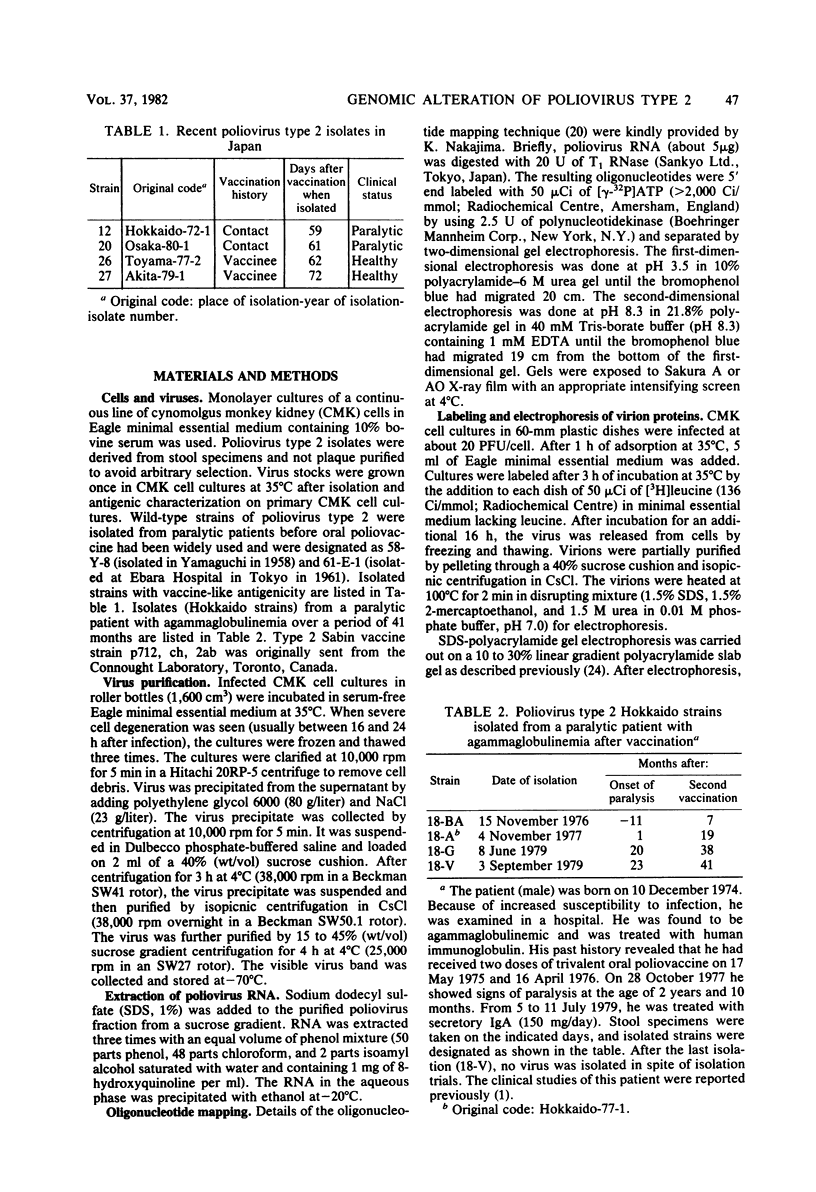
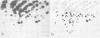Abstract
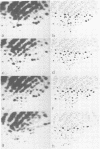
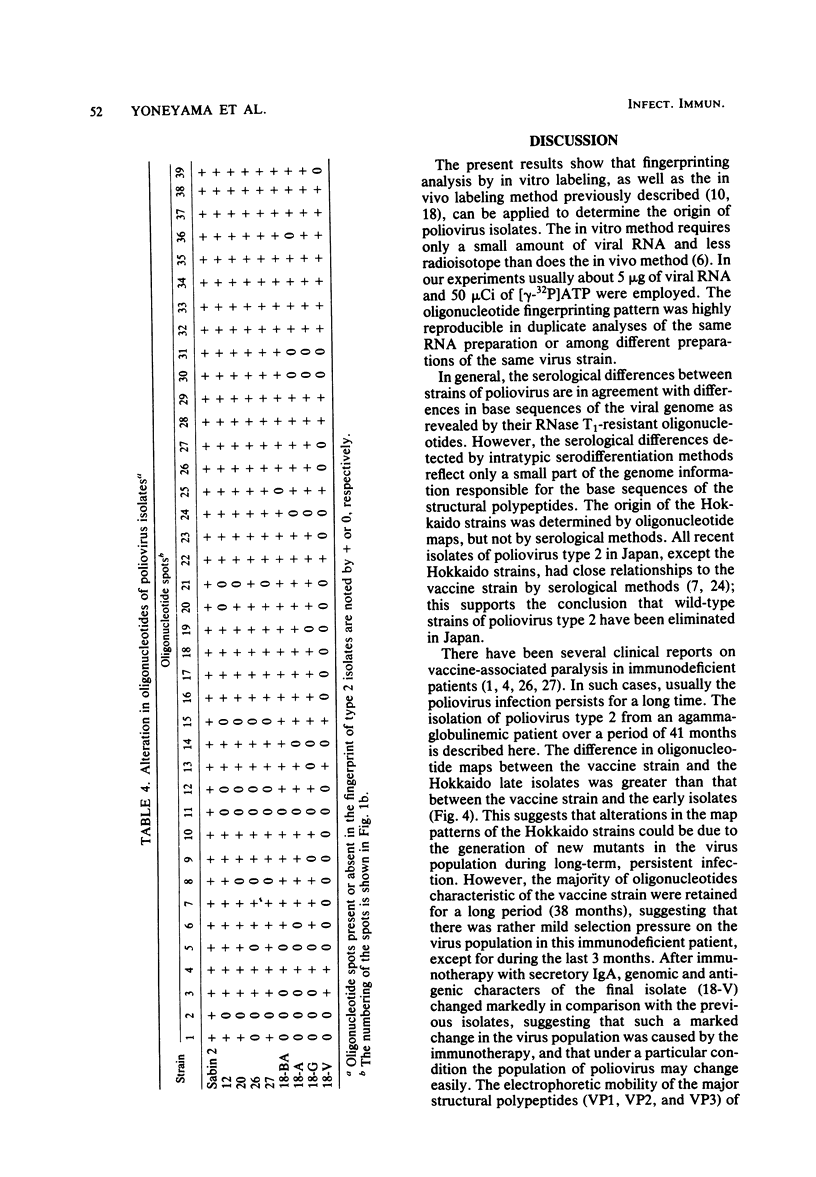
A close relationship was demonstrated by oligonucleotide fingerprinting between genomes of the poliovirus type 2 Sabin vaccine strain and recent isolates from paralytic cases associated with vaccination in Japan. The oligonucleotide maps of isolates from an agammaglobulinemic patient, who continued to excrete poliovirus type 2 for 3.5 years after the administration of oral vaccine, showed that the genomic alteration proceeded gradually, retaining the majority of the oligonucleotides characteristic of the vaccine strain for a long period, indicating vaccine origin for the isolates. The final isolate at month 41, however, lost the majority of these oligonucleotides. The heterologous antigenic relationship between the final isolate and the previous isolates was also observed. The serial alteration in electrophoretic mobility of the major structural proteins (VP1, VP2, and VP3) was observed throughout the excreting period. These results indicate that the population of the virus in this individual changed markedly during the last short period (about 3 months), in which the treatment with secretory immunoglobulin A was carried out. Genome comparisons in oligonucleotide maps show that some oligonucleotides in the genome of the vaccine strain are highly mutable after passage in humans.
