Abstract
Mounting evidence from human, animal, and in vitro studies indicates that existing drugs, developed to treat other disorders, might also be effective in preventing or slowing the progression of diabetic nephropathy to end stage renal disease. Examples of such drugs include the urate-lowering agent allopurinol, the anti-TNF agents etanercept and infliximab, and the immuno-modulating drug abatacept. Since some these medications are already on the market and have been used for a number of years for other indications, they can be immediately tested in humans for a beneficial effect on renal function in diabetes. Special emphasis should be placed on evaluating the use of these drugs early in the course of diabetic nephropathy when renal damage is most likely to be reversible and interventions can yield the greatest delay to end stage renal disease.
Keywords: diabetic nephropathy, uric acid, inflammation, immune system, novel therapeutics
Introduction
Diabetic nephropathy is the long-term complication of diabetes that imposes the highest social and economic burden, being one of the main causes of end stage renal disease (ESRD).1 Despite improvements in glycemic and blood pressure control, and the introduction of renin–angiotensin system (RAS) blockers, the overall risk of diabetic nephropathy in the population is not declining.2–4 Thus, novel therapeutic approaches are urgently needed to complement glycemic control and RAS inhibition.
One approach is to identify novel drug targets by gaining a better understanding of the pathogenesis of diabetic nephropathy at the molecular level, as discussed by other articles in this issue5–7. However, going from the identification of a drug target to the development of a clinically effective intervention is a long and costly process and only a small number of compounds that enter the development pipeline end up being approved for clinical use. The process is further complicated in the case of diabetic nephropathy by the lack of good animal models, as discussed by Breyer in this issue8.
A complementary strategy is to investigate whether existing drugs, developed and approved for other indications, may have as yet unknown therapeutic effects on diabetic nephropathy. The most obvious advantage of this approach is that clinical trials of these drugs, if justified, can be started at once. Other benefits include the extensive postmarketing surveillance conducted for many of these compounds, reducing the possibility of unknown side effects, and the fact that some of these drugs are inexpensive owing to their long presence on the market and their availability as generic preparations.
Some examples of existing drugs that could be tested for a beneficial effect on diabetic nephropathy are reported in Table 1. Below we examine the evidence suggesting that these medications might be effective in preventing or slowing down kidney damage in diabetes and discuss how clinical trials of these drugs should be designed in order to maximize their probabilities of success.
Table 1.
Examples of existing drugs that could have an application in preventing or halting the progression of diabetic nephropathy.
| Drugs | Target | Current indication | Generic available |
|---|---|---|---|
| Allopurinol | Uric Acid | Gout, chemotherapy-induced hyperuriciema | Yes |
| Febuxostat | Uric Acid | Gout, chemotherapy-induced hyperuriciema | No |
| Etanercept | TNFα | Rheumatoid and psoriatic arthritis, plaque psoriasis, ankylosing spondylitis | No |
| Infliximab | TFNα | Rheumatoid and psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, Crohn's disease, ulcerative colitis | No |
| Atsttrin | TNFα receptors | Human experimentation in progress. | No |
| Sirolimus | mTor pathway | Prevention of transplant rejection | Yes (Canada) |
| Basiliximab | CD25 | Prevention of transplant rejection | No |
| Mycophenolate mofetil (MMF) | IMPDH (purine biosynthesis) | Antoimmune disorders, prevention of transplant rejection | No |
| Abatacept | CD80/B7.1 | Rheumatoid arthritis | No |
| Belatacept | CD80/B7.1 and CD86/B7.2 | Prevention of transplant rejection | No |
Drugs targeting metabolic pathways
One possible strategy is to target metabolic pathways that are not involved in the etiology of diabetic nephropathy, but modulate the susceptibility of the kidney to the deleterious effects of hyperglycemia. Existing interventions in this category that have been investigated in clinical trials include B-vitamins, to decrease homocystinemia9–11, and statins, to decrease cholesterolemia12,13. While neither of these treatments has yielded the expected benefits14–17, new hope for such adjuvant therapies has recently come from the finding of a link between uric acid and progression of kidney damage in diabetes.
