Abstract
During antagonistic coevolution between viruses and their hosts, viruses have a major advantage by evolving more rapidly. Nevertheless, viruses and their hosts coexist and have coevolved, although the processes remain largely unknown. We previously identified Tm-1 that confers resistance to Tomato mosaic virus (ToMV), and revealed that it encodes a protein that binds ToMV replication proteins and inhibits RNA replication. Tm-1 was introgressed from a wild tomato species Solanum habrochaites into the cultivated tomato species Solanum lycopersicum. In this study, we analyzed Tm-1 alleles in S. habrochaites. Although most part of this gene was under purifying selection, a cluster of nonsynonymous substitutions in a small region important for inhibitory activity was identified, suggesting that the region is under positive selection. We then examined the resistance of S. habrochaites plants to ToMV. Approximately 60% of 149 individuals from 24 accessions were resistant to ToMV, while the others accumulated detectable levels of coat protein after inoculation. Unexpectedly, many S. habrochaites plants were observed in which even multiplication of the Tm-1-resistance-breaking ToMV mutant LT1 was inhibited. An amino acid change in the positively selected region of the Tm-1 protein was responsible for the inhibition of LT1 multiplication. This amino acid change allowed Tm-1 to bind LT1 replication proteins without losing the ability to bind replication proteins of wild-type ToMV. The antiviral spectra and biochemical properties suggest that Tm-1 has evolved by changing the strengths of its inhibitory activity rather than diversifying the recognition spectra. In the LT1-resistant S. habrochaites plants inoculated with LT1, mutant viruses emerged whose multiplication was not inhibited by the Tm-1 allele that confers resistance to LT1. However, the resistance-breaking mutants were less competitive than the parental strains in the absence of Tm-1. Based on these results, we discuss possible coevolutionary processes of ToMV and Tm-1.
Author Summary
Viruses rapidly evolve and adapt to their host organisms, and the evolutionary processes can be reproduced in the laboratory (experimental evolution). In contrast, cellular organisms (that can be viral hosts) evolve much more slowly than viruses, but the fact that they have antiviral systems suggests that viruses and their hosts have coevolved. To explore the coevolutionary histories of viruses and their hosts, we focused on Tm-1, a Solanum habrochaites gene that confers resistance to Tomato mosaic virus (ToMV). Based on analyses of the Tm-1 gene sequences in S. habrochaites, we demonstrated that a part of the gene has been under positive selection. Biochemical studies suggested that Tm-1 has evolved to strengthen its inhibitory activity rather than to diversify recognition spectra. In addition, experimental evolution analyses suggested that overcoming the Tm-1-mediated resistance by ToMV is associated with fitness costs. Based on these results, we discuss how ToMV and the plant resistance gene have coevolved.
Introduction
Because viral diseases often prevent plant reproduction, viruses affect the fitness of their host plants. To counter viruses, plants have developed defense systems such as gene-for-gene resistance and RNA silencing [1]–[5]. Viruses need to evade recognition by resistance genes and encode suppressors of RNA silencing for successful infection. This suggests that viruses and host plants have coevolved, although the processes remain largely unknown.
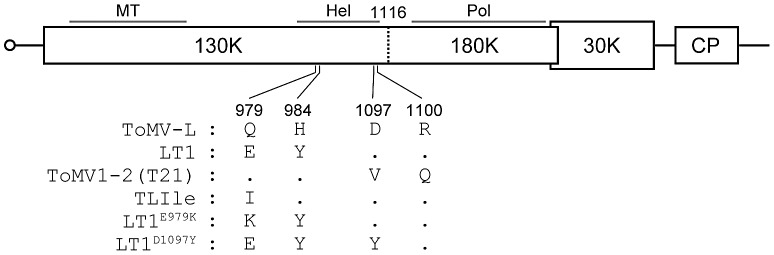
Tobacco mosaic virus, Tomato mosaic virus (ToMV), Tobacco mild green mosaic virus (TMGMV), and Pepper mild mottle virus (PMMoV) are positive-strand RNA viruses belonging to the genus Tobamovirus. The tobamovirus genome encodes at least four proteins, namely, the 130K protein, the 180K protein (translational read-through product of the 130K protein), the 30K protein, and the coat protein (CP) (Figure 1). The 130K and 180K proteins are involved in RNA replication [6] and are collectively referred to here as replication proteins. The 130K protein is a multifunctional protein that interacts with many host proteins [7], as well as small RNA duplexes to function as a suppressor of RNA silencing [8]–[10]. The 30K protein is required for cell-to-cell movement [11], [12]. The CP is the only structural protein and required for systemic spread of the virus [13].
Figure 1. Schematic representation of ToMV mutant genomes with different sensitivities to Tm-1 alleles.
Positions of amino acid residue changes in Tm-1-resistance-breaking mutants are shown. Amino acid residues identical to ToMV-L are indicated by dots. LT1 and T21 are Tm-1GCR237-breaking mutants [20], [28] and TLIle is a tm-1GCR26-sensitive mutant [30], [50]. LT1E979K and LT1D1097Y were characterized in this study. MT: methyltransferase domain, Hel: helicase domain, Pol: RNA-dependent RNA polymerase domain, CP: coat protein.
Several genes that confer resistance to tobamoviruses have been cloned, e.g., the N gene of tobacco [14], the Tm-1 gene of tomato [15], the Tm-2 gene alleles of tomato [16], [17], and the L gene alleles of pepper [18]. One of viral proteins is a determinant for resistance by each resistance gene; the 130K protein for N and Tm-1 [19]–[21], the 30K protein for Tm-2 [22], [23], and CP for L [24]–[26]. The frequency of emergence of resistance-breaking mutants varies from one resistance gene to another. For example, N and the Tm-22 allele of the Tm-2 locus are durable, while mutant viruses easily overcome the resistance by Tm-1, the Tm-2 allele of the Tm-2 locus, and the L alleles. The resistance genes that are easily overcome may have evolved more rapidly, and thus they can be good targets of studying coevolutionary processes.
The Tm-1 gene was introgressed from a wild tomato (Solanum habrochaites S. Knapp & D.M. Spooner) into tomato (Solanum lycopersicum L.) cultivars [27]. It encodes a protein that binds to ToMV replication proteins and inhibits RNA replication [15]. ToMV isolates that overcome the resistance conferred by Tm-1 have mutations in the replication protein-coding region [20], [28] (Figure 1). A resistance-breaking mutant LT1 has replication proteins that do not bind the Tm-1 protein [15], suggesting that ToMV overcame Tm-1 resistance by escaping the inhibitory interaction of its replication proteins with Tm-1. In the recently reported three-dimensional structure of the helicase domain of ToMV replication proteins, the residues involved in breaking the resistance are exposed to the surface of the molecule and locate in close spatial proximity [29], where Tm-1 likely binds. Translation product from a splicing variant of the Tm-1 mRNA that lacks the second exon did not inhibit in vitro ToMV RNA replication, which indicates that a region in the Tm-1 protein encoded by the alternative exon is important for the inhibitory activity [15]. Tm-1 homologs are widely conserved not only among plants, but also in fungi, bacteria, and archaea, suggesting that the Tm-1 protein has a primary function other than ToMV resistance and incidentally acquired the ability to bind ToMV replication proteins.
The ToMV-susceptible tomato cultivar GCR26 has a Tm-1 allele, tm-1GCR26. The amino acid sequence of the tm-1GCR26 protein shows 97% identity with Tm-1GCR237, the product of the Tm-1 gene from the ToMV-resistant tomato cultivar GCR237 [15]. The tm-1GCR26 protein does not bind the replication proteins or inhibit RNA replication of wild-type ToMV (L-strain) or LT1 [15]. However, tm-1GCR26 does bind the replication proteins and inhibit the multiplication of tobamoviruses that cannot infect tomato, namely, TMGMV, PMMoV, and the ToMV mutant TLIle in which the glutamine residue at position 979 of the replication proteins is replaced by an isoleucine residue [30] (Figure 1). TLIle, TMGMV, and PMMoV multiplication is also inhibited by Tm-1GCR237, indicating that tm-1GCR26 and Tm-1GCR237 have overlapping antiviral spectra [30].
