Abstract
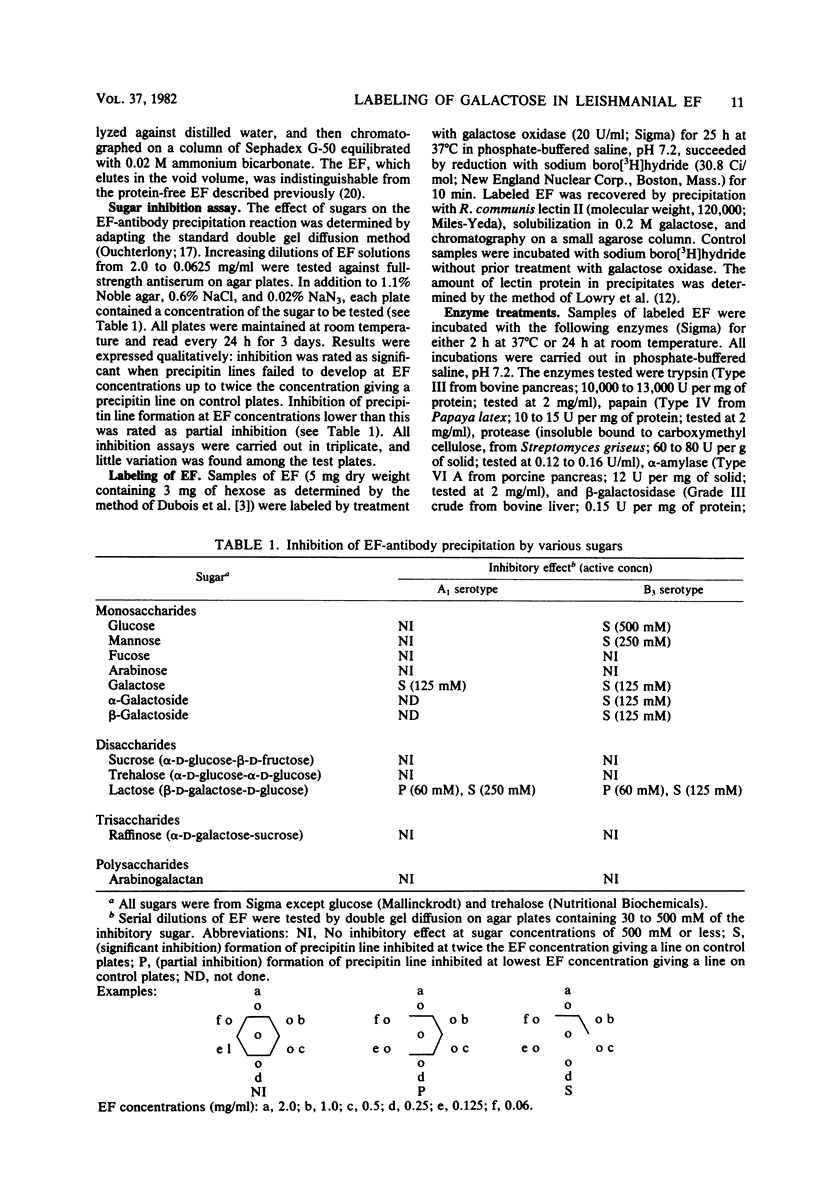
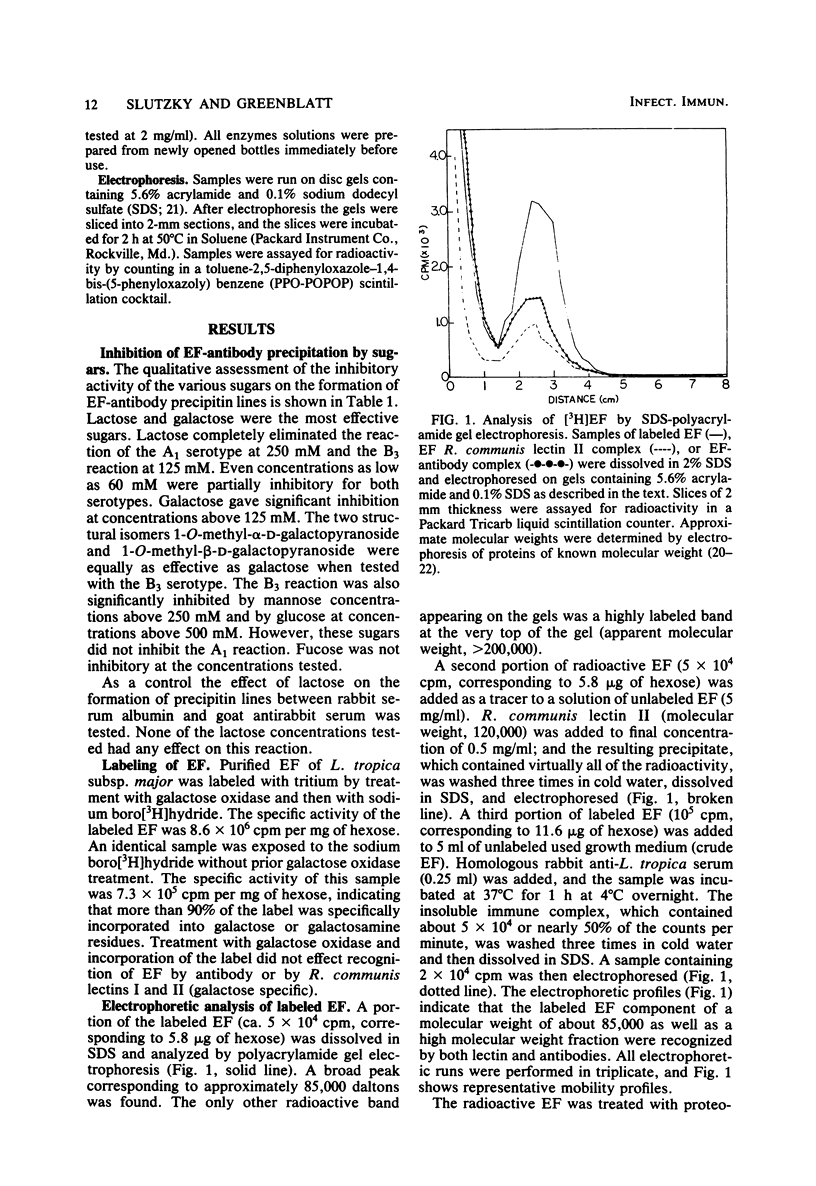
Inhibition by low-molecular-weight sugars of precipitin line formation between a polysaccharide (EF) excreted by Leishmania tropica subsp. major, Leishmania enriettii, and rabbit antileishmanial antibodies on double gel diffusion plates revealed that galactose residues, possibly as components of lactosyl groups, were the critical immunodominant sugars mediating antibody recognition of EF. The galactose residues of the EF of L. tropica subsp. major were specifically labeled with tritium via galactose oxidase and sodium boro[3H]hydride. The radioactive EF had an apparent molecular weight of about 85,000 on sodium dodecyl sulfate-polyacrylamide gels and was precipitated by antileishmanial antibodies as well as Ricinus communis lectins I and II (galactose specific). Lectins specific for glucose-mannose residues, fucose, N-acetylglucosamine, and N-acetylgalactosamine did not precipitate the labeled EF. Treatment of [3H]EF with proteolytic (trypsin, papain, protease) or glycosidic (alpha-amylase, beta-galactosidase) enzymes had no effect on either the electrophoretic pattern of the material or on its recognition by antileishmanial antibodies or R. communis lectin. This resistance to enzyme activity suggests that EF may be a useful marker for the presence of the parasite in vivo if it can be detected in minute quantities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annual meeting of the Israel Society for Parasitology, 23 April 1980, Jerusalem. Abstracts of papers. Isr J Med Sci. 1980 Jul;16(7):559–562. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- El-On J., Schnur L. F., Greenblatt C. L. Leishmania donovani: physicochemical, immunological, and biological characterization of excreted factor from promastigotes. Exp Parasitol. 