Abstract
Objective:
We tested whether maternal hypertensive disorders in pregnancy predict age-related change in cognitive ability in the offspring up to old age.
Methods:
Using mothers' blood pressure and urinary protein measurements from the maternity clinics and birth hospitals, we defined normotensive or hypertensive pregnancies in mothers of 398 men, who participated in the Helsinki Birth Cohort 1934–1944 Study. The men underwent the Finnish Defence Forces basic ability test twice: first during compulsory military service at age 20.1 (SD = 1.4) years and then in a retest at age 68.5 (SD = 2.9) years. The test yields a total score and subscores for tests measuring verbal, arithmetic, and visuospatial reasoning.
Results:
Men born after pregnancies complicated by a hypertensive disorder, compared with men born after normotensive pregnancies, scored 4.36 (95% confidence interval, 1.17–7.55) points lower on total cognitive ability at 68.5 years and displayed a greater decline in total cognitive ability (2.88; 95% confidence interval, 0.07–5.06) after 20.1 years. Of the subscores, associations were strongest for arithmetic reasoning.
Conclusion:
Maternal hypertensive disorders in pregnancy predict lower cognitive ability and greater cognitive decline up to old age. A propensity to lower cognitive ability and decline up to old age may have prenatal origins.
Increased risk of lower cognitive ability may have its origin in a suboptimal prenatal environment, as reflected in smaller body size at birth or shorter length of gestation.1 Hypertensive disorders, including chronic hypertension, gestational hypertension, and (pre)eclampsia, complicate approximately 10% of all pregnancies2 and affect the fetal developmental milieu. These disorders may thus point to mechanisms by which prenatal adversity associates with lower cognitive ability in subsequent life. We have previously reported that men born after pregnancies complicated by hypertensive disorders, in comparison with men born after normotensive pregnancies, score lower on cognitive ability at the average age of 20 years.3 Here we test whether the effects of hypertensive disorders in pregnancy on cognitive ability persist into old age among these men retested at the age of 65 to 76 years and predict age-related change in cognitive ability after age 20 years.
METHODS
Participants.
The Helsinki Birth Cohort Study (HBCS) comprises 13,345 participants—6,975 men and 6,370 women—who were born as singletons between 1934 and 1944 in 1 of the 2 public maternity hospitals in Helsinki.4 Eligible participants were still living in Finland in 1971, when a unique personal identification number was allocated to each member of the Finnish population.
A subsample (n = 931) of the 6,975 men underwent testing of cognitive ability twice: first during compulsory military service at an average age of 20.1 years (SD = 1.4; range = 17.0–28.1) and then on average 47.7 (SD = 2.9; range = 38.9–54.7) years later.
A total of 398 of them also had data from medical records on maternal blood pressure and urinary protein tests during pregnancy to diagnose hypertensive disorders and were born preterm or term. Figure e-1 on the Neurology® Web site at www.neurology.org shows the selection of the participants from the original sample. None of the mothers of these participants had only blood pressure or only urinary protein tests recorded.
The included (n = 398) men had 6.5% more often clerical fathers than men who had maternal blood pressure and urinary protein tests recorded and were born preterm or term but had participated in the cognitive testing at age 20.1 years only (n = 798 of 1,196) (p = 0.03). These groups had similar maternal age, weight, and height at delivery, birthweight, and parity. When compared with men who were excluded on the basis of not having maternal blood pressure and urinary protein tests recorded and had missing, implausible, or post-term length of gestation (n = 533 of 931), the included men had 7% more often manual worker fathers and had a 0.2-kg lower birthweight (p < 0.01) but had similar maternal age, weight, and height at delivery and similar parity.
Standard protocol approvals, registrations, and patient consents.
The Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District approved the study. The Finnish Defence Command gave permission for data linkage. All study participants signed a written informed consent.
Measures.
Hypertensive disorders in pregnancy.
For identifying hypertensive pregnancy disorders, we used mothers' blood pressure and urinary protein measurements recorded at antenatal clinics or at the birth hospital.3,5 We defined 2 groups of mothers: 1) normotensive mothers attaining neither systolic pressure of 140 mm Hg nor diastolic pressure of 90 mm Hg during pregnancy; 2) mothers with hypertensive disorder in pregnancy, including mothers with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, with or without proteinuria.