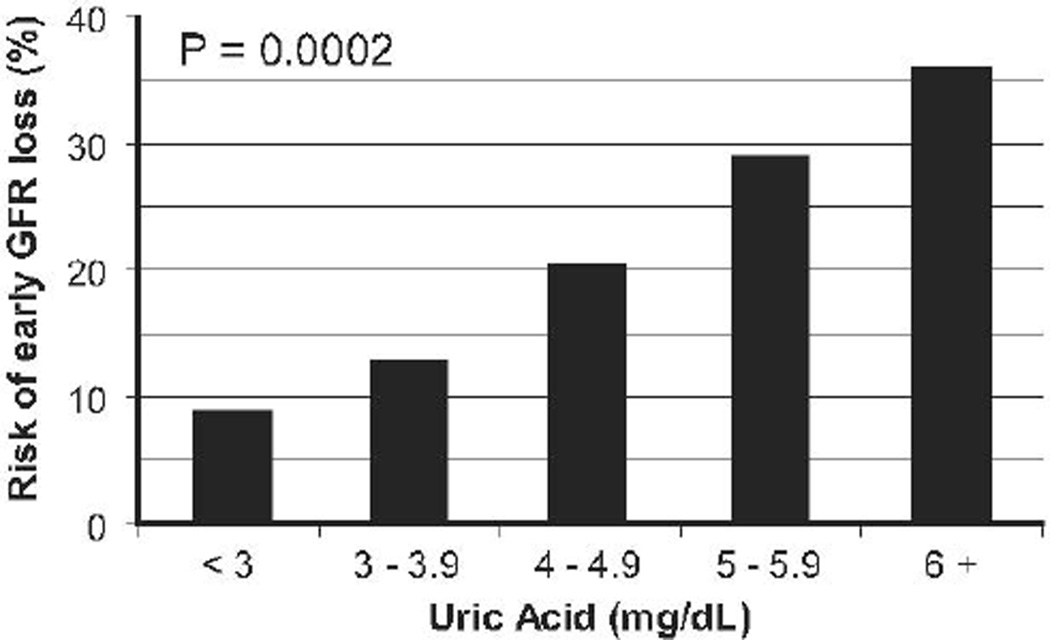
In the Second Joslin Kidney Study (JKS), elevated baseline serum uric acid was one of the strongest independent predictors of early GFR in diabetes18. In this prospective study, including 355 Joslin patients with micro- or high-normoalbuminuria and estimated GFR ≥ 60 ml/min at baseline, a direct dose-response relationship was observed between baseline serum uric acid levels and subsequent risk of early increased GFR loss, defined as a rate of GFR decline above the 97.5th percentile of the distribution in the general population (Figure 1). The unadjusted odds ratio was 1.5 (95% CI 1.3–1.9, p=0.0002) for each mg/dl increase in serum uric acid, which translates into a ~2-fold increase in the risk of early GFR loss for a serum uric acid levels ≥ 5 mg/dl as compared to levels <5 mg/dl. The magnitude of this effect did not significantly change after adjustment for urinary AER, gender, HbA1c, or, importantly, baseline GFR. Serum uric acid also predicted the transition from normoalbuminuria to micro- or macro-albuminuria in the Coronary Artery Calcification in Type 1 Diabetes Study19,20. As in the JKS, the effect of uric acid was not influenced by adjustment for other baseline variables. Similarly, in a study from Denmark, the uric acid level shortly after the onset of type 1 diabetes was a significant independent predictor of macroalbuminuria 18 years later21.
Figure 1.
Risk of early GFR loss in the JKS during 4–6 years of follow-up according to baseline serum UA levels (from Ficociello et al.)
The prospective nature of these findings and their robustness to adjustment for potential confounders strongly suggest that moderately elevated serum uric acid may play a causal role by favoring the deterioration of kidney function caused by the diabetic milieu. Alterations of nitric oxide (NO) pathways and induction of pro-inflammatory cytokines22,23, and increased oxidative stress resulting from the generation of uric acid by xanthine oxidase24,25 could be responsible for this effect. Two small clinical trials have recently provided proof of concept data for translating these findings into a novel intervention. One of these study was from Hong-Kong and included 51 subjects (25% of whom with diabetes) having CKD Stage 3 or higher, who were treated with allopurinol for 12 months26. At the end of the intervention, about 20% of individuals in the allopurinol group had had a significant increase in serum creatinine as opposed to about 70% in the placebo group. The other study was from Spain and included 113 subjects with CKD stage 3 or higher, who were treated with allopurinol for 24 months27. About 15% of the participants had diabetes. During the trial, GFR increased by 1 ml/min in the allopurinol group as compared to a 3 ml/min loss in the placebo group. A beneficial effect of urate-lowering drugs on the progression of kidney disease has also been observed in animal models28.
The availability of a safe and inexpensive uric acid-lowering drug such as allopurinol makes this intervention especially attractive. Allopurinol is an inhibitor of xanthine oxidase, which is responsible for the conversion of hypoxanthine to xanthine and of xanthine to uric acid. It has been on the market since 1964 as the main drug for the prevention of gout in hyperuricemic subjects and the prevention of acute urate nephropathy and gout in patients receiving chemotherapy for cancer. At the average dosage (300 mg/day), allopurinol causes a 30–40% reduction in serum UA29–31, but up to a 60% reduction can be obtained using the maximum dosage of 600 mg.32 Skin rashes, usually maculopapular, are the most commonly reported adverse effect. Rashes may be followed by more severe hypersensitivity reactions such as exfoliative lesions and the Stevens-Johnson syndrome, but such occurrence is very rare, in the order of 1 in 10,00033. Other potential adverse effects include gout flares (if there is a history of gout), hepatotoxicity, and, rarely, bone marrow depression. A new uric acid-lowering drug (febuxostat) that does not have the skin side effects of allopurinol has become recently available30, although its cardiovascular safety is still being investigated (NCT01101035).
