Abstract
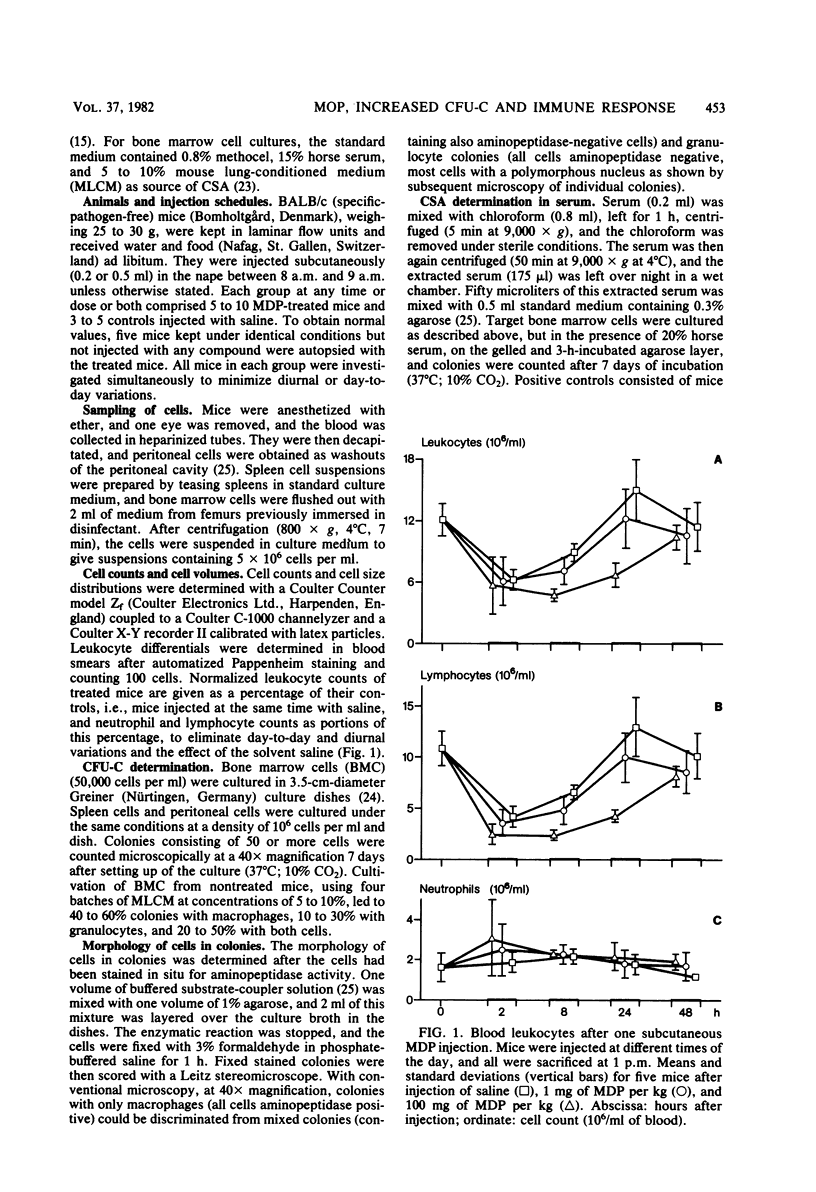
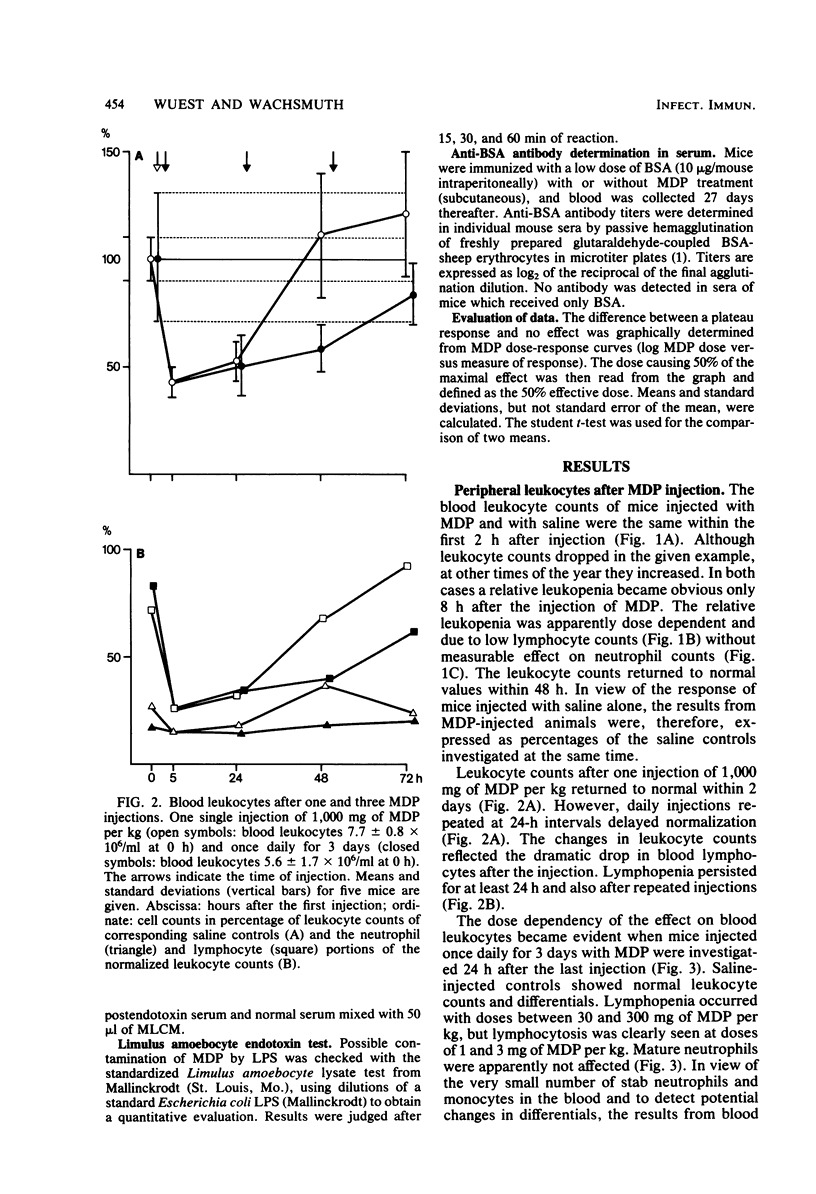
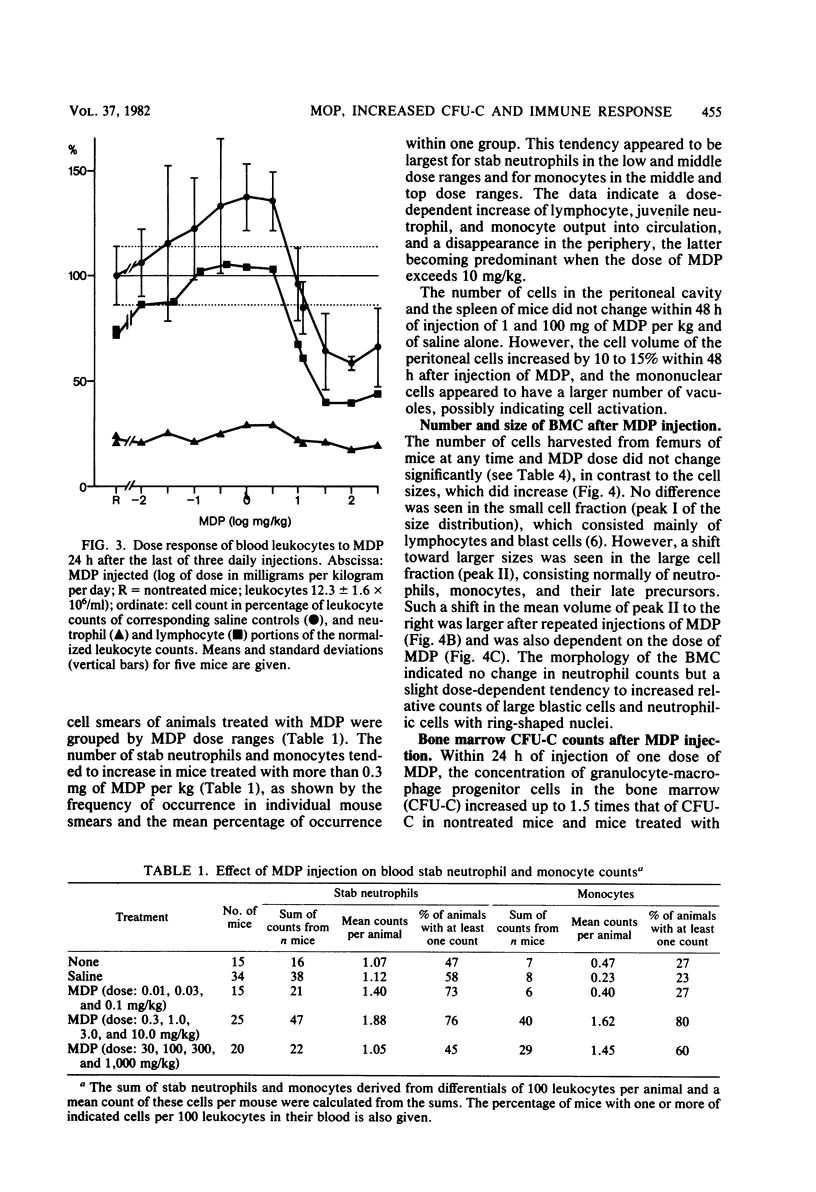
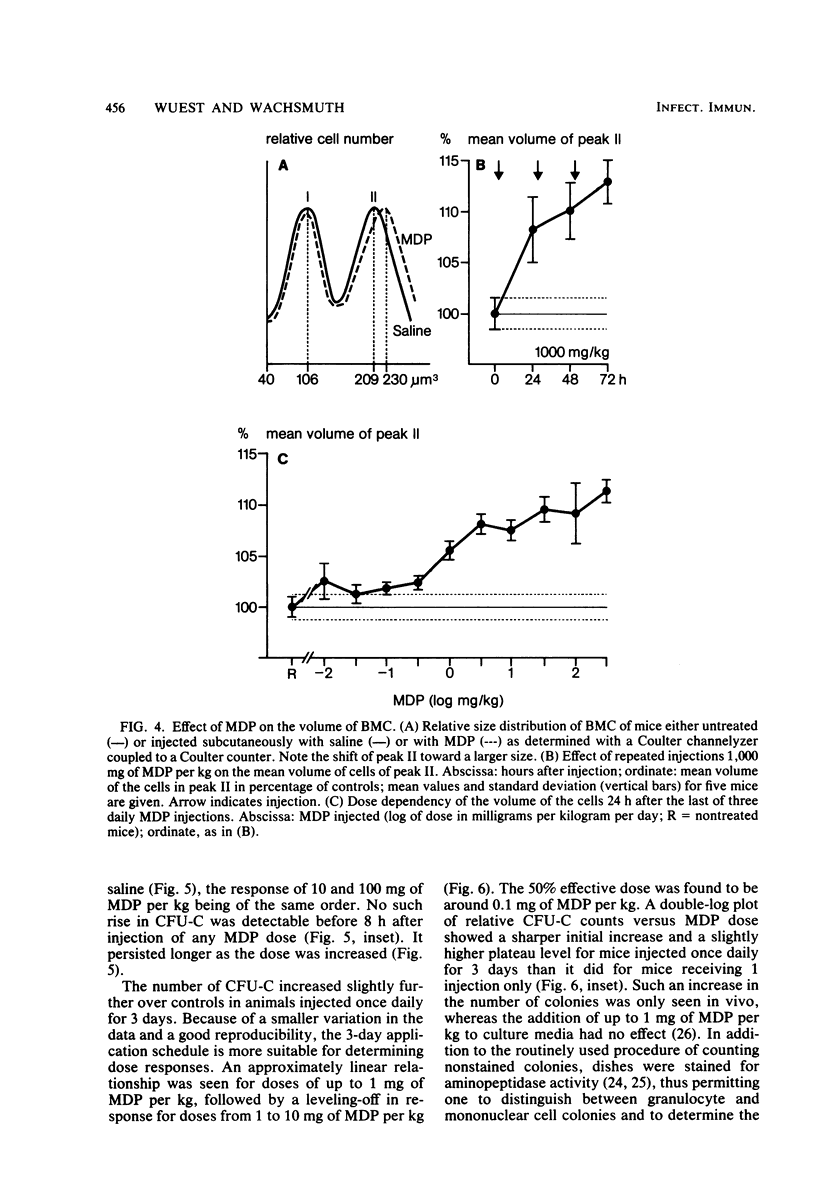
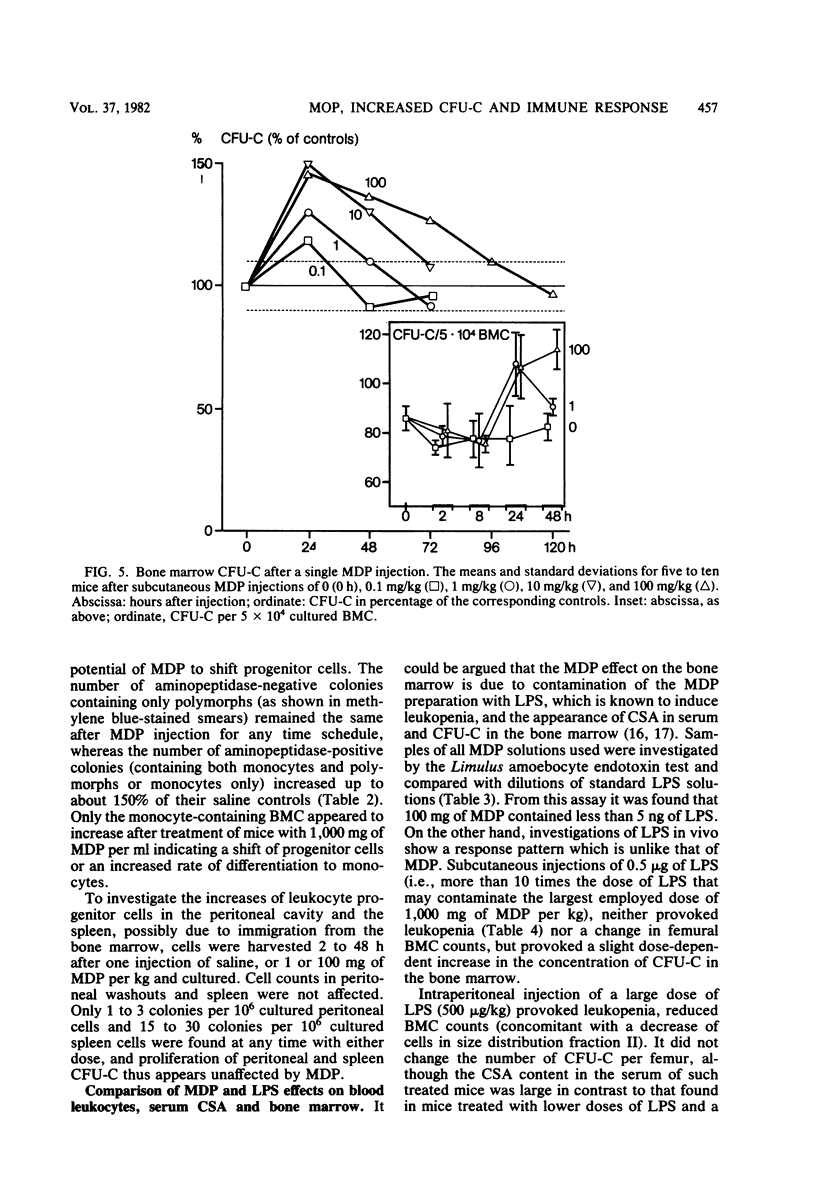
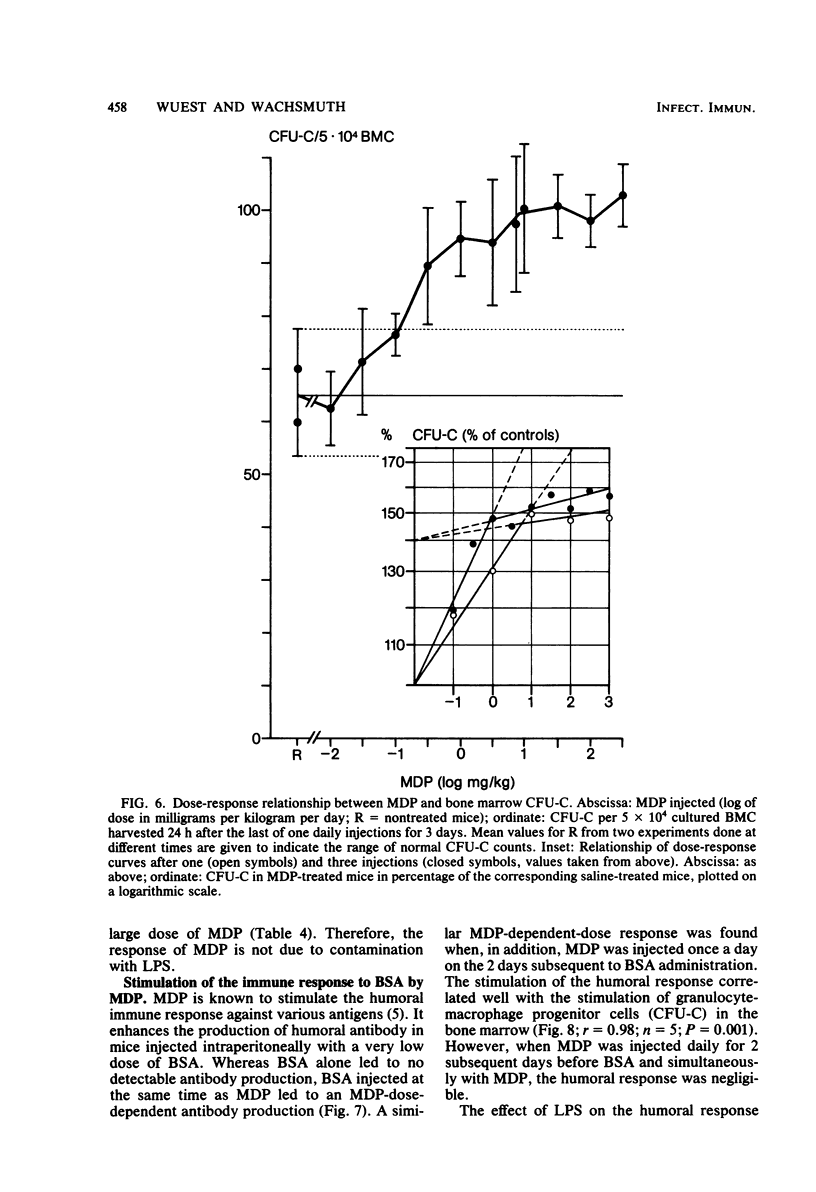
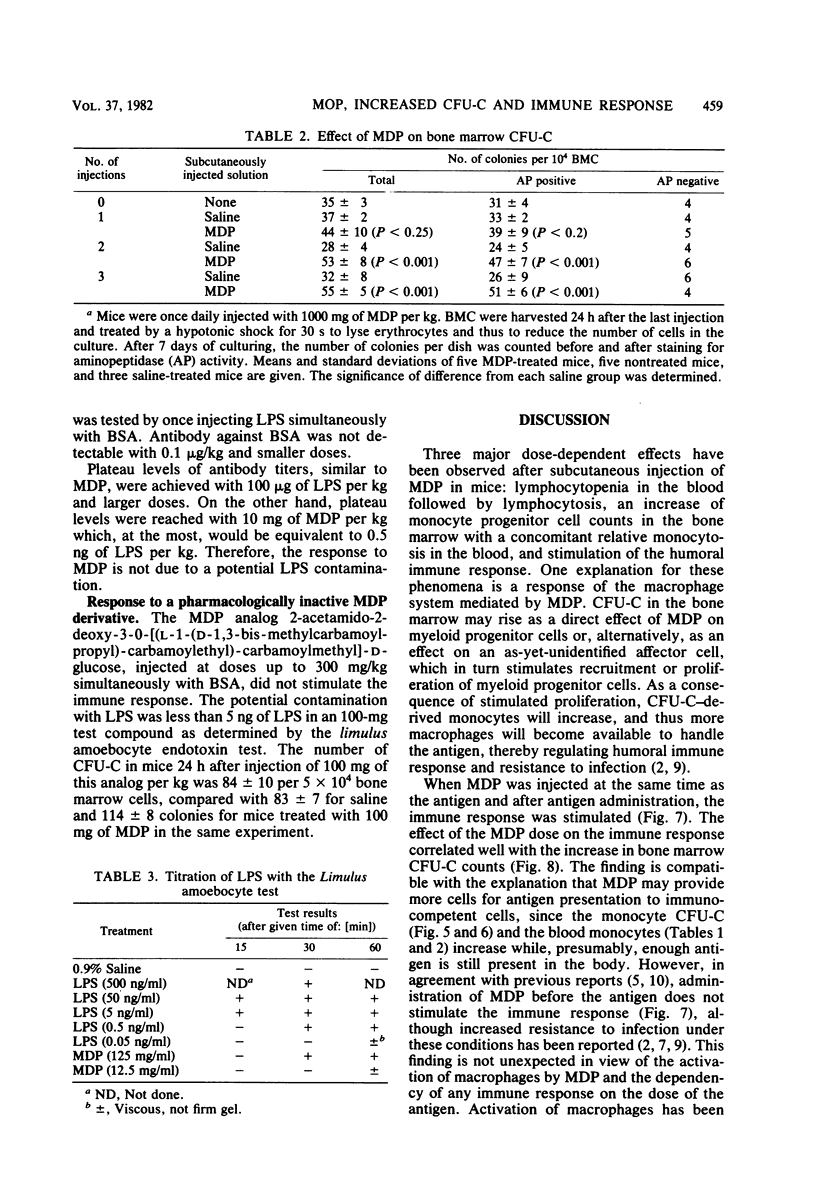
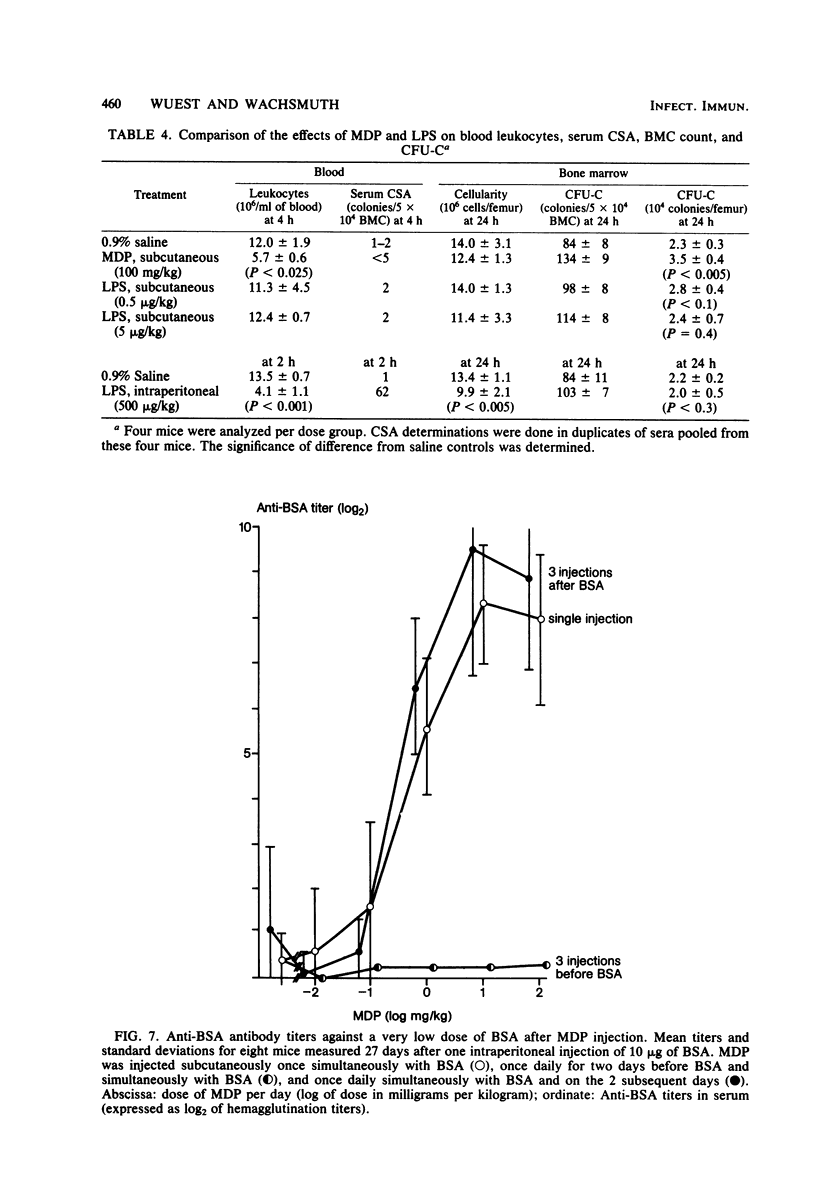
The effects of single and multiple injections of N-acetyl muramyl dipeptide (MDP) on peripheral leukocytes, colony-forming cells (i.e., bone marrow granulocyte-macrophage progenitor cells), and the humoral immune response (to bovine serum albumin) were investigated in mice. Whereas low doses of MDP (0.1 to 1 mg/kg) provoked lymphocytosis, larger doses (10 mg/kg upward) resulted in lymphocytopenia and an