Since most virus resistance genes are derived from wild relatives of the crops, studying the interactions between viruses and wild plants may elucidate the coevolutionary histories of viruses and plants. However, most molecular biological studies on plant resistance to viruses have been performed using crops or model plant species [31]. In this study, we analyzed ToMV resistance in S. habrochaites and show that a small part of the Tm-1 gene has been under positive selection. We further identified a Tm-1 allele that inhibits LT1 multiplication. On the other hand, evolution of microorganisms and their adaptation to hosts can be analyzed by experimental evolutionary methods in the laboratory [32]. In our experiments, ToMV mutants emerged that could overcome the LT1-resistant Tm-1 allele, although the mutants were less competitive than the parental strains in the absence of Tm-1.
Results
Positive selection in the Tm-1 gene of S. habrochaites
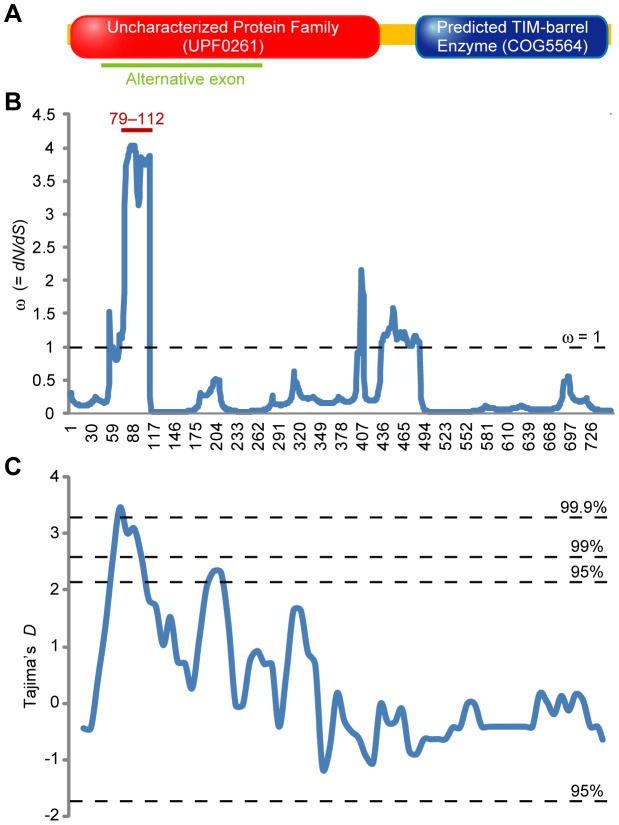
To analyze the Tm-1 gene of S. habrochaites, we obtained seeds of 24 S. habrochaites accessions from the Germplasm Resources Information Network (GRIN). All accessions were collected in South America (Peru, Ecuador, or Venezuela). From each accession, one plant was randomly chosen and the Tm-1 cDNA was sequenced. In the obtained 48 sequences, a significant negative correlation was observed between linkage disequilibrium (r2) and distance between sites in the sequences (r = −0.2975, p<0.001), suggestive of intragenic recombination between alleles. Since this result indicated that the samples were not amenable for phylogenetic analyses, we used omegaMap [33] to analyze whether the evidence of natural selection is detected from the sequences in the presence of recombination. Remarkably, positive selection (ω = ratio of the rate of nonsynonymous/synonymous substitutions >1) was detected in a small region while most of the other parts of the gene were under purifying selection (ω<1) (Figure 2). An interdomain region (residues 432–483, predicted by NCBI Conserved Domain Database [34]) likely evolved neutrally (ω = 1) (Figure 2). The posterior probability of positive selection is >95% at residues 79–112. Consistently, Tajima's D, a test of neutral evolution [35], was significantly high (p<0.001) in the positively selected region based on a sliding window analysis (Figure 2C), also indicating that the region has not evolved neutrally. Importantly, the region is located in the alternative exon (encoding amino acids 46–263) of the Tm-1 gene that is required for inhibitory activity (Figure 2) [15].
Figure 2. A small region of the Tm-1 gene is under positive selection in S. habrochaites.
(A) Predicted domain structure of the Tm-1 protein by the NCBI Conserved Domain Database. A region encoded by the alternative exon (46–263) is underlined. (B) Detection of natural selection in the Tm-1 alleles from S. habrochaites. The ratio of nonsynonymous/synonymous substitutions (ω) in each codon was inferred by omegaMap [33]. ω>1, ω = 1, and ω<1 suggest positive selection, neutral evolution, and negative selection, respectively. The region where posterior probability of positive selection (ω>1) exceeds 95% is indicated (from 79th to 112th codon). (C) Sliding window analysis of Tajima's D of the Tm-1 alleles from S. habrochaites. The confidence limits of D for neutral evolution [35] are shown as dashed lines.
ToMV resistance in S. habrochaites
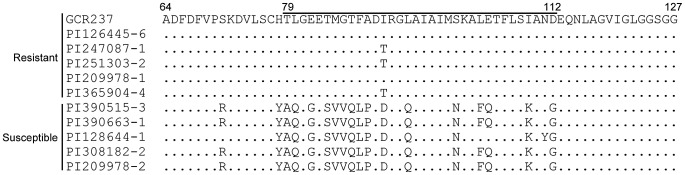
We next examined the ToMV resistance of 149 S. habrochaites plants from the 24 accessions by mechanically inoculating ToMV-L onto leaves. The accumulation of CP in the inoculated leaves was examined by SDS-PAGE, followed by Coomassie blue staining at 7 or 8 days postinoculation (dpi). Since S. habrochaites plants are self-incompatible, the accessions would not be genetically uniform. Indeed, in some accessions, both ToMV-resistant (CP undetectable) and -susceptible (CP detectable) plants were found (Table 1). Of the 149 plants tested, 94 did not accumulate detectable amounts of ToMV CP (Table 1). We then sequenced Tm-1 cDNA of randomly chosen five plants that did not accumulate ToMV CP (i.e., ToMV-resistant plants) and five plants that accumulated ToMV CP at high levels (i.e., ToMV-susceptible plants). The amino acid sequences of the positively selected region were clearly divided into two classes, consistent with their ToMV-resistant or -susceptible phenotypes (Figure 3). In this region, each of the 48 Tm-1 amino acid sequences obtained above was similar to either one of the two groups (29 to the resistant type and 19 to the susceptible type; Figure S1). These results suggest that both types of alleles were maintained by balancing selection.
Table 1. Accumulation of ToMV-L CP in S. habrochaites accessions.
| Accumulation level of ToMV-L CP | ||||
| Accession numbers | +++a | +b | −c | Number of analyzed plants |
| PI126445 | 1 | 0 | 5 | 6 |
| PI126446 | 1 | 2 | 3 | 6 |
| PI127826 | 3 | 0 | 4 | 7 |
| PI128644 | 7 | 1 | 0 | 8 |
| PI209978 | 1 | 1 | 7 | 9 |
| PI247087 | 0 | 0 | 6 | 6 |
| PI251303 | 0 | 0 | 6 | 6 |
| PI251304 | 0 | 0 | 5 | 5 |
| PI308182 | 5 | 0 | 3 | 8 |
| PI365903 | 0 | 1 | 4 | 5 |
| PI365904 | 0 | 0 | 9 | 9 |
| PI365905 | 0 | 0 | 5 | 5 |
| PI365906 | 0 | 0 | 6 | 6 |
| PI365907 | 2 | 1 | 2 | 5 |
| PI379056 | 4 | 0 | 0 | 4 |
| PI390515 | 6 | 0 | 0 | 6 |
| PI390516 | 0 | 0 | 6 | 6 |
| PI390517 | 1 | 0 | 5 | 6 |
| PI390518 | 0 | 0 | 5 | 5 |
| PI390658 | 2 | 0 | 5 | 7 |
| PI390659 | 1 | 2 | 4 | 7 |
| PI390661 | 3 | 2 | 1 | 6 |
| PI390662 | 4 | 0 | 1 | 5 |
| PI390663 | 4 | 0 | 2 | 6 |
| Total | 45 | 10 | 94 | 149 |
high level accumulation,
low level accumulation,
not detectable.