Drug targeting inflammatory pathways
Inflammation, as indicated by the presence of inflammatory cells in the tubulointerstitium, has been known for many years to occur in the diabetic kidneys. Indeed, a number of studies have shown that in diabetic subjects the degree of tubulointerstitial injury correlates better with the impairment of renal function than the degree of glomerular damage34–36. In agreement with these findings, prospective studies have shown that increase urinary levels of tubular and inflammatory markers such as kidney injury molecule-1 (KIM1) and monocyte chemotactic protein-1 (MCP-1) accompany the progression of diabetic nephropathy37,38. The demonstration in animal models that lymphocytes are not essential for these changes points to a key role of innate immunity in this process39 and suggests that drugs targeting these pathways could exert a beneficial effect in preserving renal function in diabetes.
Macrophages are the major inflammatory cells infiltrating the kidney in diabetic nephropathy40. Accumulation and activation of these cells have been described in the kidneys of db/db mice with proteinuria, their extent being correlated with hyperglycemia, HbA1c levels, albuminuria, elevated plasma creatinine, glomerular and tubular damage, renal fibrosis, and kidney expression of macrophage chemokines41. Interestingly, some of the beneficial effects of drugs commonly used in diabetes appear to result from their capacity to interfere with macrophage functions. For instance, angiotensin II receptor blockers and ACE inhibitors reduce macrophage-mediated injury in diabetic nephropathy by down-regulating macrophage NF-kB signaling42. Other agents that interfere with macrophage functions could be similarly useful to prevent diabetic nephropathy. An example is MMF – an immunosuppressant that was shown to inhibit kidney macrophage accumulation and diabetic nephropathy severity in Zucker rats43. An especially attractive target is MCP-1 - a chemokine having potent chemoattractive effects on macrophages. Inhibition of MCP-1 or its receptor reduces interstitial inflammation and macrophage accumulation in experimental models of diabetes44–46. An MCP-1 inhibitor – Bindarit - has been demonstrated to reduce albuminuria in active lupus nephritis47 and a phase II study of this drug in albuminuric subjects with type 2 diabetes has just been completed (NCT01109212), although results have not been made public yet.
Another strategy is to use drugs acting on the development of interstitial fibrosis, the sequela of inflammation that is in the end responsible for the loss of kidney function. One such drug is pirfenidone – an inhibitor of TGF-β production that has been shown to decrease matrix deposition in experimental models of kidney disease48. In a small, exploratory study, this compound had beneficial effects on GFR decline in both type 1 and type 2 diabetic subjects with advanced diabetic nephropathy, but these findings must be validated in larger studies49.
Additional suggestions about possible targets are expected from epidemiological studies. In this regard, a recent cohort study including over a thousand patients with both types of diabetes attending the Joslin Clinic has shown that elevated circulating levels of TNF receptors 1 and 2 are strong, independent predictors of subsequent kidney function loss50,51. The predictive power of these two inflammatory markers was much stronger than that of other markers of endothelial dysfunction and inflammation or other components of the TNF pathway including TNFα itself. The role of TNFα is well recognized in the etiology of rheumatoid arthritis and TNFα inhibitors, such as etanercept or infliximab, are routinely used in the treatment of this disease52. There is also a body of literature supporting a role of TNFα in the etiology of diabetic nephropathy53. However, the findings from the Joslin study raise the question as of whether interventions in diabetic nephropathy should be specifically focused on TNF receptors rather than on the TNF pathway in general. In support of this hypothesis, in vitro exposure of human kidney cells to soluble TNF receptors triggers cell death even in the absence of TNF α in the medium54. Furthermore, TNFR1 and TNFR2 knockout mice show a delay in the fibrotic response in experimental models of tubulointerstitial fibrosis55. A new antagonist of TNF receptors (progranulin), inhibiting the interaction between TNFα and TNFRs in a dose-dependent manner, has been recently identified through a global genetic screening56. This molecule and its engineered derivative Atsttrin have been shown to delay the course of rheumatoid arthritis in animal studies57. Thus, one can hypothesize that TNFR inhibition may become an attractive therapeutic tool to evaluate for the prevention of ESRD in diabetes in the near future.
Drug targeting immunological mechanisms
While diabetic nephropathy has never been considered an immunological disease, several reports in the literature have recently raised the possibility that immune-related mechanisms may be involved in its pathogenesis58,59.