increase in the number of young stab neutrophils and monocytes. MDP induced a dose-dependent increase in the number of bone-marrow macrophage progenitor cells, the maximum being reached by a dose around 10 mg/kg. A 50% increase in the maximum effect was produced by a dose around 0.1 mg/kg. The higher the dose, the longer the increase in these progenitor cells persisted. MDP mediated a dose-dependent antibody response to small amounts of bovine serum albumin, correlating with the proliferation of progenitor cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Taudou B., Chuilon S. Glutaraldehyde, cyanuric chloride and tetrazotized O-dianisidine as coupling reagents in the passive hemagglutination test. Immunochemistry. 1969 Jan;6(1):67–76. doi: 10.1016/0019-2791(69)90179-7. [DOI] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Metcalf D., Battye F., Mandel T. Analysis of rat hemopoietic cells on the fluorescence-activated cell sorter. I. Isolation of pluripotent hemopoietic stem cells and granulocyte-macrophage progenitor cells. J Exp Med. 1980 Aug 1;152(2):419–437. doi: 10.1084/jem.152.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Sharma S. D., Ferraresi R. W., Remington J. S. Effects of muramyl dipeptide treatment on resistance to infection with Toxoplasma gondii in mice. Infect Immun. 1981 Feb;31(2):716–722. doi: 10.1128/iai.31.2.716-722.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Juy D., Bourgeois E., Chedid L. In vivo regulation of humoral and cellular