Abstract
Aims
To determine whether extremely low birth weight (ELBW) infants with bilateral compared to unilateral intraventricular hemorrhage (IVH) have worse neurodevelopmental outcomes at 18–22 months.
Methods
166 ELBW infants (<1000 g) admitted to a Cincinnati NICU from 1998–2005 with a head ultrasound showing Grade I–IV IVH and neurodevelopmental assessment at 18–22 months corrected age were included. Multivariable linear and logistic regression models were developed to determine the impact of laterality and grade of IVH and other clinical variables to predict scores on the Bayley Scales of Infant Development, Second Edition, Mental Development Index (MDI) and Psychomotor Development Index (PDI) and the combined outcome of neurodevelopmental impairment (NDI).
Results
Infants with bilateral grade IV IVH had lower adjusted mean Bayley scores compared with infants with unilateral grade IV IVH. For grades I, II, and III IVH, bilaterality of IVH was not associated with lower mean Bayley scores. Infants with grade IV IVH had the highest odds of NDI. The probability of NDI increased with sepsis and postnatal steroid use.
Conclusions
ELBW infants with bilateral compared to those with unilateral grade IV IVH had worse neurodevelopmental outcomes. Infants with grades I–III IVH had similar outcomes whether they had unilateral or bilateral IVH.
Keywords: premature, sepsis, steroids, Bayley, cognitive, motor
BACKGROUND
Germinal matrix hemorrhage (GMH), intraventricular hemorrhage (IVH), and periventricular hemorrhagic infarction (PVHI) remain important causes of morbidity and mortality in premature infants. These brain lesions develop after bleeding in the subependymal germinal matrix, a highly cellular and well-vascularized region that contains precursors to neurons and glia (1). A grading system was developed by Papile et al in 1978 (2) and is still used by radiologists to describe the lesions seen on head ultrasound. Grade I refers to germinal matrix hemorrhage, Grade II to intraventricular hemorrhage without ventricular dilation, Grade III to intraventricular hemorrhage with acute ventricular dilation, and Grade IV to a venous infarction resulting in an intraparenchymal hemorrhage, or periventricular hemorrhagic infarction.
Despite the incidence declining in recent years (1), approximately 20% of very low birth weight infants develop the complication of GMH/IVH/PVHI (hereafter referred to as IVH) during their NICU stay (3–5). In multiple studies, IVH has been shown to be related to adverse neurodevelopmental outcomes at 18–24 months and at school age (6–10). More recently, even low grade IVH has been shown to be related to cerebral palsy and developmental delay (9). However, despite the knowledge that IVH is often related to poor neurodevelopmental outcomes, it is not a perfect predictor. Although 50–80% of survivors with Grade IV IVH have a moderate to severe motor deficit at 18–24 months or later (10–13), a large percentage of babies still develop normally. The factors that cause poor versus normal neurodevelopmental outcomes among infants with these brain lesions are largely unknown. One recent study showed that infants with unilateral Grade IV IVH had more favorable neurodevelopmental outcomes than those with bilateral Grade IV (14), but infants with less severe IVH were not included in the analysis. The objective of our study was to determine whether the laterality of IVH (unilateral versus bilateral) was a predictor of neurodevelopmental outcome at 18–22 months in a cohort of ELBW infants with Grades I–IV IVH.
METHODS
We analyzed data from a cohort of all ELBW infants (401–1000 g) who were born between 1/1/1998 and 1/1/2006 and admitted to the NICU at Cincinnati Children’s Hospital Medical Center, University Hospital, or Good Samaritan Hospital in Cincinnati, Ohio. Data was initially collected on these infants as part of the NICHD Neonatal Research Network Generic Database, and the infants were prospectively followed to 18–22 months corrected age as part of the Neonatal Research Network Follow Up study. For the current retrospective analysis, institutional review board (IRB) approval was obtained from each hospital. Infants were included if they were coded as having at least one abnormal head ultrasound scan in the Generic Database. Abnormal head ultrasound was defined as germinal matrix hemorrhage, intraventricular hemorrhage, ventricular dilation, cystic area in the parenchyma, cystic periventricular leukomalacia, and/or porencephalic cyst. The protocol for head ultrasound screening at the time of the study included a first head ultrasound at 7–10 days of life and a ssecond head ultrasound after 28 days of life. The most severe head ultrasound finding was used to categorize the infants. Infants with lethal congenital malformations, chromosomal abnormalities, history of meningitis, and periventricular leukomalacia were excluded.