T-cells have been found in the kidneys of animal models of diabetic nephropathy60, probably recruited into this organ by the overexpression of MCP-1, CX3CL1, and ICAM-1 by inflamed endothelial cells61. Since T-cell depletion affects the development and the natural history of renal damage in animal models62, drugs that target these cells (e.g., anti-CD3 mAb or ATG) or abrogate their proliferation/activation may have a use for diabetic nephropathy, even though they should probably be reserved to high risk patients (e.g.; fast decliners) given the potential for severe adverse events63. Interventions targeting B-cells are also available, but the evidence for a role of these cells in diabetic nephropathy is not as clear cut as for T-cells.
In addition to immune cells infiltrating the kidney, a variety of immune-related molecules have been found to be expressed by non-immune cells such as podocytes, mesangial cells, and tubular cells and to be upregulated in response to high-glucose or other stresses. For instance, Gutwein et al. found that cytokines such as IFN-γ and TNF-α induce podocyte expression of CXCL16 and ADAM10, which may in turn chemoattract T-cells64. Also, Huber et al. demonstrated that human podocytes, either grown in culture or isolated from biopsies, express many chemokines’ receptors, including CCR4, CCR8, CCR9, CCR10, CXCR1, CXCR3, CXCR4, and CXCR565. Podocytes also express CD80 (B7.1) in response to LPS and other types of stress, and lack of this molecule significantly reduces LPS-mediated podocyte injury66. Studies are underway to determine whether podocytes respond in a similar way to high glucose. If this is the case, one could hypothesize the use of CTLA4-Ig (Abatacept) – a molecule that binds and blocks B7.1 on podocytes – as a therapeutic approach to diabetic nephropathy. This drug has been safely tested in patients with new onset type 1 diabetes in a study aimed at delaying C-peptide loss67. In this multicenter trial, patients with recently diagnosed type 1 diabetes were randomly assigned to abatacept (10 mg/kg) or placebo administered intravenously on days 1, 14, 28, and monthly for a total of 27 infusions over 2 years. Abatacept-treated subjects experienced few infusion-related adverse events (22% in patients on abatacept and 17% in those on placebo) and did no show any increase in the risk of infections (42% vs. 43% in the abatacept vs. the placebo group, respectively) or neutropenia (9% vs. 14%). Thus, abatacept seems to be as safe as placebo when used in mono-therapy. In view of these findings and the extensive clinical trials of Abatacept for immune-related kidney diseases (Table 2) and Belatacept (which differs from Abatacept by only 2 aminoacids and binds both CD80/B7.1 and CD86/B7.2) in kidney-transplanted patients, these drugs appear as the most attractive immuno-modulatory interventions for diabetic nephropathy. Of note, B7.1/CD80 and CD86/B7.2 are also expressed on human tubular cells during inflammation where they may interact with their ligand CD28, either soluble or expressed by T-cells, and lead to the loss or damage of tubular cells68. Thus, blockage of B7.1 and of B7.2 may also have a benefit at this level in addition to that on podocytes.
Table 2.
Clinical Trial with CTLA4-Ig (Abatacept) in kidney/immunological diseases. Abatacept (marketed as Orencia) is a fusion protien generated with an immunoglobulin fused to the extracellular domain of CTLA4 and capable of stable binding to B7.1/CD80, thus blocking its activation.
| Study/Identifier | Purpose | Status/PI |
|---|---|---|
| Efficacy and Safety Study of Abatacept to Treat Lupus Nephritis NCT00430677 |
The purpose of this clinical research study is to learn if abatacept treatment of patients with active lupus nephritis who are also taking mycophenolate mofetil (MMF) and steroid as part of this study will control the nephritis despite a protocol-defined steroid taper; the endpoint is "confirmed complete renal response", a composite including stabilization or improvement of renal function, improvement of proteinuria, and improvement of urinary sediment. The safety of this treatment will also be studied | Ongoing Bristol-Myers Squibb |
| Abatacept and Cyclophosphamide Combination Therapy for Lupus Nephritis (ACCESS) NCT00774852 |
This study is for people with lupus who have developed complications in their kidneys, or lupus nephritis. The study will determine whether adding the experimental medication abatacept to standard cyclophosphamide therapy is more effective in improving lupus nephritis than standard cyclophosphamide therapy by itself | Ongoing David wofsy,, University of California, San Francisco Betty Diamond, MDFeinstein Institute |
| Abatacept in Treating Adults With Mild Relapsing Wegener's Granulomatosis NCT00468208 |
Wegener's granulomatosis (WG) is a rare disease that causes inflammation of blood vessele, or vasculitis. It may involve many different parts of the body, but typically affects the upper and lower respiratory tract and kidneys. The purpose of this study is to determine the safety and effectiveness of the medication abatacept in treating adults with mild relapsing WG | Ongoing Carol A. Langford, The Cleveland Clinic Peter A. Merkel, Boston University |
| Abatacept in ANCA Associated Vasculitis (ABAVAS) NCT00482066 |
The purpose of this study is to investigate whether abatacept can prevent relapse in patients with ANCA associated vasculitis(AAV). This is a randomised double blined placebo controlled trial | Terminated Alan Salama Imperial College London |
| Intravenous CTLA4-Ig Treatment in Recent Onset Type 1 Diabetes Mellitus NCT00505375 |
The purpose of this study is to determine whether treatment with CTLA4-Ig (Abatacept) in individuals with new onset T1DM will improve insulin secretion (C-peptide production) compared to placebo | Ongoing Tihamer Orban, joslin Diabetes Center |
| Islet Transplantation Using Abatacept NCT00276250 |
Islet transplantation in type 1 diabetices with hypoglycemic unawareness using abatacept as a part of a novel calcineurin-inhibitor-sparing immunosuppressive regimen. | Ongoing Christian P Larsen/Thomas C Pearson Emory University |
Clinical trials to test the efficacy of existing drugs
Since many of the drugs discussed above have been already approved for other indications, their effect on diabetic nephropathy can be readily tested in randomized clinical trials. It is crucial, however, that these trials are properly designed in order to maximize power and to exploit the full potential of these drugs to prevent or retard renal function loss.
