Abstract
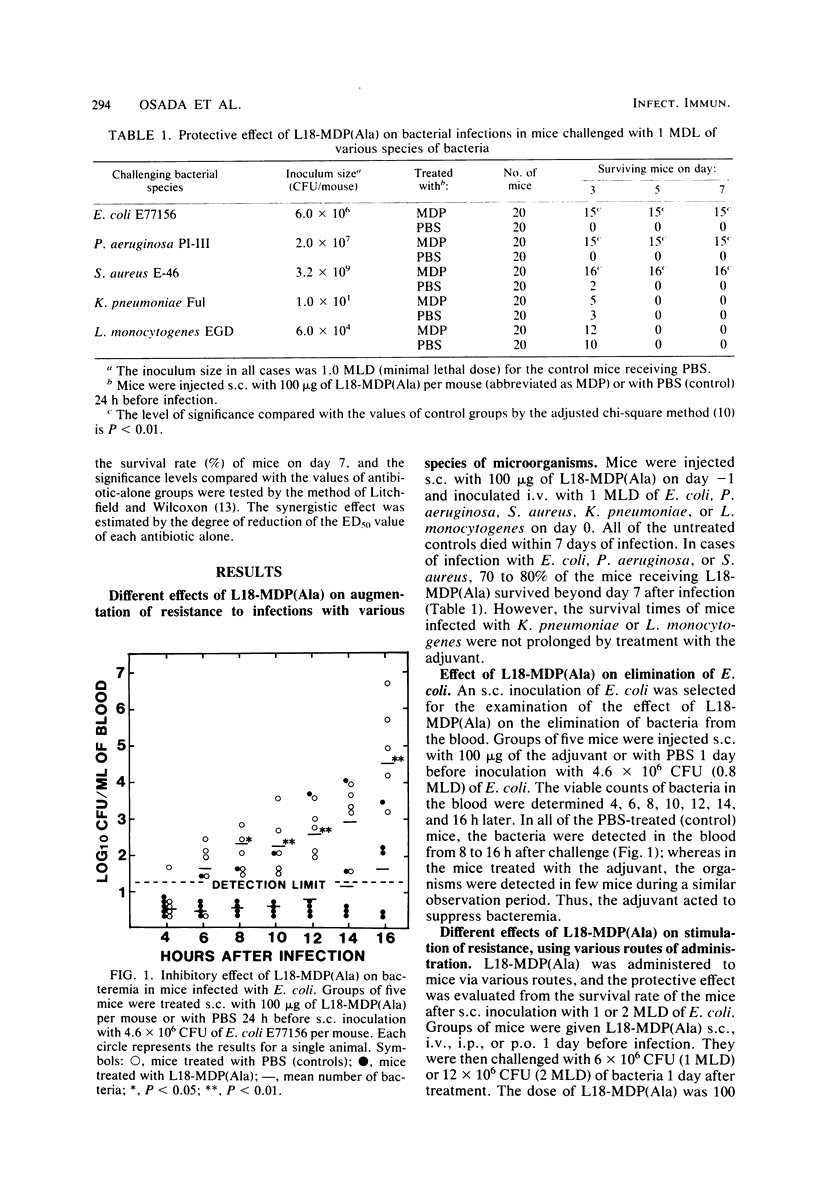
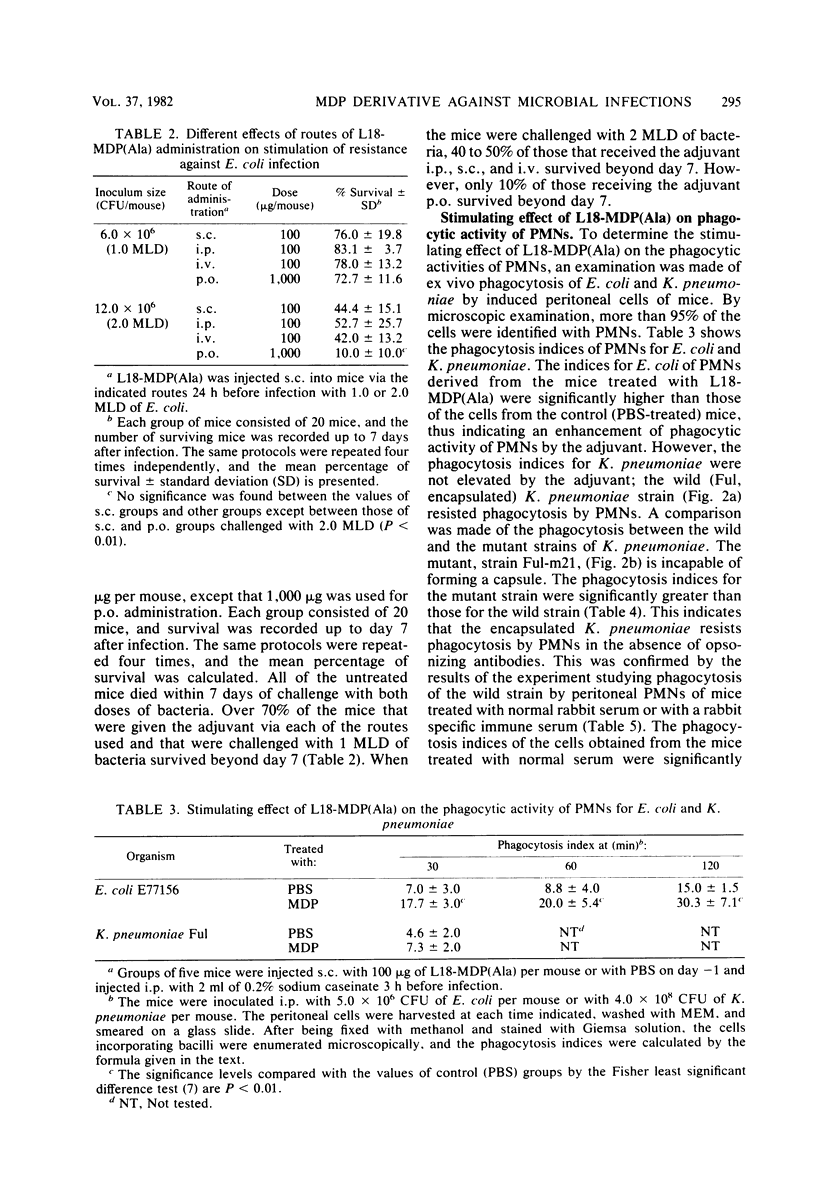
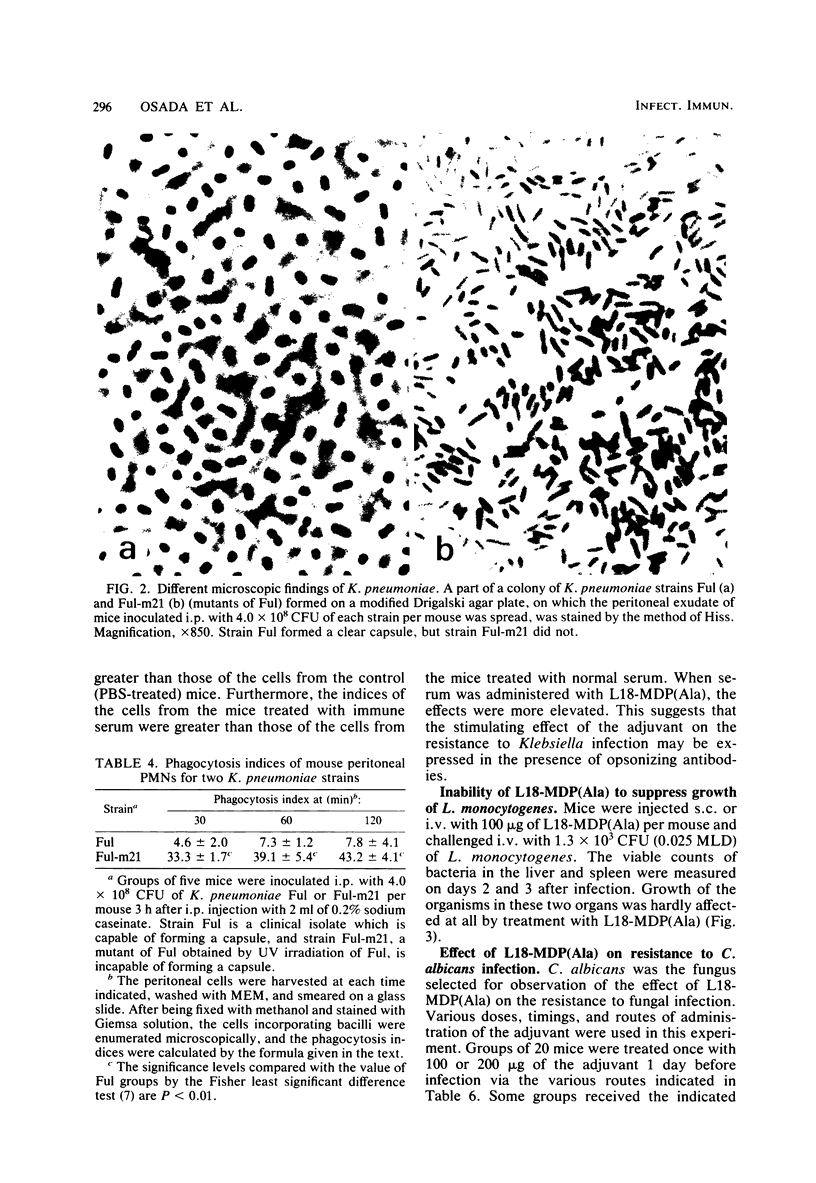
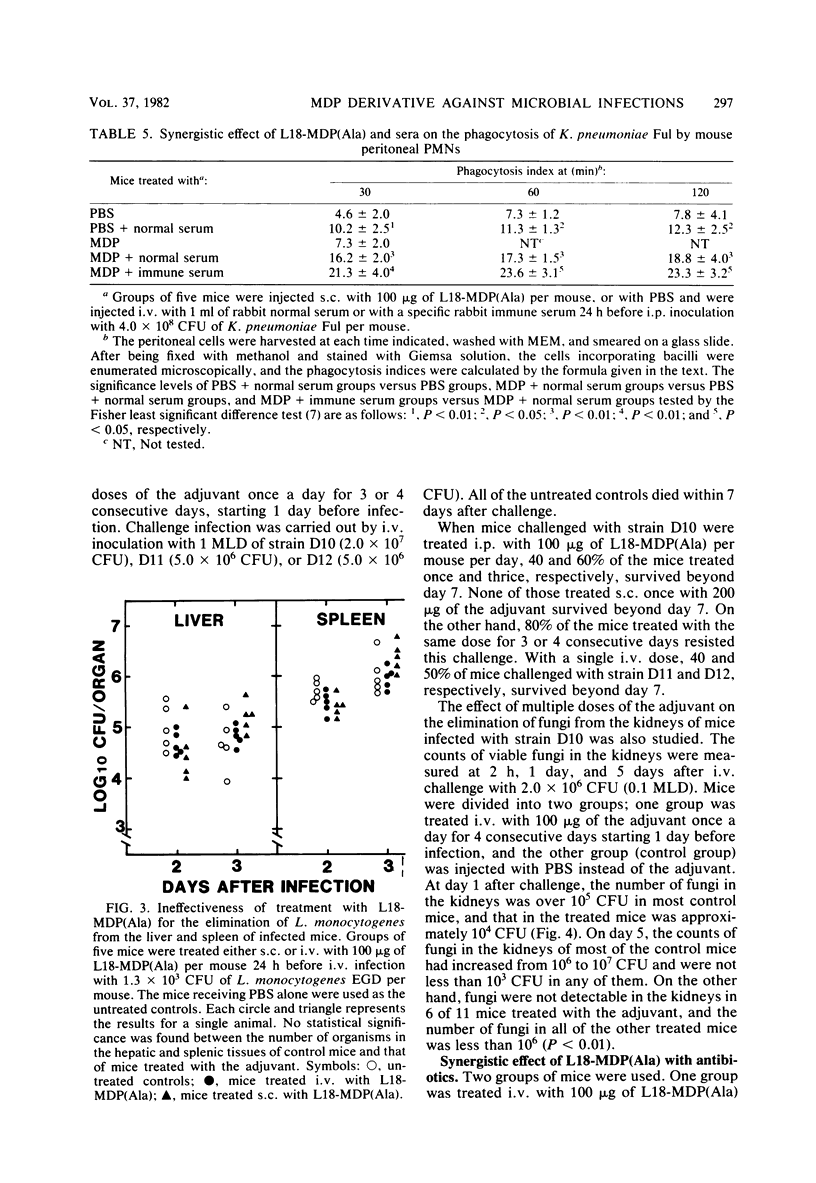
By subcutaneous treatment with an aqueous solution of 6-O-stearoyl-N-acetylmuramyl-L-alanyl-D-isoglutamine [6-O-CH3-(CH2)16-CO-MurNAc-L-Ala-D-isoGln] [referred to here as L18-MDP(Ala)], an augmentation of the resistance of mice to Escherichia coli, Pseudomonas aeruginosa. Staphylococcus aureus, and Candida albicans infections was observed, but not to infections with Klebsiella pneumoniae and Listeria monocytogenes. Against E. coli infections, L18-MDP(Ala) was highly protective, irrespective of the administration route. Bacteremia occurring at an early phase of such infections was almost completely prevented by subcutaneous treatment 1 day before infection. Single or multiple doses were also effective against C. albicans infection. The phagocytosis of E. coli by mouse peritoneal polymorphonuclear cells was enhanced by treatment with the adjuvant, and the phagocytosis of K. pneumoniae was also enhanced, but only when the mice were treated either with rabbit normal serum or with a specific immune serum. The growth of the fungus in the kidneys was significantly inhibited, and growth was eliminated from the kidneys by treatment with the adjuvant once a day for 4 consecutive days, starting 1 day before infection. However, no growth suppression of L. monocytogenes in the livers or spleens of infected mice was observed when they were treated with a single dose of the adjuvant. This difference may be ascribed to the differences in the effector mechanisms of defense and to the different degree of augmentation of each defense mechanism by L18-MDP(Ala).