After the infants meeting the above criteria were identified by their NICHD NRN number, the data was re-identified to find the infants’ names, dates of birth, and medical record numbers. Using this information, all of the head ultrasound reports for each infant were obtained. The reports were reviewed and data on laterality and highest grade of IVH was entered into a new computerized database. This data was then matched with the infant’s data from the Generic Database on potential confounders and from the Follow-up Database on the infant’s neurodevelopmental outcome at 18–22 months. Information extracted from the Follow-up Database included Bayley Scales of Infant Development Second Edition Mental Development Index (BSID II MDI), Psychomotor Development Index (PDI), presence of cerebral palsy, blindness, and hearing impairment.
The primary outcome was neurodevelopmental impairment at 18–22 months corrected age. Neurodevelopmental impairment was defined as the presence of any of the following: cerebral palsy, MDI < 70, PDI < 70, blindness, or hearing impairment. Secondary outcomes were the BSID II MDI score and BSID II PDI score. The key predictor variables were IVH grade and laterality of IVH (unilateral versus bilateral). Potential confounder variables were gender, race, birth weight, presence of bronchopulmonary dysplasia (BPD), postnatal steroids, early or late culture-positive sepsis, and necrotizing enterocolitis (NEC) requiring surgery. These variables have been reported to be associated with poor neurodevelopmental outcome in prior studies.
Statistical Analysis
A logistic regression model was developed using the key independent variables (IVH grade and laterality) and the potential confounder variables as covariates to predict the binary outcome of combined neurodevelopmental impairment. Firth’s penalized maximum likelihood estimation was used to reduce bias in the parameter estimates and to address issues pertaining to cells having zero frequency. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate overall model fit. Regardless of statistical significance, laterality (unilateral vs. bilateral IVH) remained in the models, as it was directly related to the study hypothesis. Interaction between laterality and IVH grade was tested in the model to determine if the relationship between IVH grade and neurodevelopmental outcome was modified by whether an infant had unilateral or bilateral IVH. The interaction term was not statistically significant in any of the models, and therefore this term did not remain in the final models. General linear regression models were developed using the key independent variables and the potential confounder variables to estimate the independent relationship between IVH grade with MDI and PDI scores. These models provided mean MDI and PDI scores (using least square means) with 95% confidence intervals (CI), adjusted for the confounders included in the model. A backward elimination strategy was used with p > 0.1 for exit criteria. Regression diagnostics were used to examine outliers and normality of residuals. Statistical analysis was performed using SAS (Version 9.2, SAS Institute Inc, Cary, NC).
RESULTS
Demographic and clinical characteristics
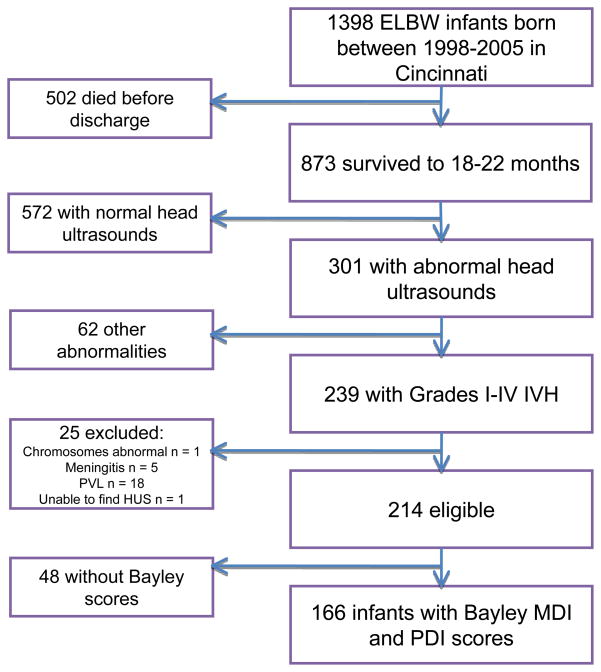
Of 1398 ELBW infants admitted to Cincinnati area NICUs with birthdates between 1/1/98 and 1/1/2006, 205 died within 12 hours and 297 died before discharge. Of the remaining 896 infants, 873 had follow up data recorded in the NICHD Neonatal Research Network Follow Up database. Infants with normal head ultrasounds were excluded, leaving 301 infants with abnormal head ultrasounds and follow up data. The head ultrasound reports for these 301 infants were obtained from hospital medical records. For the current study, only infants with Grades I–IV intraventricular hemorrhage without periventricular leukomalacia (PVL) seen on head ultrasound and Bayley MDI and PDI data were analyzed, leaving 166 infants for analysis (Figure 1). The infants without Bayley data had a similar distribution of laterality and IVH grade as the included infants. Demographic and clinical variables for the infants included in the analysis are shown in Table 1.