Confounders and covariates.
These included length of gestation (based upon date of last menstruation), weight (g) and head circumference (cm) at birth, mother's age (years) and body mass index (kg/m2) at delivery, parity (primiparous or multiparous), and fathers' occupation status in childhood (manual worker/ junior clerical/senior clerical), extracted from birth, child welfare clinic, and school health care records; highest own attained level of education (basic or less/upper secondary/lower tertiary/upper tertiary) since 1970, obtained from Statistics Finland; diagnoses of stroke and coronary heart disease (CHD) since 1969, obtained from the Finnish Hospital Discharge Register; blood pressure medication, obtained from the national register of people receiving reimbursement from the state for the costs of their medication; height at second testing and age at first and second testing of cognitive ability, and time interval between the 2 testings.
Cognitive ability.
The ability test scores were obtained from the Finnish Defence Forces basic ability test. The group-administered ability test battery is composed of verbal, arithmetic, and visuospatial reasoning subtests, each consisting of 40 timed multiple-choice questions. In the verbal reasoning subtest, the subject chooses synonyms, antonyms, or words belonging or not belonging to the same category as given words, and similar relationships between 2 word pairs. In the arithmetic reasoning subtest, the subject completes series of numbers, solves short problems, computes simple arithmetic operations, and chooses similar relationships between 2 pairs of numbers. The visuospatial reasoning subtest comprises a set of matrices containing a pattern problem with 1 part removed, and the subject has to decide which figure completes the matrix: it is analogous to widely used Raven's Progressive Matrices. Correct answers are summed, and the arithmetic mean of the 3 subtests reflects general ability and logical thinking. The test battery and its psychometric properties are described in detail elsewhere.1,3,6