immune responses of mice by a synthetic adjuvant, N-acetyl-muramyl-L-alanyl-D-isoglutamine, muramyl dipeptide for MDP. Cell Immunol. 1979 Jun;45(1):199–206. doi: 10.1016/0008-8749(79)90377-0. [DOI] [PubMed] [Google Scholar]
- Lowy I., Theze J., Chedid L. Stimulation of the in vivo dinitrophenyl antibody response to the DNP conjugate of L-glutamic acid60-L-alanine30-L-Tyrosine10 (GAT) polymer by a synthetic adjuvant, muramyl dipeptide (MDP): target cells for adjuvant activity and isotypic pattern of MDP-stimulated response. J Immunol. 1980 Jan;124(1):100–104. [PubMed] [Google Scholar]
- MacVittie T. J., Walker R. I. Endotoxin-induced alterations in canine granulopoiesis: colony-stimulating factor, colony-forming cells in culture, and growth of cells in diffusion chambers. Exp Hematol. 1978 Aug;6(7):613–618. [PubMed] [Google Scholar]
- Merser C., Sinay P., Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1316–1322. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- Nagao S., Tanaka A., Yamamoto Y., Koga T., Onoue K., Shiba T., Kusumoto K., Kotani S. Inhibition of macrophage migration by muramyl peptides. Infect Immun. 1979 May;24(2):308–312. doi: 10.1128/iai.24.2.308-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter E. Endotoxins and the immune response. Curr Top Microbiol Immunol. 1969;47:82–124. doi: 10.1007/978-3-642-46160-6_5. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P. J., Morley A., Miller M., Rickard K., Howard D., Stohlman F., Jr Effect of endotoxin on granulopoiesis and the in vitro colony-forming cell. Blood. 