Figure 1.
Table 1.
Demographic and clinical variables
| Characteristic (N=166) | Mean (SD) |
|---|---|
| Gestational age, weeks | 26 (2) |
| Birth weight, grams | 793.2 (131) |
| MDI | 83.8 (18.7) |
| PDI | 88.6 (18.6) |
|
| |
| n (%) | |
|
| |
| Male | 67 (40.4) |
| White | 112 (67.5) |
| BPD | 101 (60.8) |
| Postnatal steroids | 63 (38.2) |
| Culture positive sepsis | 67 (40.4) |
| Surgical NEC | 8 (4.8) |
| IVH grade I | 112 (67.5) |
| IVH grade II | 15 (9) |
| IVH grade III | 19 (11.5) |
| IVH grade IV | 20 (12) |
| Unilateral bleed | 81 (48.8) |
Prediction of neurodevelopmental impairment
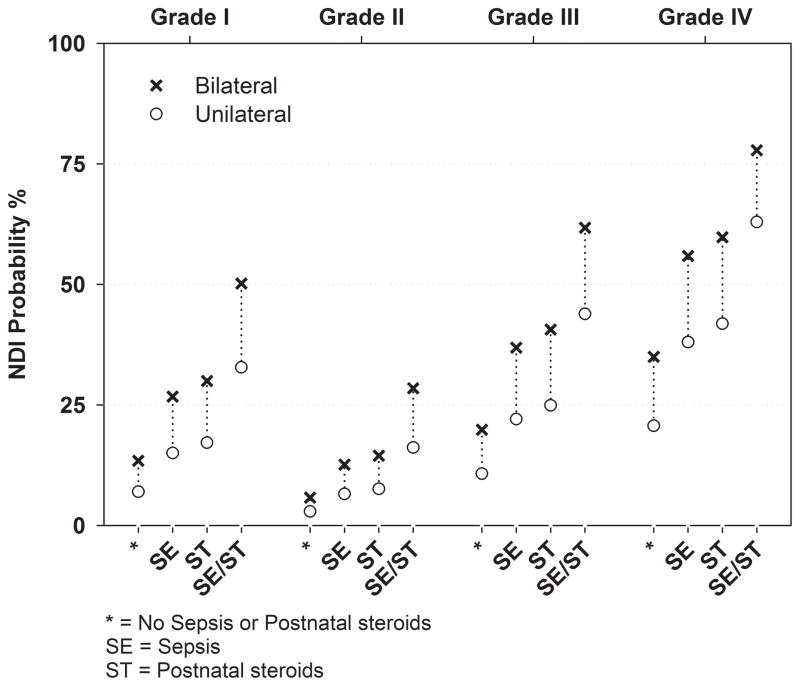
We examined predictors of neurodevelopmental impairment using multiple logistic regression. Table 2 shows the unadjusted and adjusted odds ratios with 95% confidence intervals for predictors of neurodevelopmental impairment. After controlling for other factors in the model, infants with Grade IV IVH had the highest odds of neurodevelopmental impairment. Another way to interpret the resulting model is in the calculation of predicted probabilities of NDI for the significant predictors. An example of the clinical relevance of these results is shown in Figure 2, which illustrates probabilities of neurodevelopmental impairment using the model covariates. For each grade, the probability of neurodevelopmental impairment increases from unilateral to bilateral IVH, as well as with sepsis and postnatal steroid use. For example, infants with bilateral Grade IV IVH exhibit a 35% predicted probability of NDI, which increases to 78% in sepsis cases with a course of postnatal steroids. Although the probability of impairment appears lower for Grade II compared to Grade I IVH, there is no significant difference in the odds of impairment between these two grades (odds ratio 0.40, 95% CI 0.6, 2.6). The Hosmer-Lemeshow test (p=0.79) and examination of the residuals indicated that the model fit the data well.
Table 2.