In underlined accessions, more than 70% of plant individuals did not accumulate detectable amounts of ToMV-L CP.
Figure 3. ToMV-L-resistant and -susceptible S. habrochaites have distinct amino acid sequences in the positively selected region of Tm-1.
Deduced amino acid sequences of the Tm-1 protein of five ToMV-L-resistant and -susceptible S. habrochaites plants from the indicated accessions were aligned. The positively selected region (79–112) is indicated. Identical amino acid residues to those of Tm-1GCR237 are indicated by dots.
In 10 out of the 149 plants, ToMV CP accumulated to low but detectable levels (Table 1). We sequenced the Tm-1 cDNA of one such plant (PI390659) and found that the plant is heterozygous for the putative resistant and susceptible alleles. This result was consistent with a previous report showing that Tm-1/tm-1 heterozygous tomato plants often permit delayed ToMV CP accumulation [36].
LT1 resistance in S. habrochaites
To determine whether the Tm-1 gene is responsible for the observed ToMV-L resistance, we selected 13 S. habrochaites accessions that contained relatively high proportions of ToMV-L-resistant plants (underlined in Table 1) and inoculated the Tm-1GCR237-resistance-breaking ToMV mutant LT1 onto 57 young seedlings from these accessions. For PI126445 and PI390658, CP accumulation was detected at high levels in three of four LT1-inoculated plants (Table 2). Note that Tm-1GCR237 originated from PI126445. On the other hand, many plants in the other accessions showed only low or undetectable levels of CP accumulation in LT1-inoculated leaves (Table 2). This suggests that only a fraction of the ToMV-L-resistant S. habrochaites plants, including those in PI126445, are carriers of Tm-1GCR237 or equivalent genes, and the rest have alternative or additional resistance factor(s) that prevent LT1 infection.
Table 2. Accumulation of LT1 CP in S.habrochaites accessions.
| Accumulation level of LT1 CP | ||||
| Accession numbers | +++a | +b | −c | Number of analyzed plants |
| PI126445 | 3 | 0 | 1 | 4 |
| PI209978 | 2 | 1 | 2 | 5 |
| PI247087 | 1 | 0 | 4 | 5 |
| PI251303 | 1 | 0 | 3 | 4 |
| PI251304 | 0 | 0 | 5 | 5 |
| PI365903 | 1 | 2 | 1 | 4 |
| PI365904 | 0 | 2 | 3 | 5 |
| PI365905 | 0 | 2 | 3 | 5 |
| PI365906 | 0 | 0 | 3 | 3 |
| PI390516 | 2 | 0 | 3 | 5 |
| PI390517 | 1 | 1 | 2 | 4 |
| PI390518 | 0 | 1 | 3 | 4 |
| PI390658 | 3 | 0 | 1 | 4 |
| Total | 14 | 9 | 34 | 57 |
high level accumulation,
low level accumulation,
not detectable.
Emergence of ToMV mutants that can multiply in LT1-resistant S. habrochaites
Although CP accumulation was undetectable at 8 dpi in approximately 60% of S. habrochaites plants inoculated with LT1 (Table 2), some of these plants showed disease symptoms at 15 dpi. In these plants, CP accumulation was observed. Since Tm-1-mediated resistance is easily overcome by mutations in the region coding for the helicase domain of ToMV replication proteins, the LT1 resistance in S. habrochaites may be due to a novel Tm-1 allele and the accumulated viruses may have been resistance-breaking mutants. To test this hypothesis, we extracted RNA from six plants that showed delayed accumulation of CP (three from PI390516, one from PI390517, and two from PI390518), performed RT-PCR to amplify the helicase domain-coding region of ToMV, and sequenced. The sequences obtained from four plants had the same mutation at the key residue to overcome the Tm-1-mediated resistance (G3006 in LT1 to A). The 3006th nucleotide of the isolates from the other two plants remained as G, but we identified a mutation in another residue that was also important to break Tm-1 (G3360 to T). Both G3006-to-A and G3360-to-T mutations cause amino acid substitutions (Glu979 in LT1 to Lys and Asp1097 to Tyr, respectively) (Figure 1). These findings strongly suggest that the observed LT1 resistance in S. habrochaites was conferred by an unidentified Tm-1 allele.
A single amino acid substitution in the Tm-1 protein confers the ability to inhibit LT1 multiplication
Based on the above results, we sequenced Tm-1 cDNA isolated from three LT1-resistant plants from different accessions (PI251304, PI365904, and PI365906). Deduced amino acid sequences of the Tm-1 proteins of these plants showed differences from that of Tm-1GCR237 at several residues (Figure S2), among which three residues were common in the three LT1-resistant plants (Ile91, Leu408, and Asn452 of Tm-1GCR237 were changed to Thr, Phe, and Asp, respectively). Because Ile91 resides within the positively selected region, we speculated that the I91T substitution might be important for the LT1 resistance by Tm-1. We further sequenced Ile91-encompassing Tm-1 cDNA fragments from an additional five LT1-resistant plants (PI247087, PI251303, PI390516, PI390517, and PI390518) and confirmed that they encode Thr at position 91.
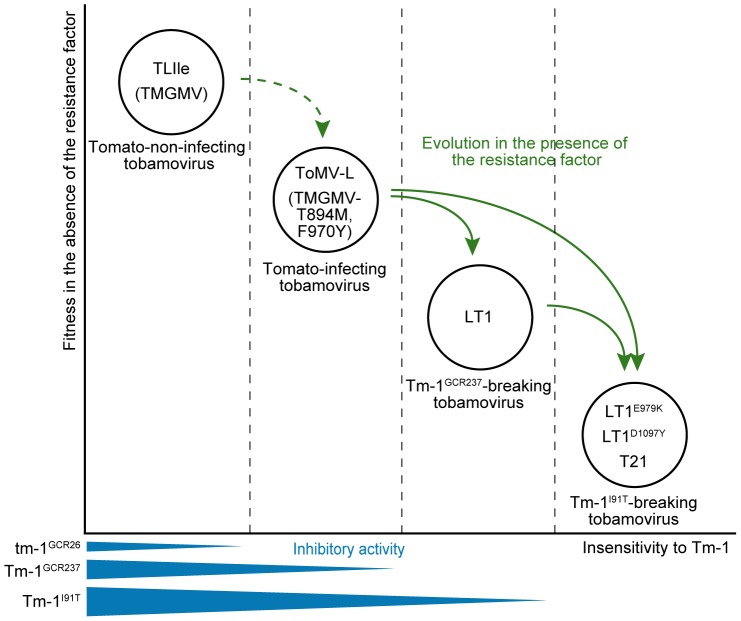
To determine whether the Thr residue at position 91 is important for LT1 resistance, we prepared transgenic tobacco BY-2 cell lines, which constitutively expressed tm-1GCR26 protein, Tm-1GCR237 protein, or Tm-1 protein with the I91T substitution (Tm-1I91T). ToMV-LT1 cDNA was also mutagenized to encode an E979K (LT1E979K) or D1097Y (LT1D1097Y) substitution in the replication proteins to determine whether these mutations are responsible for overcoming the resistance by Tm-1I91T (Figure 1). A ToMV-L mutant that has the same mutations as another Tm-1-resistance-breaking mutant (ToMV1-2) [28] was also constructed and named T21 (Figure 1). Protoplasts isolated from the transgenic BY-2 cells expressing tm-1GCR26, Tm-1GCR237, or Tm-1I91T, or non-transgenic BY-2 cells were inoculated with TLIle, ToMV-L, LT1, T21, LT1E979K, or LT1D1097Y RNA by electroporation, or mock-inoculated, and CP accumulation was analyzed at 20 hours postinoculation (hpi). In non-transgenic BY-2 cells, the CP of these viruses accumulated to similar levels (Figure 4). In tm-1GCR26-expressing cells, multiplication of TLIle was inhibited (Figure 4). In Tm-1GCR237-expressing cells, multiplication of TLIle and ToMV-L was inhibited (Figure 4). In Tm-1I91T-expressing cells, multiplication of TLIle, ToMV-L, and LT1 was inhibited (Figure 4). Multiplication of T21, LT1E979K, and LT1D1097Y was not inhibited by any of the Tm-1 variants (Figure 4). These results indicate that the I91T substitution in the Tm-1 protein confers the ability to inhibit the multiplication of LT1, while LT1E979K and LT1D1097Y emerged in LT1-resistant S. habrochaites plants by escaping from the I91T-type Tm-1 alleles. Remarkably, sensitivity of ToMV mutants to Tm-1 variants was hierarchical; a virus that was unable to overcome tm-1GCR26 was also unable to overcome Tm-1GCR237 and Tm-1I91T, and viruses that were unable to overcome Tm-1GCR237 were also unable to overcome Tm-1I91T.
