Abstract
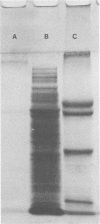
Fibronectin ("cold-insoluble globulin") has been suggested to play a role in cell-to-cell and cell-to-substratum adhesions. The 70-kilodalton terminal part of human fibronectin has recently been shown to bind to Staphylococcus aureus. In the present study, a fibronectin-binding protein was purified from sonicated S. aureus strain E2371 by affinity chromatography on fibronectin-Sepharose. The fibronectin-binding protein was isolated from an extract of sonicated S. aureus containing at least 57 different proteins as determined by crossed immunoelectrophoresis in antibodies to sonicated S. aureus. The fibronectin-binding protein was released from fibronectin-Sepharose by carbamide (8 M). No impurities in the final preparation could be detected when tested in crossed immunoelectrophoresis. By polyacrylamide gel electrophoresis in both reduced and unreduced gels, the protein showed two bands with relative molecular masses of 197,000 and 60,000, respectively. A complex between the purified S. aureus protein and fibronectin could be demonstrated by crossed immunoelectrophoresis both in monospecific antibodies against fibronectin and in S. aureus polyspecific antibody.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk I., Petersson B. A., Sjöquist J. Some physiochemical properties of protein A from Staphylococcus aureus. Eur J Biochem. 1972 Sep 25;29(3):579–584. doi: 10.1111/j.1432-1033.1972.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Clemmensen I., Thorsen S., Müllertz S., Petersen L. C. Properties of three different molecular forms of the alpha 2 plasmin inhibitor. Eur J Biochem. 1981 Nov;120(1):105–112. doi: 10.1111/j.1432-1033.1981.tb05675.x. [DOI] [PubMed] [Google Scholar]
- Doran J. E., Raynor R. H. Fibronectin binding to protein A-containing staphylococci. Infect Immun. 1981 Sep;33(3):683–689. doi: 10.1128/iai.33.3.683-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Clumping of Staphylococcus aureus by human fibronectin. Acta Pathol Microbiol Scand B. 1981 Oct;89(5):317–321. doi: 10.1111/j.1699-0463.1981.tb00195_89b.x. [DOI] [PubMed] [Google Scholar]
- Espersen F., Hertz J. B., Høiby N., Mogensen H. H. Quantitative immunoelectrophoretic analysis of Salmonella typhi antigens and of corresponding antibodies in human sera. Acta Pathol Microbiol Scand B. 1980 Aug;88(4):237–242. doi: 10.1111/j.1699-0463.1980.tb02634.x. [DOI] [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Simmons A., Myhre E. B., Jonsson S. Specific absorption of human serum albumin, immunoglobulin A, and immunoglobulin G with selected strains of group A and G streptococci. Infect Immun. 1979 Jul;25(1):1–10. doi: 10.1128/iai.25.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Immunologic "short circuits". Ann Intern Med. 1969 May;70(5):1043–1045. doi: 10.7326/0003-4819-70-5-1043. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllertz S., Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976 Dec 1;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Svendsen P. J. Fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:69–70. doi: 10.1111/j.1365-3083.1973.tb03781.x. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]