1979 Apr;47(2):254–269. doi: 10.1016/0014-4894(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L. Promotion of leishmanial infections in non-permissive host macrophages by conditioned medium. Z Parasitenkd. 1977 Sep 21;53(2):143–147. doi: 10.1007/BF00380458. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. Dimensions and specificities of recognition sites on lectins and antibodies. J Supramol Struct. 1978;8(1):79–88. doi: 10.1002/jss.400080107. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. A lectin-like receptor on mammalian macrophages. Biochem Biophys Res Commun. 1978 Nov 29;85(2):678–683. doi: 10.1016/0006-291x(78)91215-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagamura Y., Kolb H. Presence of a lectin-like receptor for D-galactose on rat peritoneal macrophages. FEBS Lett. 1980 Jun 16;115(1):59–62. doi: 10.1016/0014-5793(80)80726-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Semprevivo L. H., Honigberg B. M. Exometabolites of Leishmania donovani promastigotes. II. Spontaneous changes of exometabolite after isolation. Z Parasitenkd. 1980;62(3):201–211. doi: 10.1007/BF00926562. [DOI] [PubMed] [Google Scholar]
- Slutzky G. M., El-On J., Greenblatt C. L. Leishmanial excreted factor: protein-bound and free forms from promastigote cultures of Leishmania tropica and Leishmania donovani. Infect Immun. 1979 Dec;26(3):916–924. doi: 10.1128/iai.26.3.916-924.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutzky G. M., Greenblatt C. L. Analysis by SDS-polyacrylamide gel electrophoresis of an immunologically active factor of Leishmania tropica from growth media, promastigotes, and infected macrophages. Biochem Med. 1979 Feb;21(1):70–77. doi: 10.1016/0006-2944(79)90057-7. [DOI] [PubMed] [Google Scholar]
- Slutzky G. M., Greenblatt C. L. Isolation of a carbohydrate-rich immunologically active factor from cultures of Leishmania tropica. FEBS Lett. 1977 Aug 15;80(2):401–404. doi: 10.1016/0014-5793(77)80485-7. [DOI] [PubMed] [Google Scholar]


