Abstract
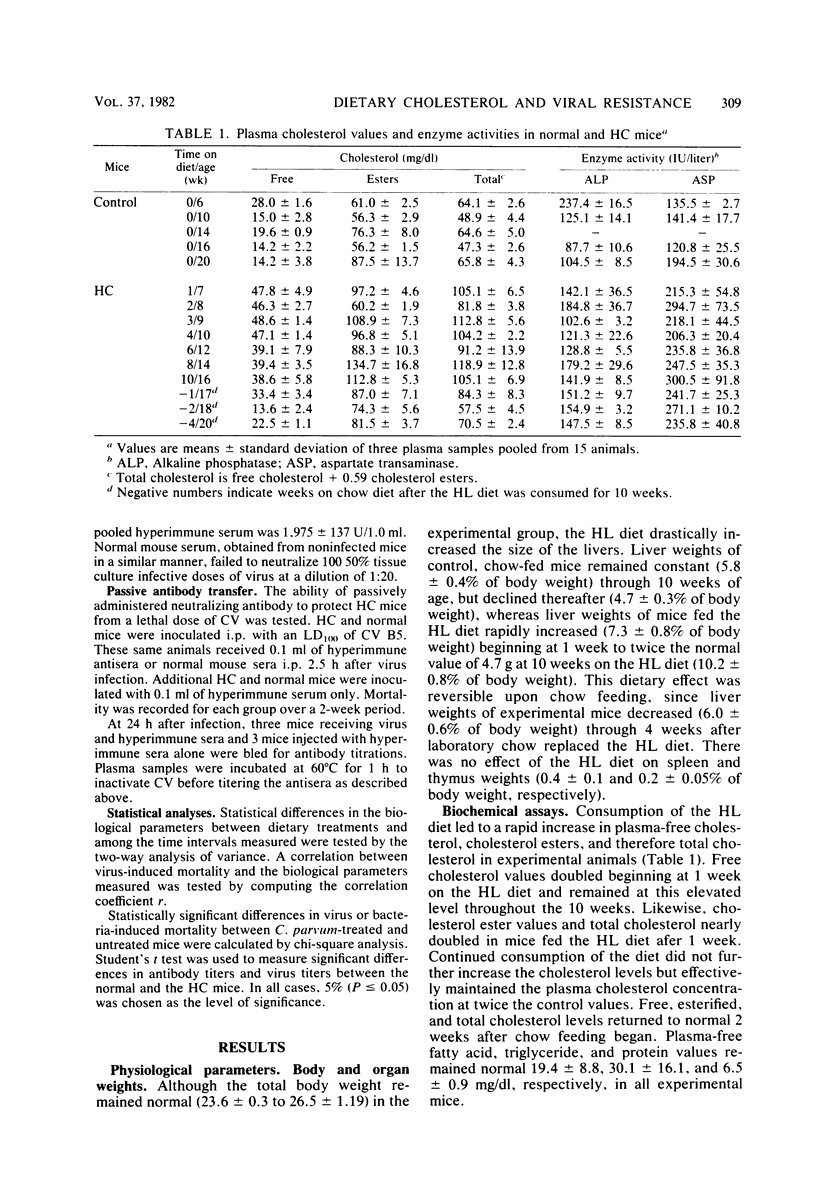
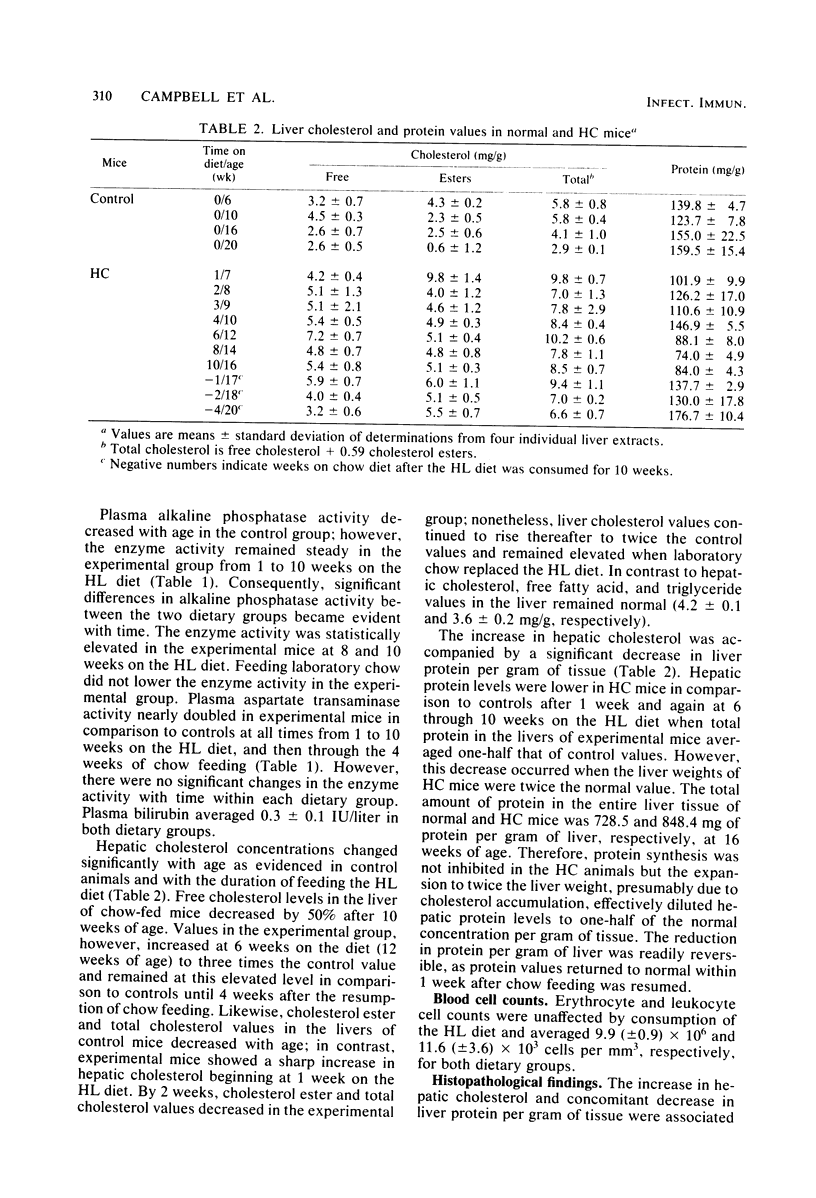
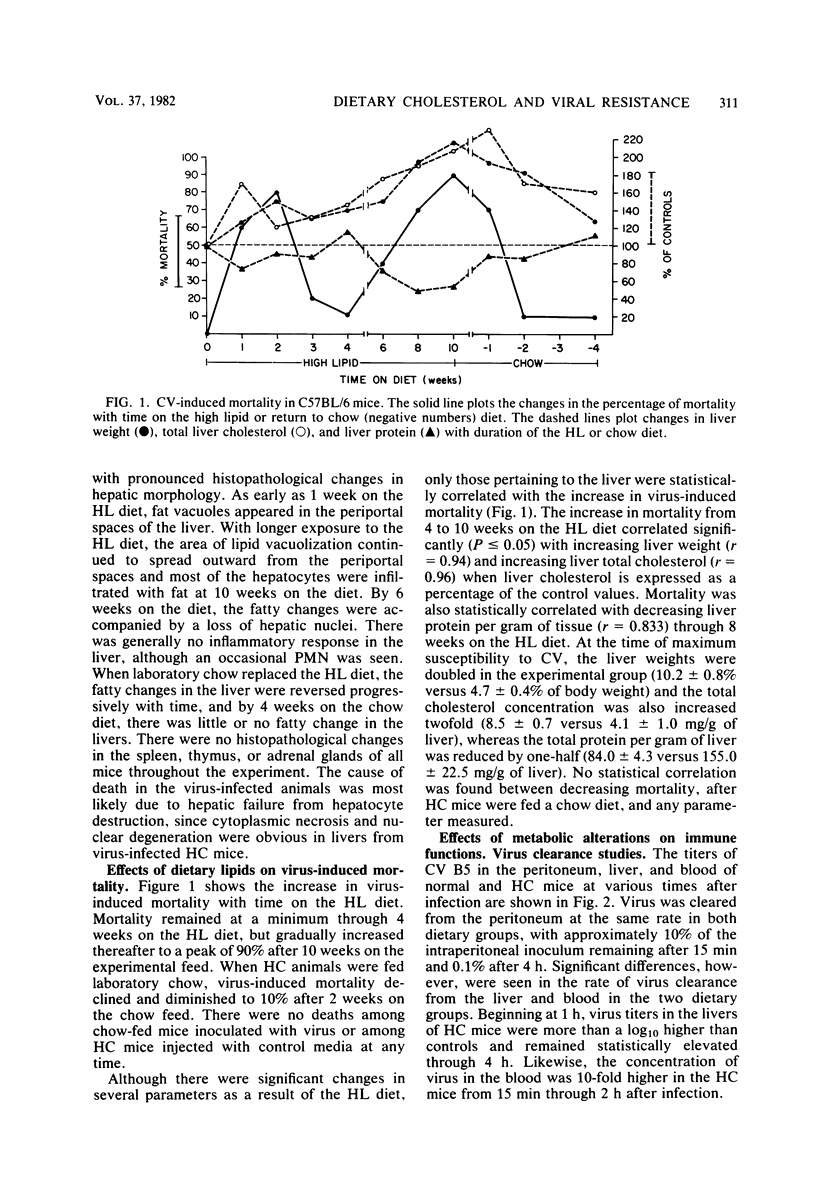
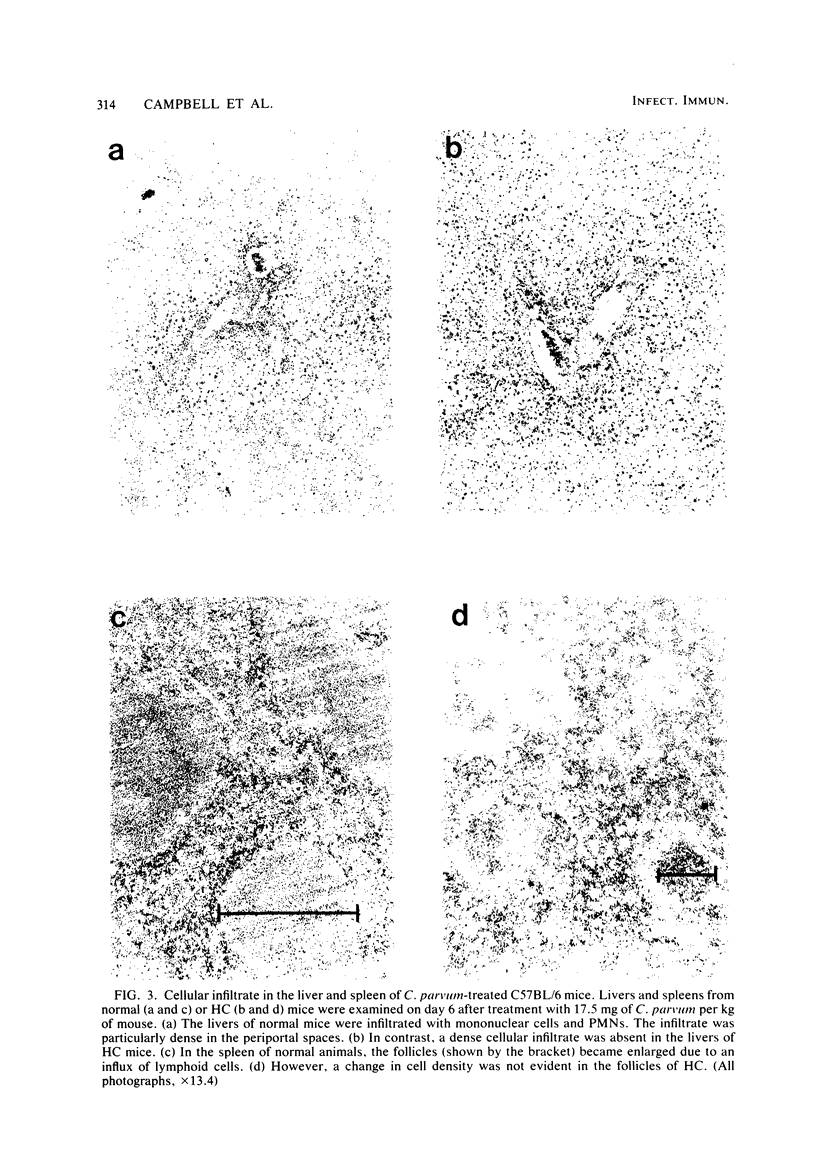
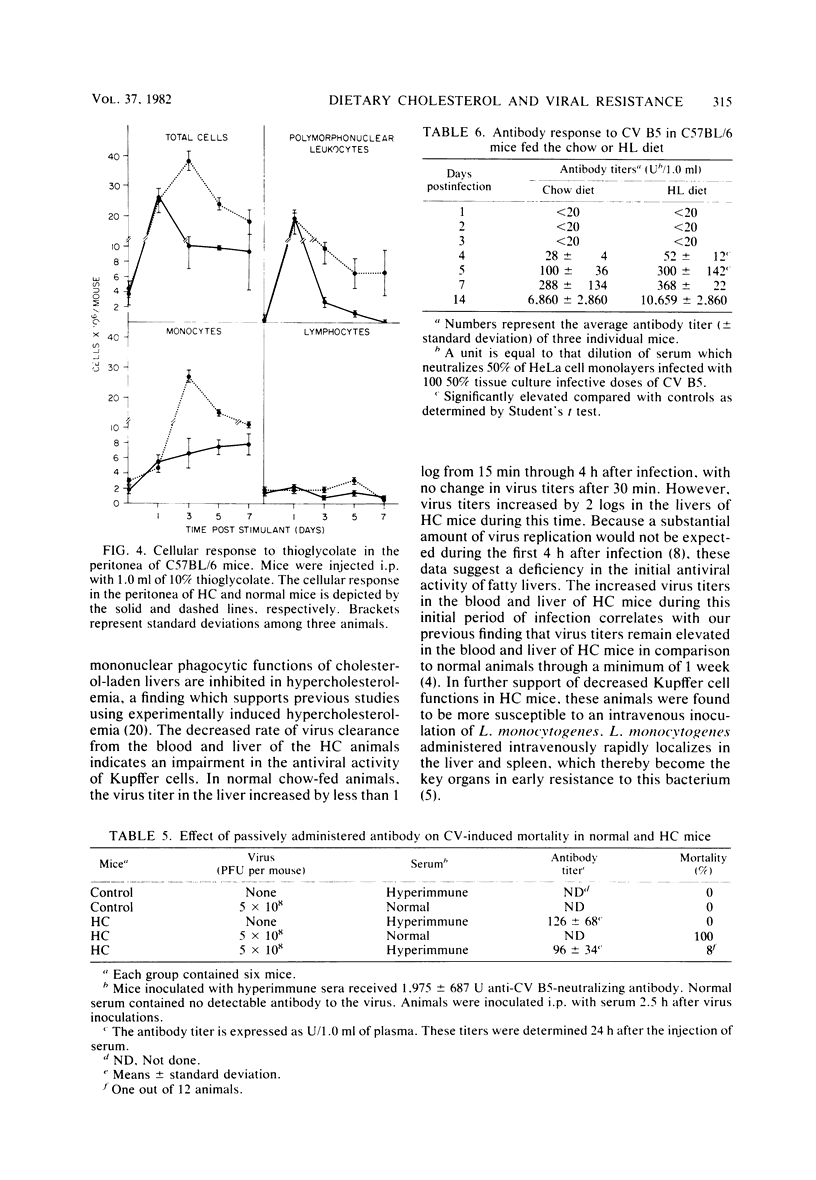
Mice made hypercholesterolemic (HC) by diet are highly susceptible to coxsackievirus (CV) B5, whereas normal adult animals remain resistant. In attempting to define those dietary-induced physiological changes which contribute to altered resistance, a strong association between accumulation of intrahepatic cholesterol and increased CV B5-induced mortality was demonstrated, with maximum susceptibility to CV coinciding with a 2.5-fold increase in the ratio of hepatic cholesterol to protein. This metabolic imbalance was associated with a lower clearance rate of CV from the blood and liver of C57BL/6 mice, although virus-specific neutralizing antibody production was unaltered. In addition to CV, HC mice