Statistical analysis.
Rank order stability and mean-level change in cognitive ability were investigated by linear regression analyses and paired t tests, respectively. The cognitive ability test scores were standardized (mean of 100 and SD of 15) for multiple linear regression analyses targeting the main study questions: Do maternal hypertensive disorders in pregnancy predict cognitive ability at age 68.5 years and age-related change in cognitive ability after age 20.1 years? Change was modeled in analyses where cognitive ability at age 68.5 years was used as the outcome and cognitive ability at age 20.1 years as the predictor.7 Interactions by “prematurity/parity/father's occupational status × hypertensive disorders in pregnancy” tested moderation by these factors.
RESULTS
Table 1 shows characteristics of the sample. Table e-1 shows the rank-order stability and mean level change of cognitive ability from 20.1 to 68.5 years. After adjustment for age at first cognitive ability test and time interval between the 2 tests, the total cognitive ability showed high rank-order stability (standardized regression coefficient = 0.75; p < 0.001); the average age-related change was −0.31 (SD = 4.82; range = −18.0 to 22.7; p = 0.20) raw total test score points; and 54.8% of the sample displayed a decline in total cognitive ability since age 20.1 years.
Table 1.
Characteristics of the sample according to mothers' hypertensive disorder during pregnancy
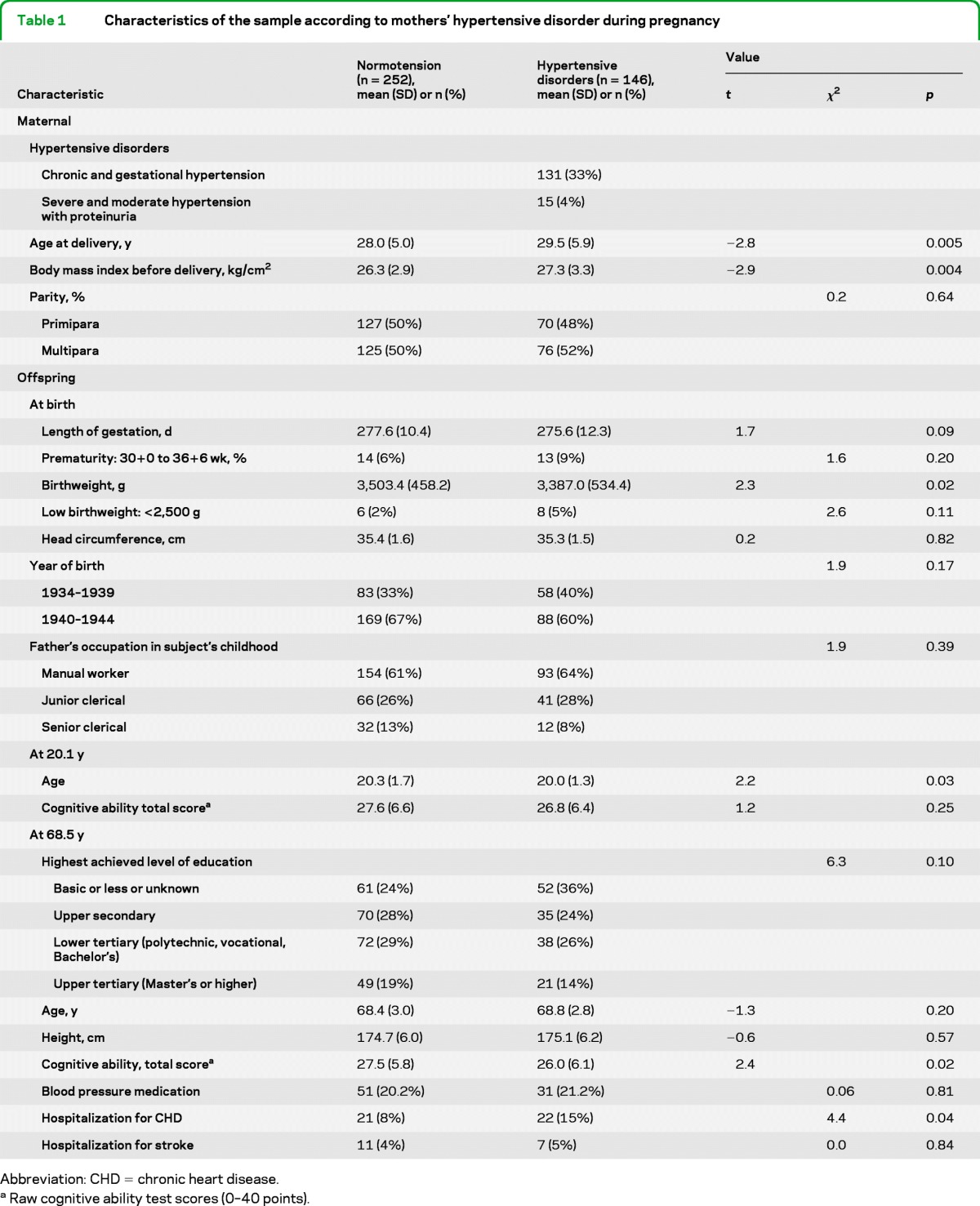
Abbreviation: CHD = chronic heart disease.
Raw cognitive ability test scores (0–40 points).
Table 2 shows that men born after pregnancies complicated by hypertensive disorders, compared with men born after normotensive pregnancies, scored lower on arithmetic and total cognitive ability at 68.5 years (p values in unadjusted and adjusted models <0.05) (table 2). They also displayed a greater decline in arithmetic and total cognitive ability after age 20.1 years (table 2).
Table 2.