Figure 4. Tm-1I91T inhibits the multiplication of LT1, but not LT1E979K or LT1D1097Y.
Protoplasts isolated from transgenic BY-2 cells expressing tm-1GCR26, Tm-1GCR237, or Tm-1I91T, or non-transgenic BY-2 cells were inoculated with TLIle, ToMV-L, LT1, T21, LT1E979K, or LT1D1097Y by electroporation. At 20 hpi, protoplasts were harvested and coat protein (CP) accumulation was analyzed by SDS-PAGE and Coomassie blue staining.
Binding of ToMV mutant replication proteins with Tm-1 variants and inhibition of in vitro RNA replication
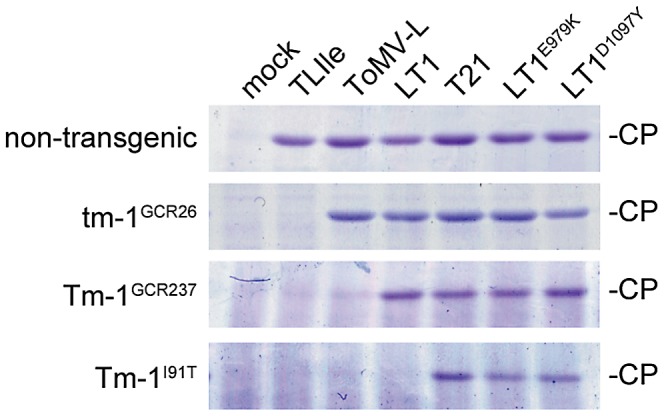
Tm-1 inhibits ToMV RNA replication by binding to the replication proteins [15]. Therefore, we examined the ability of Tm-1I91T to bind LT1 replication proteins. FLAG-tagged tm-1GCR26, Tm-1GCR237, and Tm-1I91T proteins were synthesized by in vitro translation using evacuolated tobacco BY-2 protoplast extracts from which membranes were removed by centrifugation (membrane-depleted BYL: mdBYL). The translation mixtures were mixed with mdBYL, in which TLIle, ToMV-L, LT1, T21, LT1E979K, or LT1D1097Y RNA was translated or mock-translated, and immunoprecipitation using anti-FLAG antibody-conjugated agarose was performed. As expected, the LT1, TLIle, and ToMV-L replication proteins coprecipitated with Tm-1I91T-FLAG, while the LT1E979K or LT1D1097Y replication proteins did not (Figure 5). Also, the replication proteins of ToMV mutants whose multiplication was inhibited coprecipitated with the Tm-1 variants (Figure 5).
Figure 5. Tm-1I91T binds LT1 replication proteins, but not LT1E979K or LT1D1097Y.
The genomic RNA of TLIle, ToMV-L, LT1, T21, LT1E979K, or LT1D1097Y were translated in mdBYL; mixed with mdBYL in which tm-1GCR26-FLAG, Tm-1GCR237-FLAG, or Tm-1I91T-FLAG mRNA were translated; and immunoprecipitated using anti-FLAG antibody-conjugated agarose. Mock-translation was performed as controls and indicated as no viral RNA or no FLAG RNA. Protein samples before (Input) or after (IP: anti-FLAG) FLAG purification were analyzed by Western blotting using anti-130K protein or anti-FLAG antibodies. Positions of the replication proteins (130K and 180K proteins) and FLAG-tagged tm-1GCR26, Tm-1GCR237, or Tm-1I91T proteins are indicated.
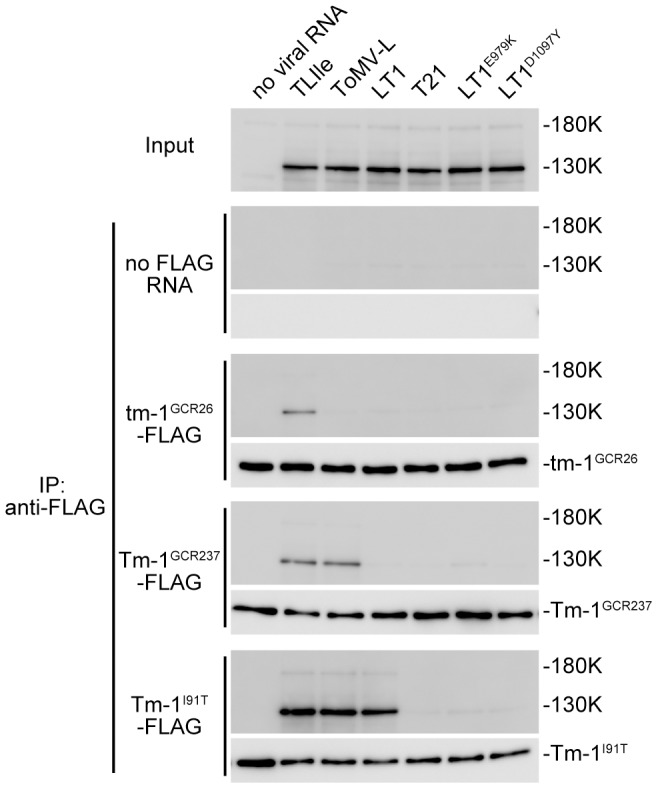
We further analyzed the inhibitory effect of Tm-1 proteins on ToMV RNA replication using an in vitro ToMV RNA replication system [37], [38]. Briefly, ToMV RNA was translated in mdBYL. Tm-1 proteins were separately synthesized by in vitro translation with mdBYL, mixed with ToMV RNA-translated mdBYL, and incubated with BYL membranes and α-32P-labeled ribonucleoside triphosphates, followed by analysis of 32P-labeled RNA. Using this assay, we observed inhibition of RNA replication of the ToMV derivatives in a pattern consistent with the results of the protoplast experiment (Figure 6). Note that the inhibitory effect of tm-1GCR26 to TLIle RNA replication in vitro is weak [30]. Moreover, the in vitro experiment showed that (i) the inhibitory effect of Tm-1 variants is dose-dependent, (ii) TLIle is more sensitive to Tm-1GCR237 than ToMV-L (Figure 6, lanes 5–7), (iii) ToMV-L is more sensitive to Tm-1I91T than LT1 (Figure 6, lanes 8–10), (iv) Tm-1GCR237 and Tm-1I91T inhibit TLIle RNA replication more strongly than tm-1GCR26, and (v) Tm-1I91T inhibits ToMV-L RNA replication more strongly than Tm-1GCR237 (Figure 6). These results suggest that I91T substitution in the Tm-1 protein strengthens its inhibitory activity enough to inhibit LT1 RNA replication, thus extending the antiviral spectrum.