were more susceptible to an intravenous inoculation of Listeria monocytogenes in comparison to controls. The macrophage stimulant Corynebacterium parvum failed to increase resistance of HC mice to a high dose of CV B4 and L. monocytogenes and failed to induce the hepatomegaly, splenomegaly, and cellular infiltrate seen in the liver and spleen of normal animals. Furthermore, the peritoneal monocytic infiltrate induced by thioglycolate in normal animals was absent in HC mice. Results from these experiments suggest that decreased resistance to CV in the HC host is attributed to a defect in the nonspecific immune responses of macrophages and monocytes which are of primary importance in resistance to this virus and other infectious agents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attia M. A., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res. 1966 Aug;26(8):1787–1800. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Campbell A. E., Loria R. M., Madge G. E. Coxsackievirus B cardiopathy and angiopathy in the hypercholesterolemic host. Atherosclerosis. 1978 Nov;31(3):295–306. doi: 10.1016/0021-9150(78)90065-5. [DOI] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R. P., Saba T. M. Vascular clearance and metabolism of lipid by the reticuloendothelial system in dogs. Am J Physiol. 1971 Nov;221(5):1511–1516. doi: 10.1152/ajplegacy.1971.221.5.1511. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, LEVINTOW L., THOREN M. M., HOOPER J. L. The time course of synthesis of poliovirus RNA. Virology. 1961 Mar;13:271–279. doi: 10.1016/0042-6822(61)90145-3. [DOI] [PubMed] [Google Scholar]
- Diesselhoff-den Dulk M. M., Crofton R. W., van Furth R. Origin and kinetics of Kupffer cells during an acute inflammatory response. Immunology. 