Cognitive ability at the ages of 20.1 and 68.5 years in the offspring who were born after normotensive pregnancies (n = 252) and pregnancies complicated by hypertensive disorders (n = 146)
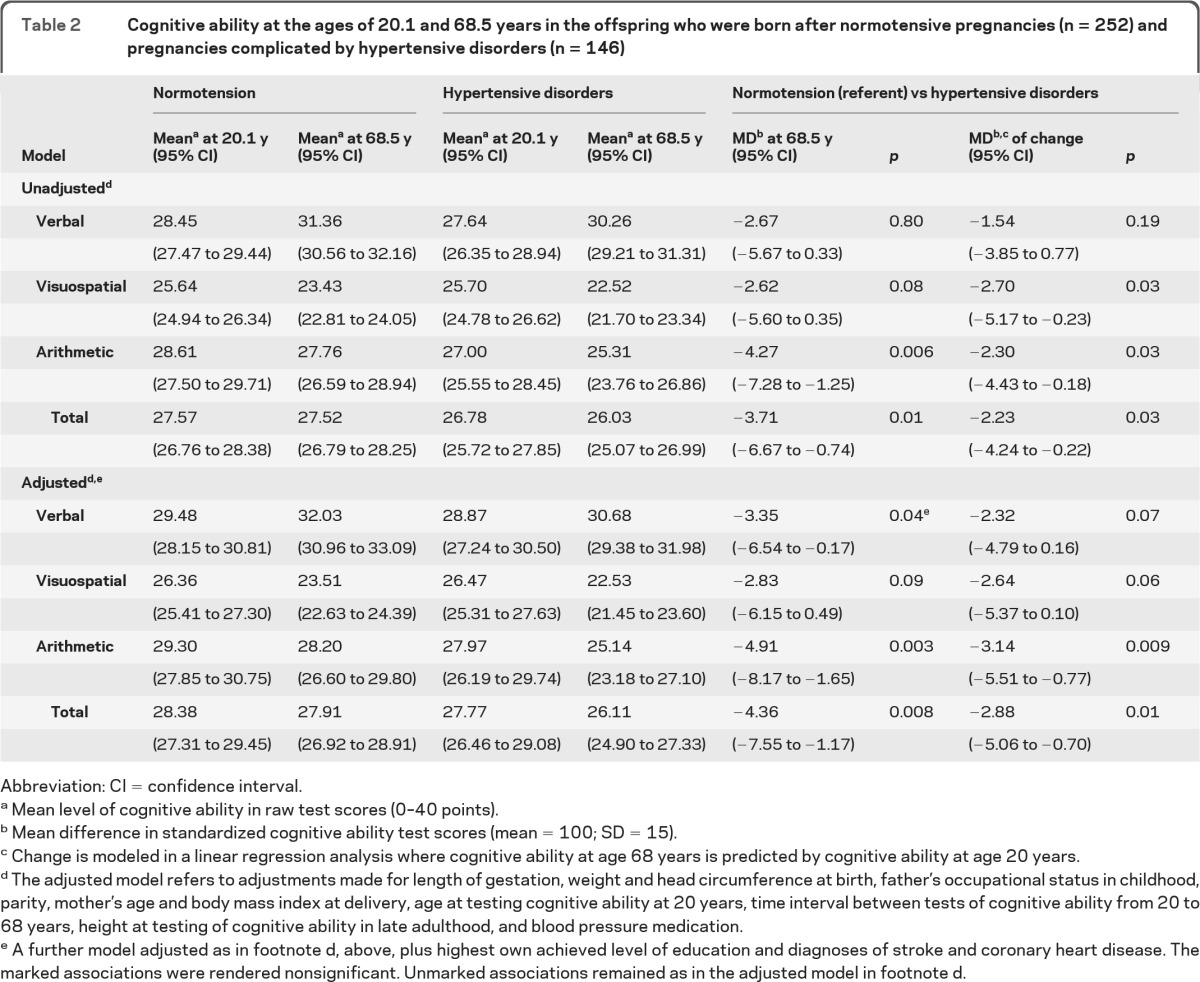
Abbreviation: CI = confidence interval.
Mean level of cognitive ability in raw test scores (0–40 points).
Mean difference in standardized cognitive ability test scores (mean = 100; SD = 15).
Change is modeled in a linear regression analysis where cognitive ability at age 68 years is predicted by cognitive ability at age 20 years.
The adjusted model refers to adjustments made for length of gestation, weight and head circumference at birth, father's occupational status in childhood, parity, mother's age and body mass index at delivery, age at testing cognitive ability at 20 years, time interval between tests of cognitive ability from 20 to 68 years, height at testing of cognitive ability in late adulthood, and blood pressure medication.
A further model adjusted as in footnote d, above, plus highest own achieved level of education and diagnoses of stroke and coronary heart disease. The marked associations were rendered nonsignificant. Unmarked associations remained as in the adjusted model in footnote d.
Prematurity/parity/father's occupational status did not moderate these associations (p > 0.10 for interactions).
DISCUSSION
We found that men who were born after pregnancies complicated by a hypertensive disorder, compared with men born after normotensive pregnancies, scored lower on tests measuring arithmetic reasoning and total cognitive ability in old age. They also displayed a greater decline in arithmetic reasoning and total cognitive ability after young adulthood. The associations were not confounded by factors that may increase the risk of hypertensive disorders or poorer cognitive ability.3 The associations were not explained on the highest own achieved level of education in adulthood or by having had a stroke or CHD.