Full text
PDF

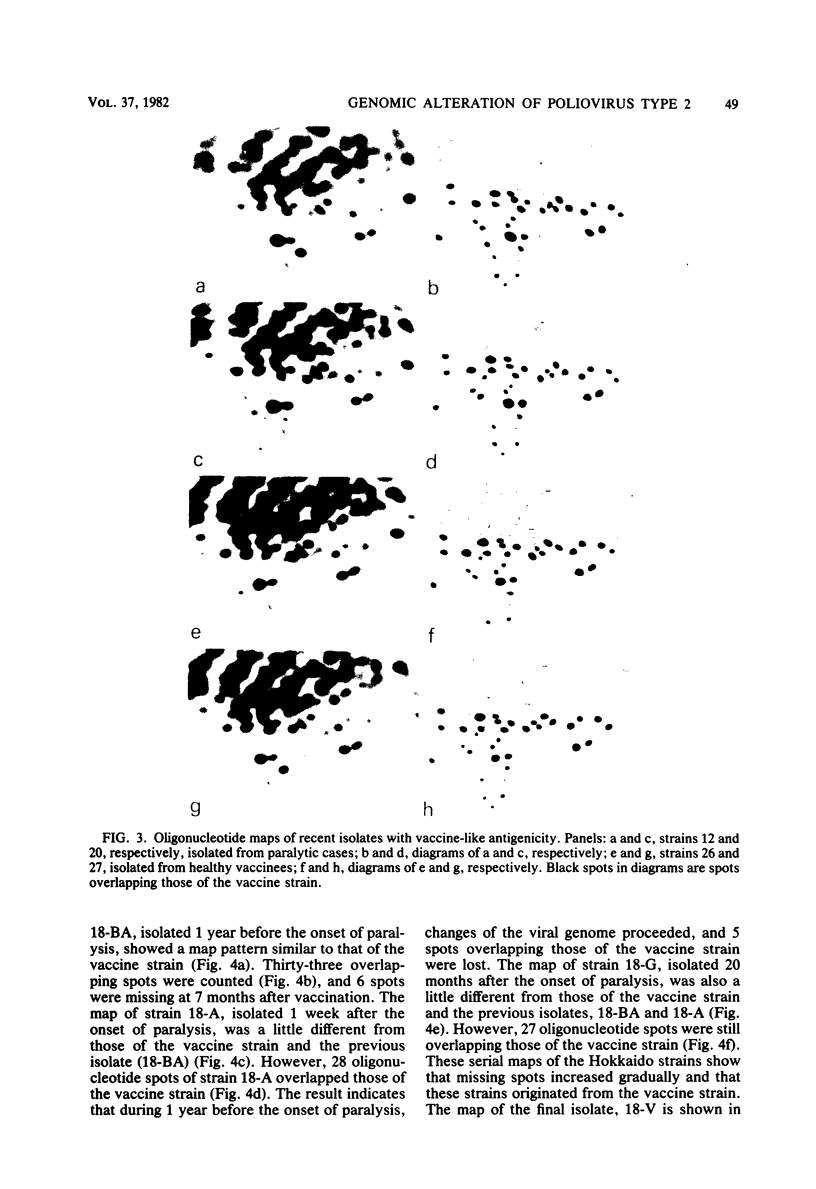


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo W., Chiba S., Yamanaka T., Nakao T., Hara M., Tagaya I. Paralytic poliomyelitis in a child with agammaglobulinemia. Eur J Pediatr. 1979 Sep;132(1):11–16. doi: 10.1007/BF00443199. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. E., Bodian D., Price D., Butler I. J., Vickers J. H. Chronic progressive poliomyelitis secondary to vaccination of an immunodeficient child. N Engl J Med. 1977 Aug 4;297(5):241–245. doi: 10.1056/NEJM197708042970503. [DOI] [PubMed] [Google Scholar]
- Frisby D. Oligonucleotide mapping of non-radioactive virus and messenger RNAs. Nucleic Acids Res. 1977 Sep;4(9):2975–2996. doi: 10.1093/nar/4.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Saito Y., Komatsu T., Kodama H., Abo W., Chiba S., Nakao T. Antigenic analysis of polioviruses isolated from a child with agammaglobulinemia and paralytic poliomyelitis after Sabin vaccine administration. Microbiol Immunol. 1981;25(9):905–913. doi: 10.1111/j.1348-0421.1981.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Pallansch M. A., Omilianowski D. R., Rueckert R. R. Changes in three of the four coat proteins of oral polio vaccine strain derived from type 1 poliovirus. J Virol. 1980 Jan;33(1):256–263. doi: 10.1128/jvi.33.1.256-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T., Hara M., Nakao C., Soda K., Tagaya I. Serodifferentiation of poliovirus strains. I. Modified Wecker test on types 1 and 2 poliovirus strains. Jpn J Med Sci Biol. 1967 Oct;20(5):349–376. doi: 10.7883/yoken1952.20.349. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Kitamura N., Nomoto A., Wimmer E. Sequence studies of poliovirus RNA. IV. Nucleotide sequence complexities of poliovirus type 1, type 2 and two type 1 defective interfering particles RNAs, and fingerprint of the poliovirus type 3 genome. J Gen Virol. 1979 Aug;44(2):311–322. doi: 10.1099/0022-1317-44-2-311. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D. Comparative biochemical studies of type 3 poliovirus. J Virol. 1980 Apr;34(1):73–84. doi: 10.1128/jvi.34.1.73-84.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Nakano J. H., Hatch M. H., Thieme M. L., Nottay B. Parameters for differentiating vaccine-derived and wild poliovirus strains. Prog Med Virol. 1978;24:178–206. [PubMed] [Google Scholar]