Full text
PDF

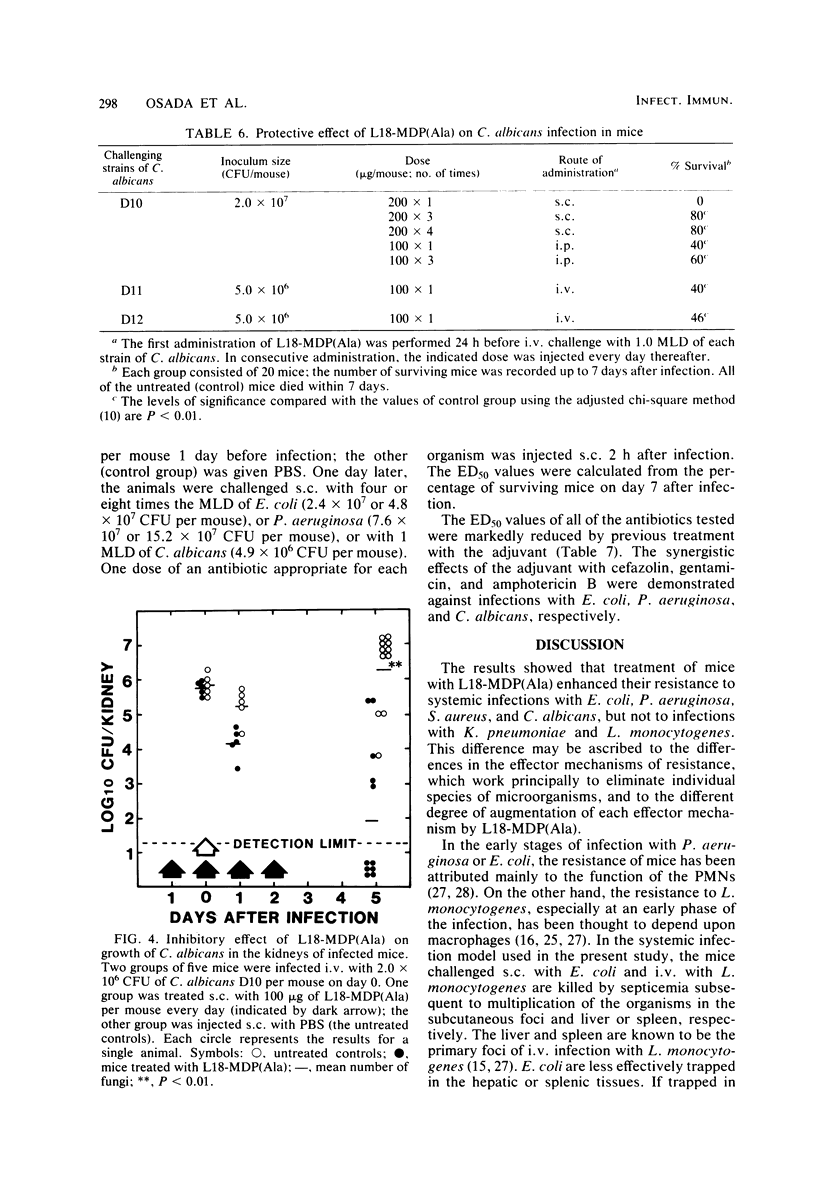


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Sugimura K., Taniyama T., Yamawaki M., Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976 Jul;14(1):18–27. doi: 10.1128/iai.14.1.18-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Audibert F., Lefrancier F., Choay J., Sela M. Enhancement of certain biological activities of muramyl dipeptide derivatives after conjugation to a multi-poly(DL-alanine)--poly(L-lysine) carrier. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6557–6561. doi: 10.1073/pnas.76.12.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A. 1977 May;74(5):2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Finger H., Wirsing von König C. H. Failure of synthetic muramyl dipeptide to increase antibacterial resistance. Infect Immun. 1980 Feb;27(2):288–291. doi: 10.1128/iai.27.2.288-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutome T., Mitsuyama M., Takeya K., Nomoto K. Importance of antiserum and phagocytic cells in the protection of mice against infection by Klebsiella pneumoniae. J Gen Microbiol. 1980 Jul;119(1):225–229. doi: 10.1099/00221287-119-1-225. [DOI] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphres R. C., Henika P. R., Ferraresi R. W., Krahenbuhl J. L. Effects of treatment with muramyl dipeptide and certain of its analogs on resistance to Listeria monocytogenes in mice. Infect Immun. 1980 Nov;30(2):462–466. doi: 10.1128/iai.30.2.462-466.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITCHFIELD J. T., Jr, WILCOXON F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949 Jun;96(2):99–113. [PubMed] [Google Scholar]
- Leclerc C., Juy D., Bourgeois E., Chedid L. In vivo regulation of humoral and cellular immune responses of mice by a synthetic adjuvant, N-acetyl-muramyl-L-alanyl-D-isoglutamine, muramyl dipeptide for MDP. Cell Immunol. 1979 Jun;45(1):199–206. doi: 10.1016/0008-8749(79)90377-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Ogawa H., Kusama T., Nagase O., Sawaki N., Inage M., Kusumoto S., Shiba T., Azuma I. Stimulation of nonspecific resistance to infection induced by 6-O-acyl muramyl dipeptide analogs in mice. Infect Immun. 1981 May;32(2):748–758. doi: 10.1128/iai.32.2.748-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M. [Analysis of protective mechanisms against infection by Listeria monocytogenes. On the contribution of non-immune phagocytes (author's transl)]. Fukuoka Igaku Zasshi. 1980 Jan;71(1):1–18. [PubMed] [Google Scholar]
