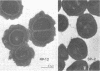
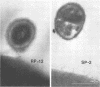
Abstract
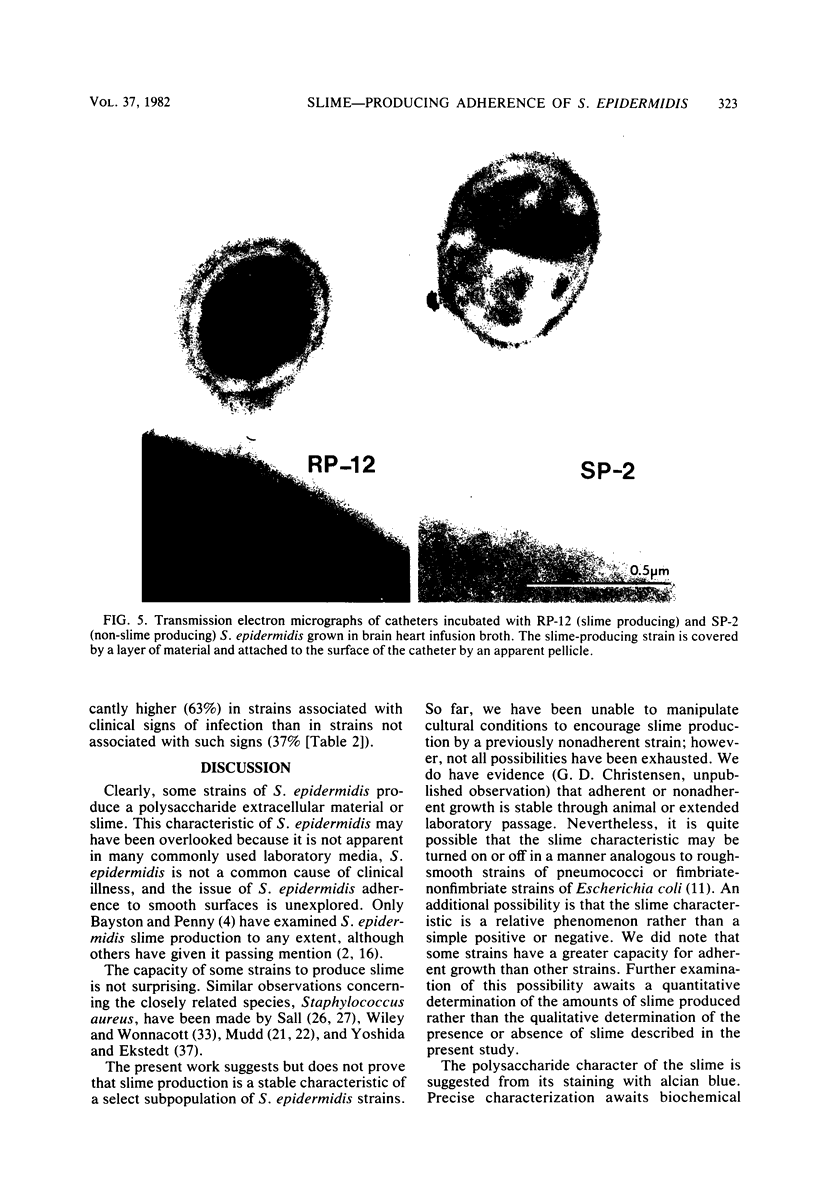
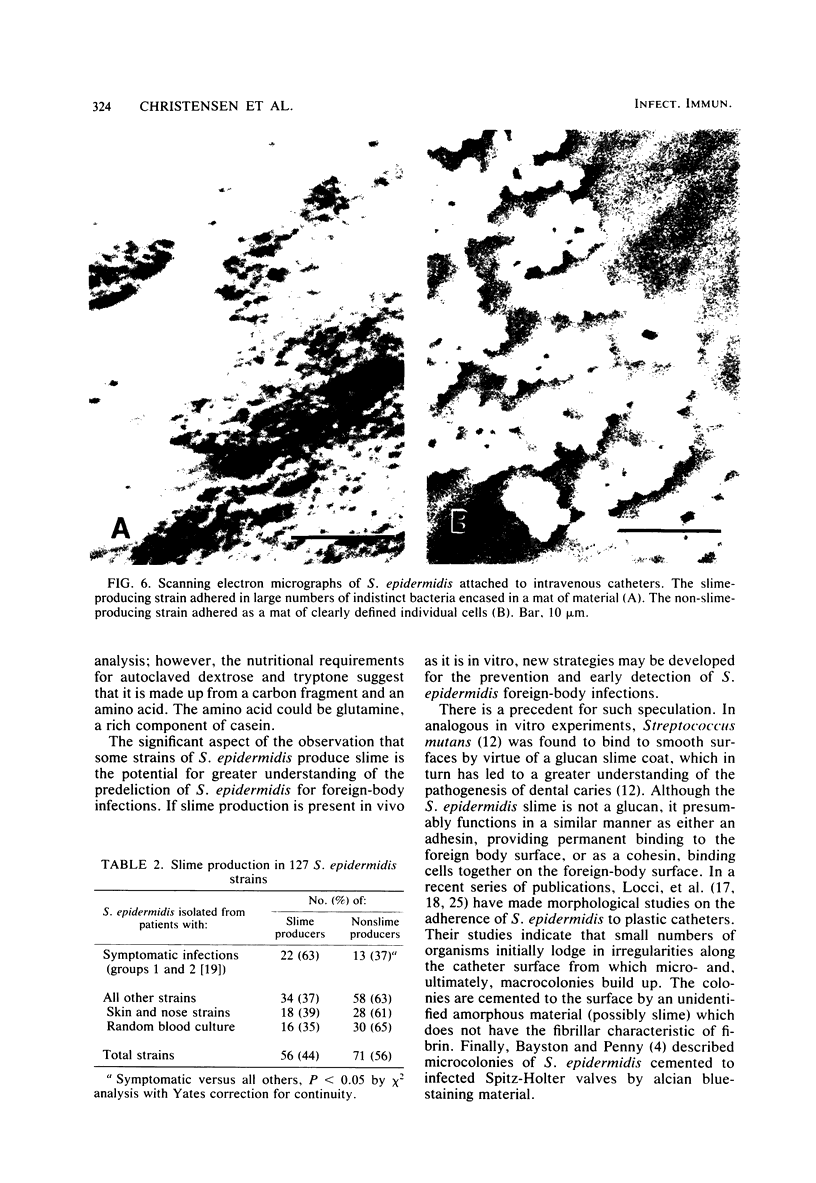
Slime production is not a generally recognized feature of Staphylococcus epidermidis. In a recent outbreak of S. epidermidis intravascular catheter-associated sepsis, we noted that 63% of clinically implicated strains grew as a slimy film coating the culture tube walls when propagated in tryptic soy broth. Only 37% of randomly collected blood culture contaminants and skin isolates demonstrated a similar phenomenon (p less than 0.05). Transmission electron micrographs of these coating bacteria showed them to be encased in an extracellular matrix that stained with alcian blue. Slime production was most evident in autoclaved media containing Casamino Acids and glucose supplementation (0.25% wt/vol). There were strain and media preparation variability of slime production in the presence of other carbohydrates. Some strains were not able to produce slime under any of the tested conditions. The production or nonproduction of slime did not influence growth rate. When grown in vitro, slime producers accumulated on the surface of intravascular catheters as macrocolonies, whereas non-slime, producers did not. Transmission and scanning electron micrographs showed slime producers to be encased in an adhesive layer on the catheter surface, whereas nonproducers were not encased. These results suggest that slime-mediated adherence may be a critical factor in the pathogenesis of S. epidermidis infections of medical devices.