- Nakano M., Matsukura T., Yoshii K., Komatsu T., Mukoyama A., Uchida N., Kodama H., Tagaya I. Genetical analysis on the stability of poliovirus type 3 Leon 12a1b. J Biol Stand. 1979 Jul;7(3):157–168. doi: 10.1016/s0092-1157(79)80019-0. [DOI] [PubMed] [Google Scholar]
- Nakao C. Antigenic characterization of poliovirus strains by neutralization kinetic studies and the modified Wecker technique. Jpn J Med Sci Biol. 1969 Aug;22(4):217–233. doi: 10.7883/yoken1952.22.217. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Babich A., Jacobson A., Dunn J. J., Wimmer E. Restricted fragmentation of poliovirus type 1, 2 and 3 RNAs by ribonuclease III. J Mol Biol. 1979 Feb 25;128(2):165–177. doi: 10.1016/0022-2836(79)90124-4. [DOI] [PubMed] [Google Scholar]
- Nottay B. K., Kew O. M., Hatch M. H., Heyward J. T., Obijeski J. F. Molecular variation of type 1 vaccine-related and wild polioviruses during replication in humans. Virology. 1981 Jan 30;108(2):405–423. doi: 10.1016/0042-6822(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Tagaya I., Nakao C., Hara M., Yamadera S. Characterization of poliovirus isolates in Japan after the mass vaccination with live oral poliomyelitis vaccine (Sabin). Bull World Health Organ. 1973 May;48(5):547–554. [PMC free article] [PubMed] [Google Scholar]
- Wege H., Stephenson J. R., Koga M., Wege H., ter Meulen V. Genetic variation of neurotropic and non-neurotropic murine coronaviruses. J Gen Virol. 1981 May;54(Pt 1):67–74. doi: 10.1099/0022-1317-54-1-67. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Hatch M. H., Kasselberg A. G., Lowry S. P., Wadlington W. B., Karzon D. T. Vaccine-associated poliomyelitis in a child with sex-linked agammaglobulinemia. J Pediatr. 1977 Sep;91(3):408–412. doi: 10.1016/s0022-3476(77)81309-7. [DOI] [PubMed] [Google Scholar]
- Wyatt H. V. Poliomyelitis in hypogammaglobulinemics. J Infect Dis. 1973 Dec;128(6):802–806. doi: 10.1093/infdis/128.6.802. [DOI] [PubMed] [Google Scholar]
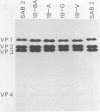
- Yoneyama T., Hagiwara A., Hara M. Structural proteins of poliovirus type 2 isolates. Microbiol Immunol. 1981;25(6):575–583. doi: 10.1111/j.1348-0421.1981.tb00059.x. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]
- van Wezel A. L., Hazendonk A. G. Intratypic serodifferentiation of poliomyelitis virus strains by strain-specific antisera. Intervirology. 1979;11(1):2–8. doi: 10.1159/000149005. [DOI] [PubMed] [Google Scholar]