Target population
One important aspect concerns the selection of the individuals to whom these interventions should be applied. As illustrated in detail in the introductory issue of this article69, the rate of renal function decline varies widely among the diabetic subjects who progress to ESRD, suggesting heterogeneity in the underlying mechanisms of kidney damage. It is also likely that the molecular pathways involved in the etiology of kidney damage undergo changes as the renal function declines from norml to ESRD. Thus, candidate drugs should be tested in specific groups of diabetic subjects defined on the basis of their rate of kidney function loss (e.g., rapid progressors vs. slow progressors) and their stage of renal function loss (e.g., early vs. late). For each drug, the group of patients to be targeted should be decided on the basis of the evidence from human and animal studies concerning the role of the drug target in the progression of kidney disease.
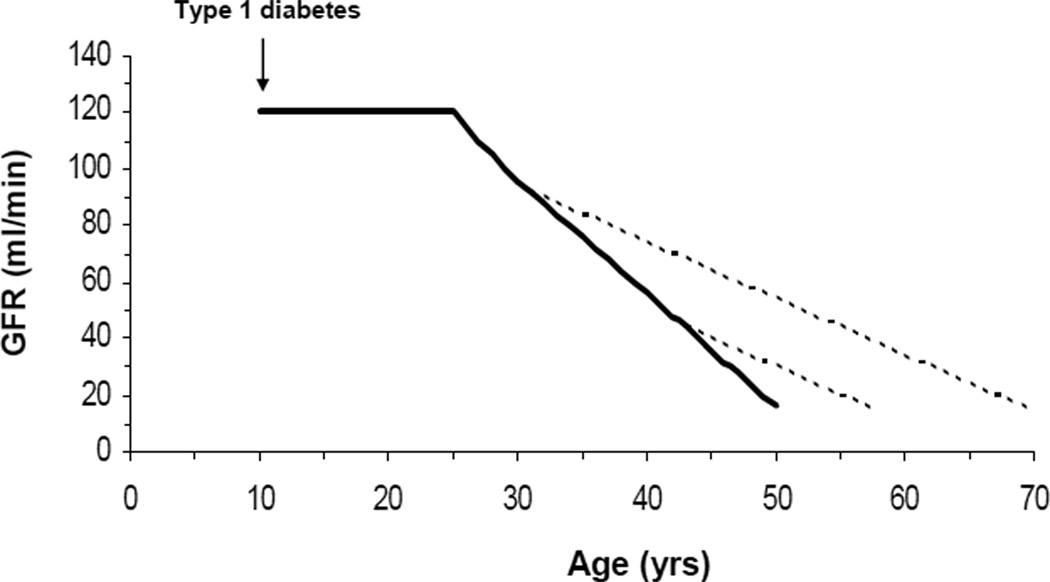
Interventions that may be effective at the early stages of renal fucntion loss are especially attractive since these can yield the greatest delay to ESRD. If a diabetic patient loses GFR at a constant rate of 4 ml/min per year, a 50% reduction in that rate of decline will delay reaching ESRD (GFR ≤ 15 ml/min) by 20 years if the intervention is started when the GFR is 90 ml/min, as opposed to a delay of only 8 years if the intervention is started when the GFR is 45 ml/min. For young type 1 diabetic patients with kidney complications, such as that depicted in Figure 2, this may make the difference between developing ESRD in their 70s as opposed to their 50s. A potential argument against early interventions is that a good proportion of the diabetic patients with mild to moderately decreased GFR may be “slow progressors” who will never reach ESRD. Thus, one may need to treat a large number of them to prevent ESRD in the relatively small proportion at risk. However, biomarkers, such as serum uric acid in the case of allopurinol18 or TNF receptors for drugs targeting the TNF system50,51, are often available to identify individuals who are at greater risk of loosing GFR and would specifically benefit from the interventions under consideration. This is in addition to increased albuminuria, which can also be used to select higher risk candidates for early interventions.