Predictors of Neurodevelopmental Impairment
| Predictor | Unadjusted Odds ratio [95% CI] | Adjusted Odds ratio [95% CI] |
|---|---|---|
| IVH grade IV | 3.1 [1.2, 8.5] | 3.5 [1.2, 10.4] |
| IVH grade III | 1.8 [.61, 5.2] | 1.6 [.52, 4.9] |
| IVH grade II | .27 [.03, 2.2] | .40 [.06, 2.6] |
| IVH grade I (reference) | -- | -- |
| Postnatal steroids | 3.6 [1.7, 7.5] | 2.8 [1.2, 6.3] |
| Sepsis | 3.2 [1.5, 6.7] | 2.4 [1.0, 5.3] |
| Bilateral vs Unilateral | 2.3 [1.1, 5.0] | 2.1 [.93, 4.6] |
Variables included in the table were those that met the inclusion/exclusion criteria for the multivariable logistic model
Figure 2.
Prediction of MDI and PDI
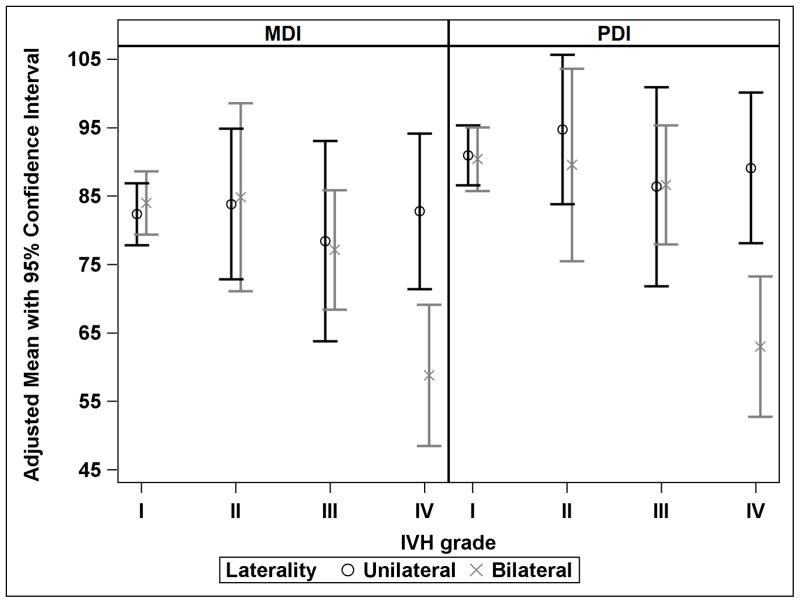
In the linear regression models, there was a significant interaction between IVH grade and laterality of IVH in the prediction of both MDI and PDI (p=.02). This interaction is evident in Grade IV IVH. While the 95% CI for the adjusted mean MDI and PDI for unilateral compared to bilateral IVH overlap in Grades I, II, and III, both adjusted mean scores differ by 25 points from unilateral to bilateral IVH among infants with Grade IV IVH (Figure 3). Table 3 shows the adjusted MDI and PDI scores by laterality and grade of IVH. We adjusted MDI for laterality, maximum IVH grade, the interaction between laterality and IVH, sex, race, birth weight, and postnatal steroids. We adjusted PDI for laterality, maximum IVH grade, the interaction between laterality and IVH, BPD, postnatal steroids, and sepsis.
Figure 3.
Table 3.
Adjusted Mean MDI and PDI with 95% Confidence Intervals by Grade and Laterality
| IVH Grade | Laterality | n | Mean MDI [95% CI] | Mean PDI [95% CI] |
|---|---|---|---|---|
| I | Unilateral | 58 | 82.4 [77.8, 86.9] | 90.9 [86.6, 95.3] |
| Bilateral | 53 | 84 [79.4, 88.6] | 90.4 [85.7, 95.1] | |
| II | Unilateral | 9 | 83.8 [72.8, 94.9] | 94.7 [83.8, 105.6] |
| Bilateral | 6 | 84.8 [71.1, 98.6] | 89.6 [75.5, 103.6] | |
| III | Unilateral | 5 | 78.4 [63.8, 93] | 86.4 [71.8, 100.9] |
| Bilateral | 14 | 77.1 [68.4, 85.9] | 86.6 [77.9, 95.3] | |
| IV | Unilateral | 9 | 82.8 [71.4, 94.2] | 89.1 [78.1, 100.1] |
| Bilateral | 11 | 58.8 [48.5, 69.1] | 63.0 [52.8, 73.3] |
MDI adjusted by IVH grade, laterality, IVH grade by laterality, birth weight, race, sex, steroids
PDI adjusted by IVH grade, laterality, IVH grade by laterality, BPD, steroids
DISCUSSION
Our study indicates that having bilateral (as compared with unilateral) IVH significantly impacts neurodevelopmental outcome only in infants with grade IV hemorrhage. Infants with bilateral grades I–III IVH have similar rates of neurodevelopmental impairment and similar mean Bayley MDI and PDI scores as infants with unilateral grades I–III IVH. In addition, the infants in our study with Grade III hemorrhage had similar neurodevelopmental outcomes as the infants with Grades I–II. This suggests that the convention of grouping together Grades III–IV IVH when predicting outcomes may not always be accurate.
