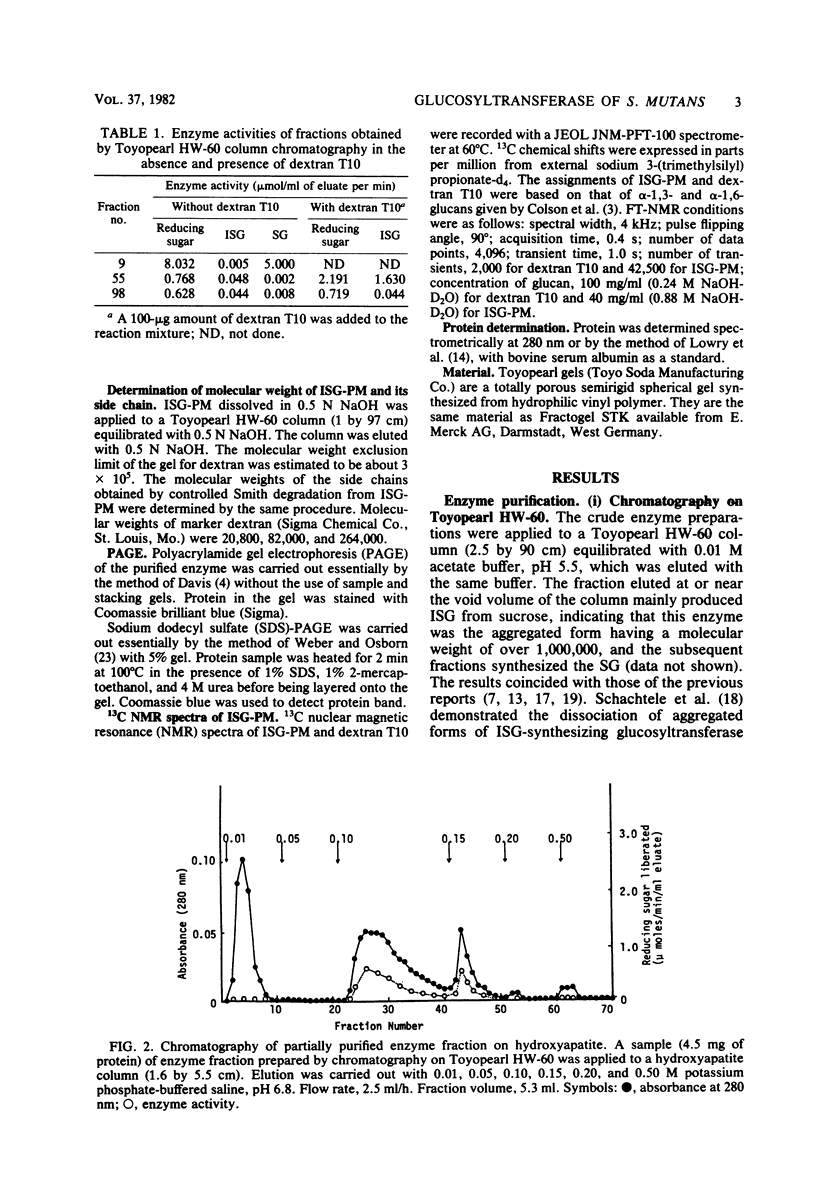
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Motoda R., Takada K., Ikeda T. Resolution of Streptococcus mutans glycosyltransferases into two components essential to water-insoluble glucan synthesis. FEBS Lett. 1981 Jun 15;128(2):213–216. doi: 10.1016/0014-5793(81)80083-x. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
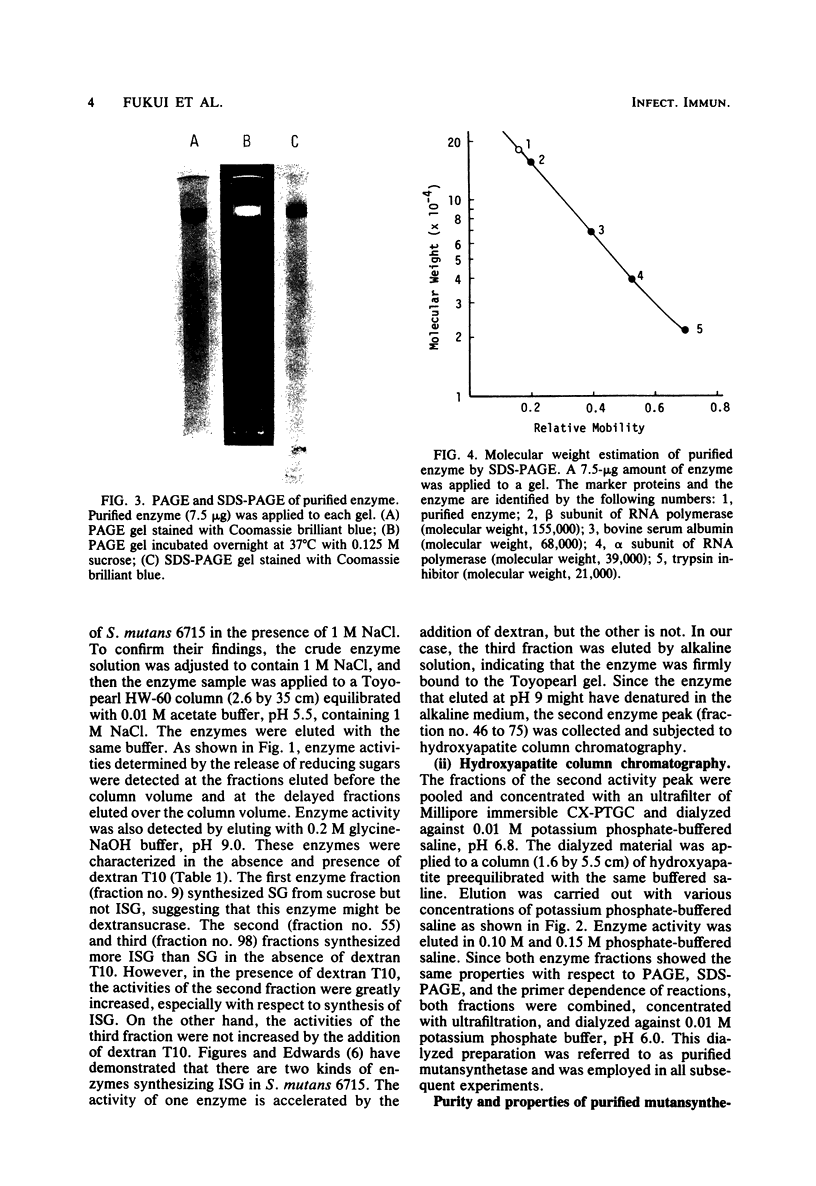
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONTGOMERY R., WU Y. C., LEE Y. C. PERIODATE OXIDATION OF GLYCOPEPTIDES FROM OVALBUMIN. Biochemistry. 1965 Mar;4:578–587. doi: 10.1021/bi00879a031. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Specific method for the purification of Streptococcus mutans dextransucrase. Infect Immun. 1977 Jun;16(3):760–765. doi: 10.1128/iai.16.3.760-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Preparation of glucosyltransferase from Streptococcus mutans by elution from water-insoluble polysaccharide with a dissociating solvent. Infect Immun. 1979 Feb;23(2):446–452. doi: 10.1128/iai.23.2.446-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J. Branching-enzyme activity of an alpha-D-glucosyltransferase of Streptococcus mutans. Carbohydr Res. 1980 Jul;82(2):404–410. doi: 10.1016/s0008-6215(00)85718-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]