Full text
PDF

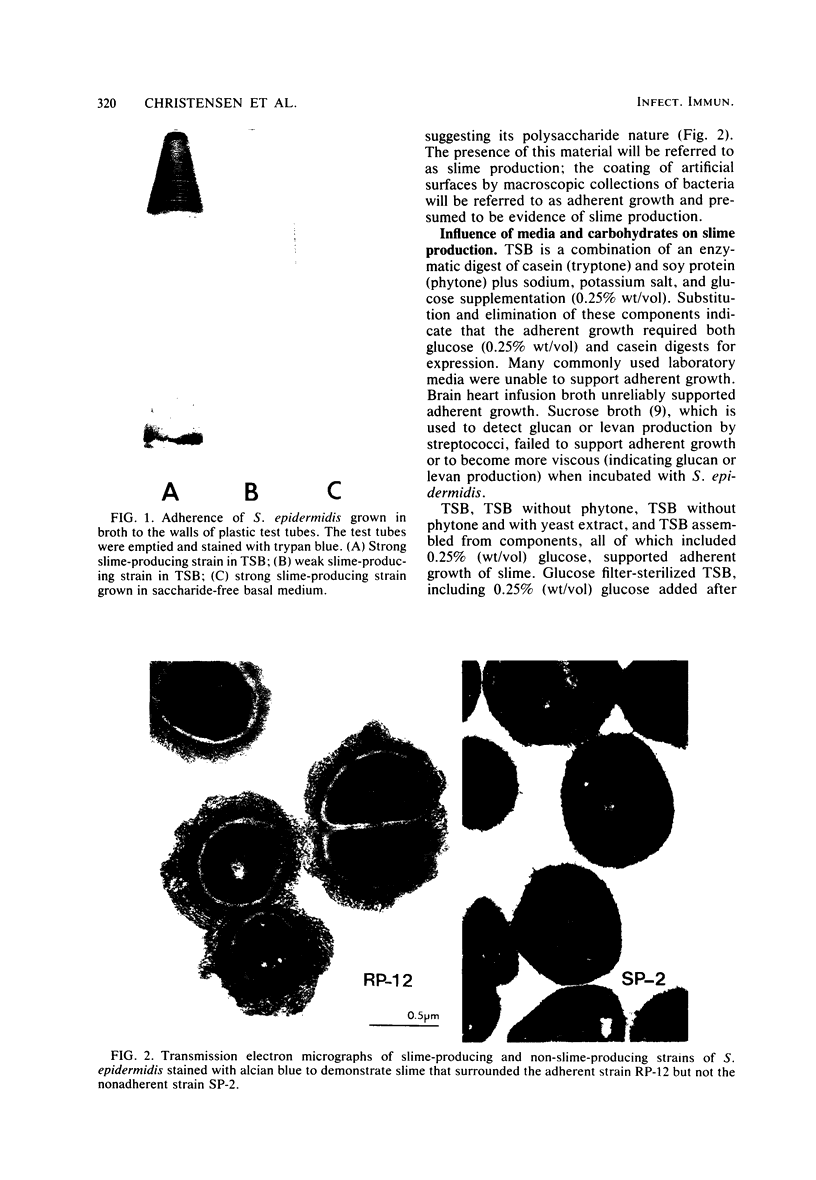
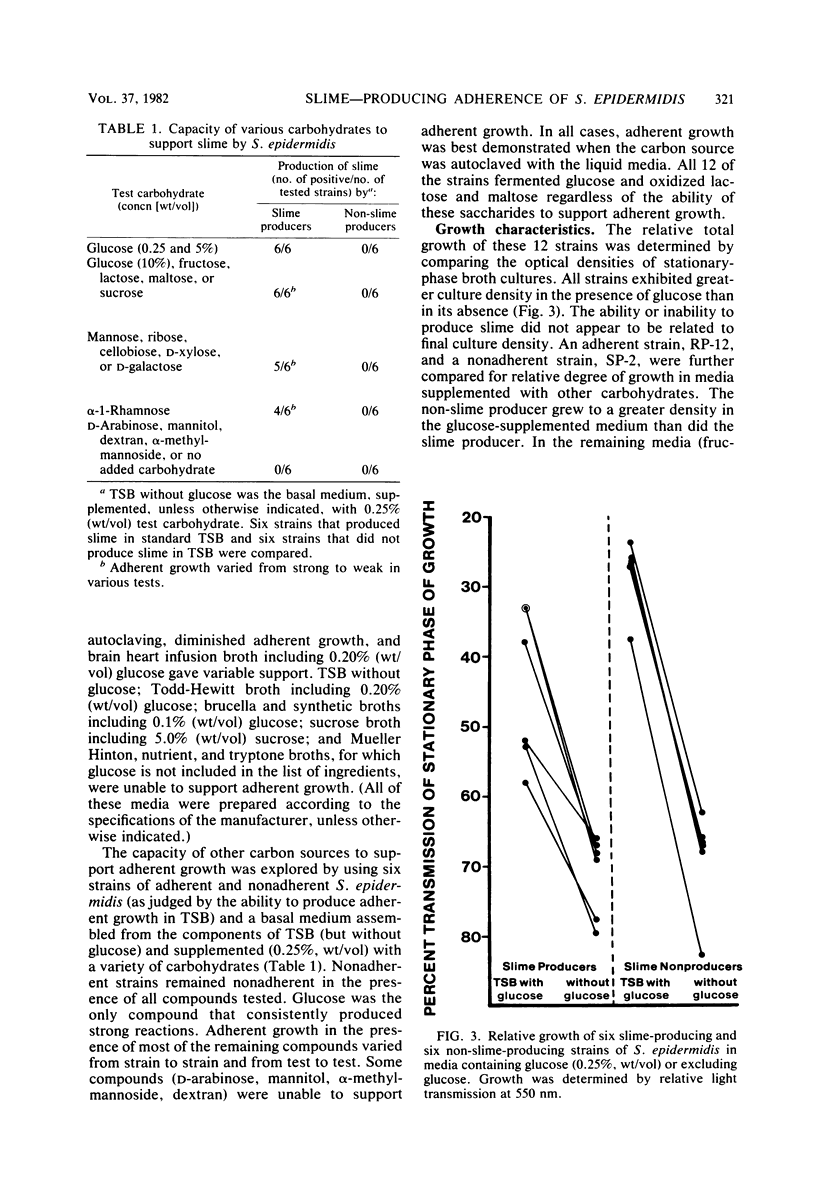



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L. Antimicrobial susceptibility and selection of resistance among Staphylococcus epidermidis isolates recovered from patients with infections of indwelling foreign devices. Antimicrob Agents Chemother. 1978 Sep;14(3):353–359. doi: 10.1128/aac.14.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Bender J. W., Hughes W. T. Fatal Staphylococcus epidermidis sepsis following bone marrow transplantation. Johns Hopkins Med J. 1980 Jan;146(1):13–15. [PubMed] [Google Scholar]
- Bentley D. W., Haque R. U., Murphy R. A., Lepper M. H. Biotyping, and epidemiological tool for coagulase-negative staphylococci. Antimicrob Agents Chemother (Bethesda) 1967;7:54–59. [PubMed] [Google Scholar]
- Blouse L. E., Lathrop G. D., Kolonel L. N., Brockett R. M. Epidemiologic features and phage types associated with nosocomial infections caused by Staphylococcus epidermidis. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):119–135. [PubMed] [Google Scholar]
- CALLAGHAN R. P., COHEN S. J., STEWART G. T. Septicaemia due to colonization of Spitz-Holter valves by staphylococci. Five cases treated with methicillin. Br Med J. 1961 Mar 25;1(5229):860–863. doi: 10.1136/bmj.1.5229.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E., Karchmer A. W., Buckley M. J., Austen W. G., Swartz M. N. Prosthetic valve endocarditis. Analysis of 38 cases. Circulation. 1973 Aug;48(2):365–377. doi: 10.1161/01.cir.48.2.365. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Stiver H. G. Combination antibiotic therapy in an outbreak of prosthetic endocarditis caused by Staphylococcus epidermidis. Can Med Assoc J. 1978 Mar 4;118(5):524–530. [PMC free article] [PubMed] [Google Scholar]
- Hellbusch L. C., Moiel R. H., Cheek W. R. Growing skull fractures. South Med J. 1977 May;70(5):555–558. doi: 10.1097/00007611-197705000-00015. [DOI] [PubMed] [Google Scholar]