- Miyake T., Takeya K., Nomoto K., Muraoka S. Cellular elements in the resistance to candida infection in mice. I. Contribution of T lymphocytes and phagocytes at various stages of infection. Microbiol Immunol. 1977;21(12):703–725. doi: 10.1111/j.1348-0421.1977.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Miyata H., Nomoto K., Takeya K. Characteristics of resistance to Listeria monocytogenes enhanced by Corynebacterium parvum in mice. Immunology. 1980 May;40(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Yapo A., Petit J. F., Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1(1):35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- Parant M., Riveau G., Parant F., Dinarello C. A., Wolff S. M., Chedid L. Effect of indomethacin on increased resistance to bacterial infection and on febrile responses induced by muramyl dipeptide. J Infect Dis. 1980 Nov;142(5):708–715. doi: 10.1093/infdis/142.5.708. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Davis A. T. Interaction of Staphylococcus aureus with human polymorphonuclear leukocytes. Contrib Microbiol Immunol. 1973;1:195–201. [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Shimotori S., Taniguchi T., Nomoto K. Cellular mechanisms in the protection against infection by Listeria monocytogenes in mice. J Gen Microbiol. 1977 Jun;100(2):373–379. doi: 10.1099/00221287-100-2-373. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Imai K., Mori R. Macrophage activation by muramyl dipeptide as measured by macrophage spreading and attachment. Microbiol Immunol. 1980;24(6):547–557. doi: 10.1111/j.1348-0421.1980.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Tsuru S., Nomoto K., Mitsuyama M., Zinnaka Y., Takeya K. Importance of polymorphonuclear leucocytes in protection of mice against Escherichia coli. J Gen Microbiol. 1981 Feb;122(2):335–338. doi: 10.1099/00221287-122-2-335. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Yarkoni E., Lederer E., Rapp H. J. Immunotherapy of experimental cancer with a mixture of synthetic muramyl dipeptide and trehalose dimycolate. Infect Immun. 1981 Apr;32(1):273–276. doi: 10.1128/iai.32.1.273-276.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Miake S., Matsumoto T., Nomoto K., Takeya K. Relationship between non-specific activity of macrophages and immune responses to Listeria monocytogenes. Immunology. 1980 Jul;40(3):295–301. [PMC free article] [PubMed] [Google Scholar]