1973 Mar;41(3):391–398. [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Riveau G., Masek K., Parant M., Chedid L. Central pyrogenic activity of muramyl dipeptide. J Exp Med. 1980 Oct 1;152(4):869–877. doi: 10.1084/jem.152.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber F. G., Gisler R. H., Schumann G., Tarcsay L., Schläfli E., Dukor P. Modulation of myelopoiesis by different bacterial cell-wall components: induction of colony-stimulating activity (by pure preparations, low-molecular-weight degradation products, and a synthetic low-molecular analog of bacterial cell-wall components) in vitro. Cell Immunol. 1978 Apr;37(1):174–187. doi: 10.1016/0008-8749(78)90185-5. [DOI] [PubMed] [Google Scholar]
- Sugimura K., Uemiya M., Saiki I., Azuma I., Yamamura Y. The adjuvant activity of synthetic N-acetylmuramyl-dipeptide: evidence of initial target cells for the adjuvant activity. Cell Immunol. 1979 Mar 1;43(1):137–149. doi: 10.1016/0008-8749(79)90157-6. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Nagao R., Kotani S., Shiba T., Kusumoto S. Stimulation of the reticuloendothelial system of mice by muramyl dipeptide. Infect Immun. 1979 May;24(2):302–307. doi: 10.1128/iai.24.2.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama T., Holden H. T. Direct augmentation of cytolytic activity of tumor-derived macrophages and macrophage cell lines by muramyl dipeptide. Cell Immunol. 1979 Dec;48(2):369–374. doi: 10.1016/0008-8749(79)90131-x. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Staber F. G. Changes in membrane-bound aminopeptidase on bone marrow-derived macrophages during their maturation in vitro. Exp Cell Res. 1977 Oct 15;109(2):269–276. doi: 10.1016/0014-4827(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Stoye J. P. Aminopeptidase on the surface of differentiating macrophages: induction and characterization of the enzyme. J Reticuloendothel Soc. 1977 Nov;22(5):469–483. [PubMed] [Google Scholar]
- Wachsmuth E. D., Wüst B. Effect of bestatin and muramyl-dipeptide on macrophage-bound aminopeptidase. Hoppe Seylers Z Physiol Chem. 1981 May;362(5):563–568. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]