Figure 6. Inhibition of in vitro RNA replication of ToMV mutants by Tm-1 variants.
The genomic RNA of TLIle, ToMV-L, LT1, T21, LT1E979K, or LT1D1097Y and the mRNA for tm-1GCR26, Tm-1GCR237, or Tm-1I91T proteins were translated in mdBYL. The translation mixtures of the Tm-1 variants were mixed with the viral RNA-translated mixtures, followed by RNA replication reaction as described in the Materials and Methods section. The amount of added Tm-1 mRNA were approximately 9 (lanes 2, 5, 8), 42 (lanes 3, 6, 9), or 126 (lanes 4, 7, 10) times as much as viral RNA on a molar basis. Mock-translated mixture was added as a control (lane 1). The positions of the genomic RNA (G) and the replicative form RNA (RF) are indicated. Asterisks represent the background signals.
Fitness costs for ToMV to overcome resistance by Tm-1 alleles
The observed hierarchical ToMV–Tm-1 interactions predict that LT1 or other resistance-breaking mutants should have emerged and dominated Tm-1-sensitive viruses in nature. However, many field isolates of ToMV from tomato are Tm-1GCR237-sensitive, although resistance by this gene was broken within a year of its introduction to commercial tomato cultivars in 1960s [39]. Thus, resistance-breaking mutants may have lower fitness than the wild-type in the absence of Tm-1. In fact, previous studies reported that a series of spontaneously isolated or nitrous acid-induced ToMV mutants capable of overcoming Tm-1 (but not a field isolate) multiplied to lower levels and caused milder symptoms than wild-type virus in nonresistant tm-1 tomato [40]. Also, a TMGMV mutant that can overcome resistance by tm-1GCR26 (TMGMV-T894M,F970Y) had a compromised ability to suppress RNA silencing, an antiviral defense system of plants [41].
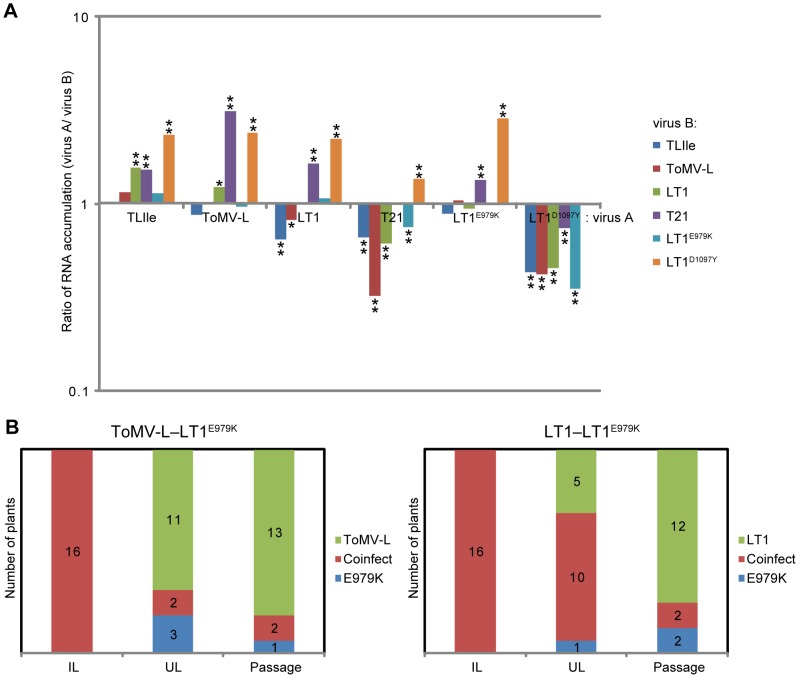
Although CP accumulation levels were not significantly different among the ToMV derivatives when they were individually inoculated into non-transgenic BY2 protoplasts (Figure 4), we examined the relative fitness between the ToMV derivatives by co-inoculation of BY2 protoplasts with a 1∶1 mixture of two ToMV derivative RNAs. As we had six ToMV derivatives, 15 combinations were tested. As a control, individual derivative RNAs were separately inoculated and the protoplasts were cocultured. At 20 hpi, RNA was extracted from the protoplasts and RT-PCR-amplified cDNA fragments of progeny viruses were sequenced by GS-FLX titanium (Roche, Basel, Switzerland). The ratio of the two strains in the progeny of the co-inoculation experiment was normalized to the control (individual) infection, and dominance by one of the two strains was examined using a chi-square test (Table S1). LT1D1097Y was less competitive than the other five variants, as was T21 (excluding LT1D1097Y) (Figure 7A). Having amino acid substitutions at the same residue (D1097V for T21 and D1097Y for LT1D1097Y; Figure 1), the replication proteins of LT1D1097Y and T21 would be disadvantageous with regard to multiplication within protoplasts, probably replicating the viral RNA. Similarly, LT1 RNA accumulation was lower than TLIle or ToMV-L RNA when co-inoculated (Figure 7A). Thus, LT1 is less competitive than ToMV-L and TLIle in the absence of Tm-1.
Figure 7. Fitness costs to the ToMV mutants in the absence of Tm-1.
(A) Competition of two ToMV derivatives in BY-2 protoplasts. Protoplasts isolated from non-transgenic BY-2 cells were co-inoculated with two of the six ToMV derivatives used in this study. As a control, individual derivatives were separately inoculated and the protoplasts were cocultured. At 20 hpi, RNA was extracted, and amplified cDNA was sequenced using the GS-FLX titanium. Ratios of the viral count (virus A/virus B) normalized to the respective control experiment (individual infection) are shown. *: p<0.05, **: p<0.01 based on a chi-square test for the ratio of the two derivatives in the coinfection experiment against the ratio expected from the control experiment. A result of each competition is represented twice so that each virus A histogram shows the results of competition against all the other derivatives (virus B). (B) Competition of LT1E979K with ToMV-L or LT1 in tomato plants. Mixtures of viral RNA were mechanically inoculated onto the leaves of 16 tomato (GCR26: tm-1) plants. RNA was extracted from the inoculated leaves (IL) and upper non-inoculated leaves (UL) at 10 and 42 dpi, respectively. At least four young leaflets of each co-inoculated plant at 46 dpi were homogenized; each homogenate was inoculated onto a healthy plant, and RNA was extracted from upper non-inoculated leaves at 42 dpi (Passage). RT-PCR-amplified cDNA fragments were directly sequenced and the numbers of plants accumulating either both or one of the co-inoculated derivatives are shown.
In contrast, LT1E979K RNA accumulated to levels similar to those of TLIle, ToMV-L, or LT1 when co-inoculated (Figure 7A), suggesting that LT1E979K is not at a disadvantage in protoplasts. Thus, we performed co-inoculation experiments of LT1E979K with ToMV-L or LT1 to 16 tomato plants (cv. Craigella GCR26; tm-1/tm-1). RNAs were purified from the inoculated leaves at 10 dpi and from upper non-inoculated leaves at 42 dpi. RT-PCR-amplified viral cDNA fragments were sequenced to determine whether both of the inoculated strains accumulated or if one strain was eliminated. In the inoculated leaves, all plants accumulated both of the co-inoculated strains (Figure 7B). In upper non-inoculated leaves, only two (for ToMV-L–LT1E979K) and 10 (for LT1–LT1E979K) co-inoculated plants showed coinfection, while the remainder of the plants accumulated only one of the strains (11∶3 for ToMV-L∶LT1E979K, 5∶1 for LT1∶LT1E979K) (Figure 7B). Next, viruses in the young leaves of these 16 co-inoculated plants were reinoculated to uninfected plants. After the passage, LT1E979K was eliminated in 13 or 12 of 16 inoculated plants in competition with ToMV-L or LT1, respectively (Figure 7B). From the results, we estimated relative fitness values for ToMV-L and LT1 against LT1E979K as 4.31±0.05 and 2.43±0.07, respectively (for details see Text S1 and Figure S3). Thus, the replication proteins of LT1E979K are likely to have a compromised function required for virus spread in plants. Taken together, the ability to overcome Tm-1GCR237 or Tm-1I91T by ToMV is accompanied by pleiotropic fitness costs, i.e., impaired RNA replication in single cells or virus spread in plants.