Figure 2.
GFR trajectory of a hypothetical type 1 diabetes patients who developed diabetes at age 10 and started to lose renal function at age 25 at a constant rate of 4 ml/min per year. The solid line represents the GFR trajectory without treatment, the dotted lines are the trajectories with an intervention that reduce GFR decline from 4 to 2 ml/min/year and is started at a GFR of 90 ml/min or at a GFR of 45/ml/min.
Response/outcome variable
Past clinical trials of therapies for diabetic nephropathy have often used albuminuria as the response variable. However, prospective studies have shown that, in a substantial proportion of type 1 diabetes patients, there may be a dissociation between natural history of renal function and that of albuminuria.70–72 Since it is the loss of renal function that drives the increased morbidity and mortality associated with diabetic nephropathy, it seems obvious that this, rather than albuminuria, should be the outcome on which the efficacy of an intervention is measured. If the goal is to intervene early, as we have argued in the previous paragraph, the most effective response variable would be the GFR at the end of the intervention considered on a continuous scale after adjustment for the baseline value. This approach is equivalent to comparing pre- to post-treatment changes in GFR between treatment arms, but yields greater power when the correlation between pre- and post-treatment values is only moderate73. A related approach is to measure the GFR at different time points in order to estimate the slope of GFR decline during the intervention period. The problem with this strategy, however, is that GFR slopes may be unduly influenced by transitory changes in the GFR early in the course of treatment as discussed by Stevens et al.74. Furthermore, the methods for comparing slopes are more complex, since they involve the analysis of time × treatment interactions. Such complexity may offset the gain in power provided by the multiple GFR measures. Survival analyses based on hard endpoints, such as ESRD or serum creatinine doubling, are not useful for the study of interventions on early GFR loss since an extremely long trial duration and large sample size would be required in order to have enough events for a meaningful comparison between treatment arms. For instance, an untreated patients having a baseline GFR of 80 ml/min and losing GFR at a constant rate of 4 ml/min/year would need 12 years to experience a doubling of serum creatinine and 17 years to reach ESRD. It should be added that it is not strictly necessary to demonstrate efficacy on hard endpoints in the case of drugs that are already on the market and for which there is no interest in applying to the FDA for a new indication. If such evidence is needed, as in the case of a new drug, one approach can be to restrict the study population to subjects who have a GFR closer to the end-point and are at especially high risk of losing renal function based their previous clinical history or biomarker profile.
Time frame
Another important aspect concerns the optimal duration of the trial. Many of the trials conducted thus far have been relatively short, mostly being less than two year long, in order to minimize attrition and contain financial and human costs. However, it is critical that trials are long enough to go beyond functional, short-term effects that drugs may have on GFR74. Even more importantly, the longer is the trial, the larger are the differences in GFR that can be attained between treatment arms, increasing the power of the study for any given sample size.
Summary and conclusions
Despite the progress that has occurred during the pat 20 years, preventing end stage renal disease in diabetes is still an unmet need. While research is ongoing to develop new medications, several drugs that are already on the market for the treatment of metabolic, inflammatory, and immunological disorders can be hypothesized to have beneficial effects on diabetic nephropathy based on the known links between their molecular targets and kidney damage in diabetes. Given their known safety profile, some of these drugs could and should be immediately tested for a beneficial effect on kidney function in humans. In doing so, we should take the opportunity to rethink the design of diabetic nephropathy clinical trials in order to increase power and maximize the impact of interventions on the natural history of kidney disease in diabetes.