- Holt R. The classification of staphylococci from colonized ventriculo-atrial shunts. J Clin Pathol. 1969 Jul;22(4):475–482. doi: 10.1136/jcp.22.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES D., DEIBEL R. H., NIVEN C. F., Jr Identity of Staphylococcus epidermidis. J Bacteriol. 1963 Jan;85:62–67. doi: 10.1128/jb.85.1.62-67.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. I. Microtopographical characteristics of intravenous catheters as detected by scanning electron microscopy. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):285–292. [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. III. Adhesion of staphylococci to lumina of intravenous catheters perfused with bacterial suspensions. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):300–307. [PubMed] [Google Scholar]
- MUDD S., DECOURCY S. J., Jr INTERACTION OF VISCID MATERIAL OF STAPHYLOCOCCUS AUREUS WITH SPECIFIC IMMUNE SERUM. J Bacteriol. 1965 Mar;89:874–879. doi: 10.1128/jb.89.3.874-879.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples R. R., Hone R., Notley C. M., Richardson J. F., Crees-Morris J. A. Ivestigation of coagulase-negative staphylococci from infections in surgical patients. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):140–156. [PubMed] [Google Scholar]
- McCabe R. M., Donkersloot J. A. Adherence of Veillonella species mediated by extracellular glucosyltransferase from Streptococcus salivarius. Infect Immun. 1977 Dec;18(3):726–734. doi: 10.1128/iai.18.3.726-734.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. Capsulation, pseudocapsulation, and the somatic antigens of the surface of Staphylococcus aureus. Ann N Y Acad Sci. 1965 Jul 23;128(1):45–58. doi: 10.1111/j.1749-6632.1965.tb11628.x. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Patterson F. P., Brown C. S. The McKee-Farrar total hip replacement. Preliminary results and complications of 368 operations performed in five general hospitals. J Bone Joint Surg Am. 1972 Mar;54(2):257–275. [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- SALL T. Interrelationship of extracellular enzymes and pseudocapsulation in a strain of Staphylococcus aureus. J Bacteriol. 1962 Jun;83:1238–1243. doi: 10.1128/jb.83.6.1238-1243.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALL T., MUDD S., TAUBLER J. Concerning the surfaces of cells of Staphylococcus pyogenes. I. A pseudocapsulation phenomenon under certain experimental conditions. J Exp Med. 1961 Apr 1;113:693–700. doi: 10.1084/jem.113.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., BLACK P. H., MARK V. H., SWARTZ M. N. Indolent Staphylococcus albus or aureus bacteremia after ventriculoatriostomy. Role of foreign body in its initiation and perpetuation. N Engl J Med. 1961 Feb 9;264:264–270. doi: 10.1056/NEJM196102092640602. [DOI] [PubMed] [Google Scholar]
- Schoenbaum S. C., Gardner P., Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975 May;131(5):543–552. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- Shea S. M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971 Dec;51(3):611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILEY B. B., WONNACOTT J. C. Isolation and partial characterization of a capsular material from Staphylococcus aureus. J Bacteriol. 1962 Jun;83:1169–1176. doi: 10.1128/jb.83.6.1169-1176.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. D., Jr, Amstutz H. C., Czerniecki A., Salvati E. A., Mendes D. G. Total hip replacement with fixation by acrylic cement. A preliminary study of 100 consecutive McKee-Farrar prosthetic replacements. J Bone Joint Surg Am. 1972 Mar;54(2):207–236. [PubMed] [Google Scholar]
- Wilson P. D., Jr, Salvati E. A., Aglietti P., Kutner L. J. The problem of infection in endoprosthetic surgery of the hip joint. Clin Orthop Relat Res. 1973 Oct;(96):213–221. [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]