Discussion
Since viruses cannot multiply without a host cell, they evolve under selective pressure imposed by their hosts. In contrast, little evidence exists that wild plants coevolved with viruses [31]. A notable exception is the eukaryotic translation initiation factor (eIF) 4E gene in Capsicum annuum and other plants [42]–[44]. For potyviruses, a successful interaction between the viral protein VPg and eIF4E is required for virus multiplication, and disruption of this interaction results in resistance. Thus, mutations in the eIF4E gene that affect the interaction with VPg confer recessive resistance to the corresponding potyviruses. The loci encoding eIF4E are known to be under diversifying selection and each virus evolves so that VPg can bind to eIF4E in the corresponding host [42]. The presence of multiple eIF4E alleles generated by diversifying selection may effectively protect plant populations from potyvirus infection, since viruses that have adapted to a host that harbors an eIF4E allele often lose infectivity to plants with other alleles. In contrast, no information is currently available regarding how dominant virus resistance genes evolve against viruses. Products of dominant resistance genes interact, whether directly or indirectly, with viral factors (avirulence factors) for resistance. Resistance-breaking virus mutants emerge by mutations that escape the inhibitory interaction with the resistance factor. Even if mutations occur in dominant resistance loci to generate diversified alleles, most of the alleles would not be useful to counter escaped viruses since gaining the ability to interact with new factor is much more difficult than losing an established interaction. Therefore, diversification may not be equally effective for the evolution of dominant resistance genes as for recessive resistance genes.
In this study, we found that a small region (residues 79–112) of the dominant resistance gene Tm-1 has been under positive selection in S. habrochaites (Figure 2). The positively selected region is important to inhibit ToMV RNA replication [15], and an amino acid substitution in this region (I91T) extends the antiviral spectrum (Figure 4). In addition, the amino acid sequences under positive selection were grouped into two groups corresponding to ToMV resistance phenotypes (Figure 3). These observations suggest that infection by tobamoviruses served as a selective pressure during S. habrochaites evolution. Although little information regarding ToMV strains infecting wild S. habrochaites population is currently available, the results of the experimental evolution analyses suggest that ToMV easily evolves to escape from the inhibition by Tm-1 alleles. Thus, ToMV and the resistance gene Tm-1 have likely coevolved.
We demonstrated that interactions between ToMV mutants and Tm-1 variants are hierarchical. The hierarchical classification may also apply to other tobamoviruses; the multiplication of tm-1GCR26-sensitive TMGMV and PMMoV are also inhibited by Tm-1GCR237 [30], and a TMGMV mutant that can replicate in the presence of tm-1GCR26 (TMGMV-T894M,F970Y) cannot overcome the resistance by Tm-1GCR237 (K.I. and M.I., unpublished result). Thus, wild-type TMGMV and TMGMV-T894M,F970Y are categorized into the TLIle class and ToMV-L class, respectively (Figure 8). Based on these considerations and the results of in vitro RNA replication inhibition by Tm-1 variants, we suggest that the relative strengths of binding to the replication proteins and inhibition of RNA replication by each Tm-1 protein variant decreases in the order of TLIle, TMGMV>ToMV-L, TMGMV-T894M,F970Y>LT1>T21, LT1E979K, and LT1D1097Y (Figure 8). Additionally, for each ToMV variant, the binding strengths to the replication proteins and inhibition of RNA replication by Tm-1 variants decrease in the order of Tm-1I91T>Tm-1GCR237>tm-1GCR26 (Figure 8). Under selective pressure by tobamoviruses, Tm-1 may have modified the strength of its inhibitory activity, but not diversified the recognition spectra.
Figure 8. Model of the hierarchical interactions between ToMV and Tm-1.
The horizontal axis indicates the insensitivity (i.e., weakness of binding to) of the tobamovirus replication proteins to Tm-1. The vertical axis indicates fitness of tobamoviruses in Tm-1-lacking hosts. The dashed lines represent thresholds that determine whether viruses can infect plants harboring tm-1GCR26, Tm-1GCR237, or Tm-1I91T. Schematic representation of the inhibitory activities of tm-1GCR26, Tm-1GCR237, and Tm-1I91T are shown at the bottom. Whether ToMV-L evolved from a tm-1-sensitive prototype remains unknown, although TMGMV evolved to TMGMV-T894M,F970Y in the presence of tm-1GCR26 with apparent fitness costs [30], [41].
Currently, two modes of pathogen–host coevolution have been proposed: an ‘arms race’ model in which short-lived alleles are repeatedly fixed in both pathogens and hosts, and a ‘trench warfare’ model in which balanced polymorphisms in relevant genes are maintained [45]–[47]. With regard to the ToMV resistance, the only known function of the Tm-1 gene to date, the resistant alleles in S. habrochaites would be more beneficial than susceptible ones and thus the susceptible alleles could be eliminated in the ‘arms race’ model. However, in this study, we found that 45 of 149 S. habrochaites plants from 24 accessions permit efficient ToMV-L multiplication (Table 1), and the positively selected region of 19 of the 48 sequences of Tm-1 cDNA have identical or very similar sequences to those of ToMV-L-susceptible plants (Figure S1). Although some biases may have resulted from the seed collection and propagation processes, ToMV-susceptible S. habrochaites plants should exist to some extent in nature. Considering that the region under positive selection of the putative ToMV-L-susceptible Tm-1 alleles have very low amino acid sequence diversity (Figures 3 and S1), the alleles may also be adaptive and maintained by balancing selection. Possible driving forces of balancing selection includes costs of resistance; i.e., the ToMV-L susceptible allele may be beneficial in particular situations regarding the original function of Tm-1 or show resistance to other (tobamo)viruses. On the other hand, for ToMV, overcoming Tm-1 resistance was associated with fitness costs (Figure 7), which may help avoid fixation of the resistance-breaking mutations in the ToMV population, especially when the viruses frequently infect ToMV-L-susceptible S. habrochaites subpopulations. Taken together, ToMV and Tm-1 may have been under a coevolutionary process following a trench warfare-like model.
The above speculation predicts that Tm-1-sensitive (ToMV-L class) and resistance-breaking (LT1 class or higher hierarchy) ToMV strains should coexist in nature. Thus, even if S. habrochaites evolves a new resistance allele that inhibits multiplication of resistance-breaking strains, it may not be very beneficial unless it maintains the ability to inhibit lower hierarchy strains. This may explain, at least in part, why Tm-1 appears to have evolved to strengthen its inhibitory activity but does not produce diversified alleles that have different antiviral spectra. Such an evolutionary process of a resistance gene and subsequent viral escape would result in hierarchical interactions. Similar hierarchical interactions were observed between tobamoviruses and the L gene alleles of pepper [18]. The L gene recognizes the CP of tobamoviruses and elicits defense reactions [24]–[26]. In addition, a recent report showed that the ability of tobamoviruses to overcome L alleles is associated with high fitness costs [48]. Thus, regardless of the mechanisms of action, coevolutionary processes that we proposed above for ToMV and Tm-1 may often occur between viruses and the corresponding dominant resistance genes.