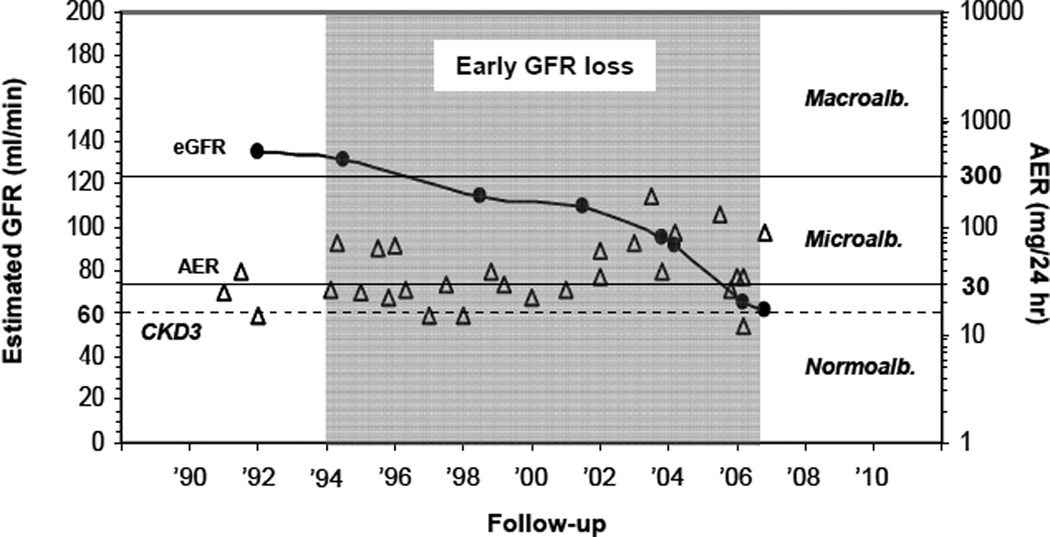
Figure 3.
Course of urinary albumin excretion (triangles) and renal function (circles) over the 14 years preceding the onset of CKD3 in a Joslin type 1 diabetic patient.
Acknowledgments
Part of this work was supported by NIH grant R03 DK094484 (A.D.) and MIUR grant "Staminali" RF-FSR-2008-1213704 (P.F.). P.F. is the recipient of a JDRF-Career Development Award and an ASN Career Development Award. MAN is the recipient of ADA mentor-based fellowship 7-03-MN-28 and Joslin DRC P&F grant 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: AD, MAN, and PF have nothing to declare.
Reference List
- 1.Krolewski AS, Warram JH. Epidemiology of late complications of diabetes: A basis for the development and evaluation of preventive program. In: Kahn CR, Weir GC, King GL, Jacobson AM, Moses AC, Smith RJ, editors. Joslin's Diabetes Mellitus. New York: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 2.U S Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 3.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, et al. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol. 2011;22(3):545–553. doi: 10.1681/ASN.2010040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012 doi: 10.1016/j.semnephrol.2012.07.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey B. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012 doi: 10.1016/j.semnephrol.2012.07.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mima A, Qi W, King GL. Implications of treatment that target protective mechanisms against diabetic nephropathy. Semin Nephrol. 2012 doi: 10.1016/j.semnephrol.2012.07.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breyer MD. Drug development for diabetic glomerular disease. Semin Nephrol. 2012 In press. [Google Scholar]
- 9.Looker HC, Fagot-Campagna A, Gunter EW, Pfeiffer CM, Narayan KM, Knowler WC, et al. Homocysteine as a risk factor for nephropathy and retinopathy in Type 2 diabetes. Diabetologia. 2003;46(6):766–772. doi: 10.1007/s00125-003-1104-x. [DOI] [PubMed] [Google Scholar]
- 10.Buysschaert M, Dramais AS, Wallemacq PE, Hermans MP. Hyperhomocysteinemia in type 2 diabetes: relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care. 2000;23(12):1816–1822. doi: 10.2337/diacare.23.12.1816. [DOI] [PubMed] [Google Scholar]
- 11.Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131(5):363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet F, Cooper ME. Potential influence of lipids in diabetic nephropathy: insights from experimental data and clinical studies. Diabetes Metab. 2000;26(4):254–264. [PubMed] [Google Scholar]
- 13.Valensi P, Picard S. Lipids, lipid-lowering therapy and diabetes complications. Diabetes Metab. 2011;37(1):15–24. doi: 10.1016/j.diabet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303(16):1603–1609. doi: 10.1001/jama.2010.490. [DOI] [PubMed] [Google Scholar]
- 15.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54(5):810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17(7):2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 17.Sukhija R, Bursac Z, Kakar P, Fink L, Fort C, Satwani S, et al. Effect of statins on the development of renal dysfunction. Am J Cardiol. 2008;101(7):975–979. doi: 10.1016/j.amjcard.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues TC, Maahs DM, Johnson RJ, Jalal DI, Kinney GL, Rivard C, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33(11):2471–2473. doi: 10.2337/dc10-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 23.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 24.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51(4):1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 25.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Lozada LG, Tapia E, Soto V, Avila-Casado C, Franco M, Wessale JL, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008;108(4):69–78. doi: 10.1159/000127837. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher HR, Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59(11):1540–1548. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 30.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 31.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375(9732):2161–2167. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 34.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187(2–3):251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 36.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011;79(4):464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolkow PP, Niewczas MA, Perkins B, Ficociello LH, Lipinski B, Warram JH, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19(4):789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim AK, Ma FY, Nikolic-Paterson DJ, Kitching AR, Thomas MC, Tesch GH. Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia. 2010;53(8):1772–1782. doi: 10.1007/s00125-010-1757-1. [DOI] [PubMed] [Google Scholar]