1979 May;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- Fisher B., Gebhardt M. C., Saffer E. A., Fisher E. R. Effect of Corynebacterium parvum on liver proliferation and regeneration. Cancer Res. 1979 Apr;39(4):1361–1368. [PubMed] [Google Scholar]
- Kirchner H., Scott M. T., Hirt H. M., Munk K. Protection of mice against viral infection by Corynebacterium parvum and Bordetella pertussis. J Gen Virol. 1978 Oct;41(1):97–104. doi: 10.1099/0022-1317-41-1-97. [DOI] [PubMed] [Google Scholar]
- Kos W. L., Loria R. M., Snodgrass M. J., Cohen D., Thorpe T. G., Kaplan A. M. Inhibition of host resistance by nutritional hypercholesteremia. Infect Immun. 1979 Nov;26(2):658–667. doi: 10.1128/iai.26.2.658-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loria R. M., Kibrick S., Madge G. E. Infection of hypercholesterolemic mice with Coxsackievirus B. J Infect Dis. 1976 Jun;133(6):655–662. doi: 10.1093/infdis/133.6.655. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Smith I. I., Stuart A. E. Effects of simple lipids on macrophages in vitro. J Pathol. 1975 Jan;115(1):13–16. doi: 10.1002/path.1711150103. [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Katayama Y., Koga M., Yamamura Y., Yamaguchi Y., Uchida K. Cholesterol from serum and cholesterol-phospholipid dispersions by isolated rat hepatocytes in vitro. J Lab Clin Med. 1978 Aug;92(2):228–238. [PubMed] [Google Scholar]
- Webb S. R., Loria R. M., Madge G. E., Kibrick S. Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J Exp Med. 1976 May 1;143(5):1239–1248. doi: 10.1084/jem.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F. Lack of correlation between neutralizing antibody production and suppression of coxsackievirus B-3 replication in target organs: evidence for involvement of mononuclear inflammatory cells in host defense. J Immunol. 1979 Jul;123(1):31–36. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]