To conclude, we would like to discuss from a practical view. In the in vitro system, increased amounts of Tm-1 protein enhance the inhibition of ToMV RNA replication (Figure 6). In Tm-1/tm-1 heterozygous plants, resistance-breaking ToMV mutants emerge more frequently than in Tm-1/Tm-1 homozygous plants [36], indicating that the intracellular level of the Tm-1 protein influences durability. In addition, Tm-1I91T inhibits ToMV RNA replication more strongly than Tm-1GCR237 (Figure 6). Results of the competition assay suggest that a correlation exists between the level of RNA replication inhibition by Tm-1 and fitness costs (Figure 7), i.e., a higher quality and/or quantity of the Tm-1 protein are associated with increased fitness costs for ToMV to overcome the resistance (Figure 8). Thus, one effective strategy to create durable or sustainable tobamovirus-resistant crops would be to identify stronger Tm-1 alleles from genetic resources or create such alleles by mutagenesis and subsequent overexpression of these genes.
Materials and Methods
Viruses
For inoculation into S. habrochaites, crude leaf homogenates (50 mg of leaf tissues in 1 ml of 5 mM sodium phosphate buffer, pH 7) of ToMV-L [49] or LT1 [20] infectious transcript-inoculated tomato were mechanically inoculated onto the first or second true leaves. The infectious cDNA clone of TLIle [50] was provided by Dr. Yuichiro Watanabe (the University of Tokyo), those of LT1E979K and LT1D1097Y were created by site-directed mutagenesis, and that of T21 was created by replacing the region encompassing the mutation sites of pTLW3 [49] with the RT-PCR-amplified fragment from the genome of L11A237, a ToMV mutant capable of overcoming Tm-1 (MAFF260005, obtained from the NIAS Genebank), which has the same mutations as ToMV1-2 [28] (Figure 1). In vitro transcripts synthesized from the infectious clones using an AmpliCap T7 High Yield Message Maker kit (CELLSCRIPT, Inc., Madison, WI) were used for electroporation, in vitro translation/replication, and co-inoculation onto GCR26 leaves.
Plants
The seeds of S. habrochaites accessions were obtained from GRIN. Plants were grown at 24°C under a 16-h light/8-h dark cycle. Tobacco BY-2 cells were grown, maintained, and transformed as described previously [51], [52]. For transformation of BY-2 cells, tm-1GCR26-FLAG, Tm-1GCR237-FLAG [15], and Tm-1I91T-FLAG (created by site-directed mutagenesis from Tm-1GCR237) cDNA were cloned into the binary vector pBI121.
Analysis of Tm-1 cDNA of S. habrochaites
RT-PCR was performed using RNA extracted from leaves of a randomly chosen individual of each S. habrochaites accession as a template with the following primers: 5′-tccattttgaaatctcgattgtaaca-3′ and 5′-taaagaaagaggtgaagaccataca-3′. The amplified fragments were sequenced directly as well as after cloning to obtain two sequences from a diploid individual. Some plants showed no polymorphisms in the coding region, which we assumed to be homozygous. Accession numbers of the sequences that were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases are AB713134–AB713181. Obtained sequences were analyzed by PERMUTE in the OMEGAMAP package [33] to examine a correlation between distance and linkage disequilibrium, and by OMEGAMAP [33] to detect natural selection in the presence of recombination. OMEGAMAP analysis was conducted using 10 randomly chosen orderings of the haplotypes and the following priors: μ = Improper inverse, κ = Improper inverse, φ = Improper inverse. For ω, we used inverse distribution with a range of 0.001–100 and set the average length of blocks for ω at 30 codons. For ρ, we used inverse distribution with a range of 0.001–100 and set the average length of blocks for ρ at 80 codons. The inverse distribution corresponds to a uniform distribution on the log scale. We assumed that all codons have equal frequencies. Two independent Markov chain Monte Carlo chains were run for 500,000 iterations, with a 25,000 iteration burn-in. Upon convergence the two chains were merged to infer ω. Tajima's D was calculated by DNAsp ver. 5.1 [53] using 120-bp window slides in steps of 30 bp. Although seed propagation processes of each accession would reduce the genetic diversity of the population and could affect the analyses, we considered this effect to be negligible since several alleles from different accessions have identical or very similar sequences, and we sequenced the Tm-1 cDNA of only one individual from each accession. The positively selected region in the Tm-1 cDNA of five S. habrochaites plants that did not accumulate ToMV-L CP (i.e., resistant plants) and five plants that accumulate ToMV-L CP (i.e., susceptible plants) were sequenced as described above. Each of these plants had a single sequence in this region as shown in Figure 3.
Protoplast experiments
Isolation of protoplasts from tobacco BY-2 cells followed by electroporation of viral RNA and preparation of mdBYL was performed essentially as described previously [38], [51], [54]. For detection of CP, approximately 5×105 protoplasts were inoculated with 2 µg of ToMV genomic RNA. CP accumulation at 20 hpi was examined by SDS-PAGE followed by Coomassie blue staining. For the competition assay, approximately 5×105 protoplasts were inoculated with mixtures of genomic RNA (3 µg each) from two ToMV variants or 6 µg of a single variant. The protoplasts inoculated with a single variant were mixed with those with another variant and cocultured for 20 hours. RNA was extracted from one-tenth of the inoculated protoplasts and the cDNA fragments encompassing the mutation sites were amplified by RT-PCR. Sequencing of the amplified cDNA fragment using GS-FLX titanium was performed by Takara Bio Inc. (Shiga, Japan).
Immunoprecipitation
Immunoprecipitation analysis of FLAG-tagged Tm-1 variants was performed essentially as described previously [30]. The protein samples were analyzed by Western blotting using anti-ToMV replication protein antibody [52] and anti-FLAG antibody (Sigma, St. Louis, MO).
In vitro translation and replication reactions of viral RNA
Messenger RNAs for tm-1GCR26, Tm-1GCR237, or Tm-1I91T were synthesized from the plasmids harboring the corresponding cDNAs [15] using the mScript mRNA Production System (CELLSCRIPT, Inc.). The messenger RNAs (64 fmol/µl of reaction mixture) or tobamovirus RNA (7.12 fmol/µl of reaction mixture) were translated in mdBYL-based translation mixtures [38], [51] at 23°C for 1 hour. Tobamovirus RNA-translated mixtures (1 µl) were mixed with a mock-translated mixture (14 µl), translation mixture for Tm-1 variants (1 µl) plus mock-translated mixture (13 µl), translation mixture for Tm-1 variants (4.67 µl) plus mock-translated mixture (9.33 µl), or 14 µl of translation mixture for Tm-1 variants, and incubated at 23°C for 20 minutes. The mixtures were further incubated with 5 µl of P30 BYL (membrane fraction of BYL) at 15°C for 2 hours, followed by incubation with 5 µl of ribonucleoside triphosphate mixture containing [α-32P]CTP [51] at 23°C for 1 hour. The reaction was terminated by phenol extraction, and the RNA products were purified and analyzed by electrophoresis in an 8 M urea–2.4% polyacrylamide gel and autoradiography.
Supporting Information
Amino acid sequences under positive selection in the Tm-1 protein of S. habrochaites . 48 amino acid sequences of the Tm-1 protein from 24 S. habrochaites accessions were aligned. The positively selected region (79–112) is indicated. Identical amino acid residues to those of Tm-1GCR237 are indicated by dots. a and b indicate two sequences obtained from a single plant. The same sequence is represented twice as both a and b when the plant had no sequence heterogeneity in the indicated region.
(TIF)
Amino acid sequence alignments of the Tm-1 protein from LT1-resistant S. habrochaites . Deduced amino acid sequences of the Tm-1 protein from three S. habrochaites plant individuals showing the LT1-resistant phenotypes (PI251304, PI365904, PI365906), GCR237 (LT1-susceptible but ToMV-L-resistant), and GCR26 (susceptible to both ToMV-L and LT1) are compared. Common changes in LT1-resistant S. habrochaites are highlighted.