Only one large study has looked at laterality and outcomes in IVH (14). This study evaluated only infants with Grade IV IVH and found that infants with bilateral Grade IV IVH had significantly worse cognitive and motor outcomes than those with unilateral Grade IV hemorrhage. They also determined that infants with unilateral Grade IV IVH had cognitive outcomes that were similar to those of the general population. These findings are consistent with our study and suggest that the brain can adapt when there has been significant destruction of brain tissue on one side, but severe impairment usually results when there is parenchymal injury on both sides of the brain.
Until recently, infants with Grade I–II IVH were thought to have a similar risk of adverse neurodevelopmental outcomes as infants without IVH. In 2006, Patra and colleagues found in a single center study that mean MDI scores were lower and rates of cerebral palsy and neurodevelopmental impairment were higher in infants with Grade I–II bleeds as compared to infants with no IVH (9). However, the difference in MDI score was probably not clinically significant (74 vs 79) and there was no difference in PDI score. A more recent study looking at 1472 infants born from 2006–2008 found that infants with Grades I–II IVH had no difference in neurodevelopmental outcomes at 18–22 months as compared with infants with no IVH (A. Payne, personal communication, July 25 2011). These studies suggest that if Grade I and II IVH do affect outcome, the effect is not large. Our study adds to the body of knowledge on lower grade IVH by showing that bilateral Grade I and II hemorrhage do not change neurodevelopmental outcome as compared to unilateral Grade I–II IVH. The lower predicted probabilities of neurodevelopmental impairment for Grade II compared to Grade I IVH were most likely due to the small number of patients with Grade II IVH and NDI. We found postnatal steroids and sepsis to be major confounders in the predictive models of NDI and Bayley scores. Controversy still remains over the effect of postnatal steroids on neurodevelopment, as outcomes in patients given courses of steroids are affected by multiple other factors, including chronic lung disease. Infants from the current study were born between 1998–2005, an era in which postnatal steroids were given at earlier ages and at higher doses than they are today. Multiple studies have suggested that sepsis contributes to NDI (15–18), which may be mediated through white matter injury (19). Figure 2 illustrates that all of these factors (grade of IVH, bilaterality of IVH, sepsis, and postnatal steroids) should be considered when discussing neurodevelopmental prognosis with families.
Limitations of this study include the retrospective nature, although the data were collected prospectively by experienced research personnel. Head ultrasounds were obtained for clinical reasons at various times (based on a suggested protocol) and read by different radiologists, so the presence and grade of IVH could be subject to misclassification bias. Only infants who returned for follow up and had Bayley scores performed were included in the analysis, which may have resulted in a selection bias. A portion of the adverse outcome may be associated with non-cystic white matter injury not detected by head ultrasound.
Strengths of the study include the adjustment for multiple confounders in the model, and the re-review of all head ultrasound reports by a single researcher to ensure accuracy of coding of IVH data.
The results of this study may be helpful in counseling families about expected outcomes in infants with IVH. Starting in 2005, laterality of IVH was collected as part of the NICHD Neonatal Research Network Generic Database, which will facilitate future studies to determine the effect of unilateral versus bilateral IVH on neurodevelopmental outcomes. Future studies should evaluate the effect of steroids in the current era as a confounder of outcome in infants with IVH. Studies with longer follow up and larger populations could also evaluate the laterality of involvement as a predictor of outcomes such as language development.
KEY NOTES.