We are unaware of any previous studies testing whether maternal hypertensive disorders are associated with cognitive ability in old age and decline in cognitive ability after young adulthood. Our findings thus contribute significantly to the literature on cognitive aging and suggest that a propensity toward lower cognitive ability has its origins in the prenatal period, when the majority of the development of brain structure and function occurs. Our results may also offer mechanistic insight into why short length of gestation and small body size at birth are linked with lower cognitive ability, as hypertensive disorders are among the key reasons for prematurity and intrauterine growth restriction.
The possible factors underlying the associations between hypertensive disorders and long-term neurodevelopmental consequences may relate to placental implantation and hence less placental perfusion,8 glucocorticoid metabolism,9 inflammatory mechanisms,10 and genetic and epigenetic mechanisms.
Generalization of the findings is limited to men. Loss of follow-up over time may introduce a bias, as our findings may apply only to the healthiest men who have survived to old age. Moreover, our data did not allow us to require 2 elevated blood pressure measurements to establish a diagnosis.11 Consequently, the cumulative incidence of hypertensive disorders was higher than that reported in the literature. These limitations undermine rather than increase our ability to detect associations.
Hypertensive disorders in pregnancy predict lower cognitive ability and greater cognitive decline over decades in the adult offspring. A propensity to lower cognitive ability and greater cognitive decline may have its origins in fetal life.
Supplementary Material
GLOSSARY
- CHD
coronary heart disease
- HBCS
Helsinki Birth Cohort Study
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Conception and design, acquisition of data, analysis and interpretation of data: Ms. Tuovinen, Pyhälä, Alastalo, Mr. Lahti, Drs. Räikkönen, Kajantie, Henriksson, Leskinen, Pesonen, Heinonen, Lahti, Osmond, Barker, Eriksson; drafting the article or revising it critically for important intellectual content: Ms. Tuovinen, Pyhälä, Alastalo, Mr. Lahti, Drs. Räikkönen, Kajantie, Henriksson, Leskinen, Pesonen, Heinonen, Lahti, Osmond, Barker, Eriksson; final approval of the version to be published: Ms. Tuovinen, Pyhälä, Alastalo, Mr. Lahti, Drs. Räikkönen, Kajantie, Henriksson, Leskinen, Pesonen, Heinonen, Lahti, Osmond, Barker, Eriksson.
DISCLOSURE
The authors report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Räikkönen K, Forsén T, Henriksson M, et al. Growth trajectories and intellectual abilities in young adulthood. Am J Epidemiol 2009; 170: 447– 455 . [DOI] [PubMed] [Google Scholar]
- 2. Roberts CL, Ford JB, Algert CS, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open 2011; 1: e000101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tuovinen S, Raikkonen K, Kajantie E, et al. Hypertensive disorders in pregnancy and intellectual abilities in the offspring in young adulthood: The Helsinki Birth Cohort Study. Ann Med 2011; 44: 394– 403 . [DOI] [PubMed] [Google Scholar]
- 4. Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BJM 2001; 322: 949– 953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 2009; 40: 1176– 1180 . [DOI] [PubMed] [Google Scholar]
- 6. Tiihonen J, Haukka J, Henriksson M, et al. Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. Am J Psychiatry 2005; 162: 1904– 1910 . [DOI] [PubMed] [Google Scholar]
- 7. Twisk JWR. Applied Longitudinal Data Analysis of Epidemiology. Cambridge, UK: Cambridge University Press; 2003 [Google Scholar]
- 8. Newnham JP, Moss TJ, Nitsos I, Sloboda DM, Challis JRG. Nutrition and the early origins of adult disease. Asia Pac J Clin Nutr 2002; 11: S537– S542 . [DOI] [PubMed] [Google Scholar]
- 9. Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 2006; 11: 309– 316 . [DOI] [PubMed] [Google Scholar]
- 10. Räikkönen K, Pesonen A-K, Heinonen K, et al. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am J Epidemiol 2009; 170: 1137– 1146 . [DOI] [PubMed] [Google Scholar]
- 11. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy Am J Obstet Gynecol 2000; 183: S1– S22 . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