- 40.Tesch GH. Macrophages and diabetic nephropathy. Semin Nephrol. 2010;30(3):290–301. doi: 10.1016/j.semnephrol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65(1):116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 42.Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H. The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. J Cardiovasc Pharmacol. 2006;48(4):135–142. doi: 10.1097/01.fjc.0000245241.79959.d6. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Iturbe B, Quiroz Y, Shahkarami A, Li Z, Vaziri ND. Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int. 2005;68(3):1041–1047. doi: 10.1111/j.1523-1755.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 44.Giunti S, Barutta F, Perin PC, Gruden G. Targeting the MCP-1/CCR2 System in diabetic kidney disease. Curr Vasc Pharmacol. 2010;8(6):849–860. doi: 10.2174/157016110793563816. [DOI] [PubMed] [Google Scholar]
- 45.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294(4):F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 46.Tesch GH, Lim AK. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2011;300(2):F301–F310. doi: 10.1152/ajprenal.00607.2010. [DOI] [PubMed] [Google Scholar]
- 47.Ble A, Mosca M, Di Loreto G, Guglielmotti A, Biondi G, Bombardieri S, et al. Antiproteinuric effect of chemokine C-C motif ligand 2 inhibition in subjects with acute proliferative lupus nephritis. Am J Nephrol. 2011;34(4):367–372. doi: 10.1159/000330685. [DOI] [PubMed] [Google Scholar]
- 48.Shihab FS, Bennett WM, Yi H, Andoh TF. Pirfenidone treatment decreases transforming growth factor-beta1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am J Transplant. 2002;2(2):111–119. doi: 10.1034/j.1600-6143.2002.020201.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22(6):1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gohda T, Niewczas MA, Skupien J, Walker WH, Ficociello LH, Sciutto FR, et al. Circulating Tumor Necrosis Factor Receptors 1 and 2 and risk of Early Renal Function Decline in Type 1 Diabetes. J Am Soc Nephrol. 2011 [Google Scholar]
- 51.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Sciutto FR, et al. Serum markers of Tumor Necrosis Factor Pathway And Risk of End-Stage Renal Disease in Type 2 Diabetes. J Am Soc Nephrol. 2011 [Google Scholar]
- 52.McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 53.Navarro JF, Mora-Fernandez C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17(6):441–450. doi: 10.1016/j.cytogfr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Al Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19(12):1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 55.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. American Journal of Physiology - Renal Physiology. 1999;277(5):F766–F772. doi: 10.1152/ajprenal.1999.277.5.F766. [DOI] [PubMed] [Google Scholar]
- 56.Liu CJ, Bosch X. Progranulin: A growth factor, a novel TNFR ligand and a drug target. Pharmacol Ther. 2011 doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The Growth Factor Progranulin Binds to TNF Receptors and Is Therapeutic Against Inflammatory Arthritis in Mice. Science. 2011;332(6028):478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20(6):1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 59.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22(6):1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 60.Breyer MD, Bottinger E, Brosius FC, III, Coffman TM, Harris RC, Heilig CW, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 61.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17(2):368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 62.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60(11):2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Getts DR, Shankar S, Chastain EM, Martin A, Getts MT, Wood K, et al. Current landscape for T-cell targeting in autoimmunity and transplantation. Immunotherapy. 2011;3(7):853–870. doi: 10.2217/imt.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutwein P, Abdel-Bakky MS, Doberstein K, Schramme A, Beckmann J, Schaefer L, et al. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J Cell Mol Med. 2009;13(9B):3809–3825. doi: 10.1111/j.1582-4934.2009.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huber TB, Reinhardt HC, Exner M, Burger JA, Kerjaschki D, Saleem MA, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168(12):6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- 66.Reiser J, von GG, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong CK, Ho AW, Tong PC, Yeung CY, Chan JC, Kong AP, et al. Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in type 2 diabetic patients with nephropathy. J Clin Immunol. 2008;28(1):36–43. doi: 10.1007/s10875-007-9137-4. [DOI] [PubMed] [Google Scholar]
- 69.Krolewski AS, Bonventre JV. New therapies are desperately needed to reduce risk of ESRD in type 1 diabetes, a call to action. Semin Nephrol. 2012 doi: 10.1016/j.semnephrol.2012.07.001. In press. [DOI] [PubMed] [Google Scholar]
- 70.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 71.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 72.Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31(5):971–973. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 73.Tu YK, Blance A, Clerehugh V, Gilthorpe MS. Statistical power for analyses of changes in randomized controlled trials. J Dent Res. 2005;84(3):283–287. doi: 10.1177/154405910508400315. [DOI] [PubMed] [Google Scholar]
- 74.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol. 2006;1(4):874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]