Neurodevelopmental outcomes at 18–22 months for 166 ELBW infants with Grade I–IV IVH were analyzed and multivariable linear and logistic regression models were developed to predict outcomes based on IVH grade, IVH laterality, and other clinical variables. As expected, infants with bilateral grade IV IVH had worse neurodevelopmental outcomes than those with unilateral grade IV IVH; however, infants with grades I–III IVH had similar outcomes whether their IVH was unilateral or bilateral.
Acknowledgments
Supported in part by a grant from the National Institute of Child Health and Human Development Eunice Kennedy Shriver Neonatal Research Network (U10 HD 027853)
We thank the following individuals and hospital staff that contributed to this research:
Eunice Kennedy Shriver NICHD NRN investigators1, administrator2, and research coordinators and staff3: Edward Donovan1, Jean Steichen1, Estelle Fischer2, Kate Bridges3, Teresa Gratton3, Cathy Grisby3, Jody Hessling3, Lenora Jackson3, Kristin Kirker3, Marcia Worley Mersmann3, Holly Mincey3, Greg Muthig3, Stacie Tepe3
The staff of the NICUs at Cincinnati Children’s Hospital Medical Center, Good Samaritan Hospital, and University Hospital, Cincinnati
COMPLETE LIST OF ABBREVIATIONS USED
- BPD
bronchopulmonary dysplasia
- BSID
Bayley Scales of Infant Development
- ELBW
extremely low birth weight
- GMH
germinal matrix hemorrhage
- IRB
institutional review board
- IVH
intraventricular hemorrhage
- MDI
mental development index
- NDI
neurodevelopmental impairment
- NEC
necrotizing enterocolitis
- PDI
psychomotor development index
- PVHI
periventricular hemorrhagic infarction
Footnotes
Conflicts of interest: none
References
- 1.Volpe JJ. Intracranial hemorrhage: Germinal matrix-intraventricular hemorrhage of the premature infant. In: Volpe JJ, editor. Neurology of the Newborn. 5. Philadelphia: WB Saunders; 2008. [Google Scholar]
- 2.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 3.Batton DG, Holtrop P, DeWitte D, Pryce C, Roberts C. Current gestational age-related incidence of major intraventricular hemorrhage. J Pediatr. 1994;125:623–5. doi: 10.1016/s0022-3476(94)70023-0. [DOI] [PubMed] [Google Scholar]
- 4.Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Arch Dis Child Fetal Neonatal Ed. 2002;86:F86–90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheth RD. Trends in incidence and severity of intraventricular hemorrhage. J Child Neurol. 1998;13:261–4. doi: 10.1177/088307389801300604. [DOI] [PubMed] [Google Scholar]
- 6.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, Network NR. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167–77. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648–54. doi: 10.1016/j.jpeds.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Futagi Y, Toribe Y, Ogawa K, Suzuki Y. Neurodevelopmental outcome in children with intraventricular hemorrhage. Pediatr Neurol. 2006;34:219–24. doi: 10.1016/j.pediatrneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I–II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149:169–73. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Sherlock RL, Anderson PJ, Doyle LW, Group VICS. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81:909–16. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Bassan H, Benson CB, Limperopoulos C, Feldman HA, Ringer SA, Veracruz E, et al. Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics. 2006;117:2111–8. doi: 10.1542/peds.2005-1570. [DOI] [PubMed] [Google Scholar]
- 12.de Vries LS, Roelants-van Rijn AM, Rademaker KJ, Van Haastert IC, Beek FJ, Groenendaal F. Unilateral parenchymal haemorrhagic infarction in the preterm infant. Eur J Paediatr Neurol. 2001;5:139–49. doi: 10.1053/ejpn.2001.0494. [DOI] [PubMed] [Google Scholar]
- 13.De Vries LS, Van Haastert IL, Rademaker KJ, Koopman C, Groenendaal F. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144:815–20. doi: 10.1016/j.jpeds.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Maitre NL, Marshall DD, Price WA, Slaughter JC, O’Shea TM, Maxfield C, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124:e1153–60. doi: 10.1542/peds.2009-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of Sepsis on Neurodevelopmental Outcome in a Swiss National Cohort of Extremely Premature Infants. Pediatrics. 2011;128:e348–57. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 16.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–8. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125:e736–40. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 18.van der Ree M, Tanis JC, Van Braeckel KN, Bos AF, Roze E. Functional impairments at school age of preterm born children with late-onset sepsis. Early Hum Dev. 2011 doi: 10.1016/j.earlhumdev.2011.06.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–5. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]