(TIF)
Estimation of relative fitness of a virus variant to the other co-inoculated virus variant in plants. A model developed for estimation of relative fitness of a virus variant to a co-inoculated virus is schematically shown. The ratios of exclusive infections by one of the two variants and coinfection by the two variants were calculated by this model using different parameter sets, and were compared with the frequencies of exclusive infections and coinfections that were experimentally observed to estimate most likely parameter values for r, λ 1, λ 2, and λ 3. The most-likely estimates and their standard errors or standard deviations are also shown in a table. See Text S1 for detailed procedures.
(TIF)
Pyrosequencing examinations of the proportion of viral strains accumulated in co-inoculated protoplasts.
(DOCX)
Estimation of relative fitness of ToMV derivatives in co-inoculated tomato plants.
(DOCX)
Acknowledgments
We thank GRIN for providing the S. habrochaites seeds, Dr. K. Hirai and Dr. Y. Watanabe for the plasmids encoding T21 and TLIle cDNA, respectively, Dr. A. Arakawa for helpful suggestions, and members of our laboratory for productive discussions.
Funding Statement
This study was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) to M.I. and by a grant from PRESTO of JST to S.M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ruiz-Ferrer V, Voinnet O (2009) Roles of Plant Small RNAs in Biotic Stress Responses. Annu Rev Plant Biol 60: 485–510. [DOI] [PubMed] [Google Scholar]
- 2. Csorba T, Pantaleo V, Burgyán J (2009) RNA silencing: an antiviral mechanism. Adv Virus Res 75: 35–71. [DOI] [PubMed] [Google Scholar]
- 3. Kang B-C, Yeam I, Jahn MM (2005) Genetics of Plant Virus Resistance. Annu Rev Phytopathol 43: 581–621. [DOI] [PubMed] [Google Scholar]
- 4. Maule AJ, Caranta C, Boulton MI (2007) Sources of natural resistance to plant viruses: status and prospects. Mol Plant Pathol 8: 223–231. [DOI] [PubMed] [Google Scholar]
- 5. Gómez P, Rodríguez-Hernández AM, Moury B, Aranda MA (2009) Genetic resistance for the sustainable control of plant virus diseases: breeding, mechanisms and durability. Eur J Plant Pathol 125: 1–22. [Google Scholar]
- 6. Ishikawa M, Meshi T, Motoyoshi F, Takamatsu N, Okada Y (1986) In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res 14: 8291–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishibashi K, Nishikiori M, Ishikawa M (2010) Interactions between tobamovirus replication proteins and cellular factors: their impacts on virus multiplication. Mol Plant Microbe Interact 23: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 8. Kurihara Y, Inaba N, Kutsuna N, Takeda A, Tagami Y, et al. (2007) Binding of tobamovirus replication protein with small RNA duplexes. J Gen Virol 88: 2347–2352. [DOI] [PubMed] [Google Scholar]
- 9. Mérai Z, Kerényi Z, Kertész S, Magna M, Lakatos L, et al. (2006) Double-Stranded RNA Binding May Be a General Plant RNA Viral Strategy To Suppress RNA Silencing. J Virol 80: 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Csorba T, Bovi A, Dalmay T, Burgyán J (2007) The p122 Subunit of Tobacco Mosaic Virus Replicase Is a Potent Silencing Suppressor and Compromises both Small Interfering RNA- and MicroRNA-Mediated Pathways. J Virol 81: 11768–11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deom CM, Oliver MJ, Beachy RN (1987) The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science 237: 389–394. [DOI] [PubMed] [Google Scholar]
- 12. Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, et al. (1987) Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J 6: 2557–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takamatsu N, Ishikawa M, Meshi T, Okada Y (1987) Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J 6: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, et al. (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78: 1101–1115. [DOI] [PubMed] [Google Scholar]
- 15. Ishibashi K, Masuda K, Naito S, Meshi T, Ishikawa M (2007) An inhibitor of viral RNA replication is encoded by a plant resistance gene. Proc Natl Acad Sci U S A 104: 13833–13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanfermeijer FC, Dijkhuis J, Sturre MJ, de Haan P, Hille J (2003) Cloning and characterization of the durable tomato mosaic virus resistance gene Tm-22 from Lycopersicon esculentum . Plant Mol Biol 52: 1037–1049. [DOI] [PubMed] [Google Scholar]
- 17. Lanfermeijer FC, Warmink J, Hille J (2005) The products of the broken Tm-2 and the durable Tm-22 resistance genes from tomato differ in four amino acids. J Exp Bot 56: 2925–2933. [DOI] [PubMed] [Google Scholar]
- 18. Tomita R, Sekine K-T, Mizumoto H, Sakamoto M, Murai J, et al. (2011) Genetic Basis for the Hierarchical Interaction Between Tobamovirus spp. and L Resistance Gene Alleles from Different Pepper Species. Mol Plant Microbe Interact 24: 108–117. [DOI] [PubMed] [Google Scholar]
- 19. Padgett HS, Beachy RN (1993) Analysis of a Tobacco Mosaic Virus Strain Capable of Overcoming N Gene-Mediated Resistance. Plant Cell 5: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meshi T, Motoyoshi F, Adachi A, Watanabe Y, Takamatsu N, et al. (1988) Two concomitant base substitutions in the putative replicase genes of tobacco mosaic virus confer the ability to overcome the effects of a tomato resistance gene, Tm-1 . EMBO J 7: 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padgett HS, Watanabe Y, Beachy RN (1997) Identification of the TMV Replicase Sequence That Activates the N Gene-Mediated Hypersensitive Response. Mol Plant Microbe Interact 10: 709–715. [Google Scholar]
- 22. Meshi T, Motoyoshi F, Maeda T, Yoshiwoka S, Watanabe H, et al. (1989) Mutations in the Tobacco Mosaic Virus 30-kD Protein Gene Overcome Tm-2 Resistance in Tomato. Plant Cell 1: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weber H, Schultze S, Pfitzner AJP (1993) Two amino acid substitutions in the tomato mosaic virus 30-kilodalton movement protein confer the ability to overcome the Tm-22 resistance gene in the tomato. J Virol 67: 6432–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berzal-Herranz A, De La Cruz A, Tenllado F, Díaz-Ruíz JR, López L, et al. (1995) The Capsicum L3 gene-mediated resistance against the tobamoviruses is elicited by the coat protein. Virology 209: 498–505. [DOI] [PubMed] [Google Scholar]
- 25. De La Cruz A, López L, Tenllado F, Díaz-Ruíz J, Sanz A, et al. (1997) The coat protein is required for the elicitation of the Capsicum L2 gene-mediated resistance against the tobamoviruses. Mol Plant Microbe Interact 10: 107–113. [DOI] [PubMed] [Google Scholar]
- 26. Gilardi P, García-Luque I, Serra MT (1998) Pepper mild mottle virus coat protein alone can elicit the Capsicum spp. L 3 gene-mediated resistance. Mol Plant Microbe Interact 11: 1253–1257. [Google Scholar]
- 27. Pelham J (1966) Resistance in tomato to tobacco mosaic virus. Euphytica 15: 258–267. [Google Scholar]
- 28. Strasser M, Pfitzner AJP (2007) The double-resistance-breaking Tomato mosaic virus strain ToMV1-2 contains two independent single resistance-breaking domains. Arch Virol 152: 903–914. [DOI] [PubMed] [Google Scholar]
- 29. Nishikiori M, Sugiyama S, Xiang H, Niiyama M, Ishibashi K, et al. (2012) Crystal Structure of the Superfamily 1 Helicase from Tomato Mosaic Virus. J Virol 86: 7565–7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishibashi K, Naito S, Meshi T, Ishikawa M (2009) An inhibitory interaction between viral and cellular proteins underlies the resistance of tomato to nonadapted tobamoviruses. Proc Natl Acad Sci U S A 106: 8778–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fraile A, García-Arenal F (2010) The Coevolution of Plants and Viruses: Resistance and Pathogenicity. Adv Virus Res 76: 1–32. [DOI] [PubMed] [Google Scholar]
- 32. Elena SF, Lenski RE (2003) Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat Rev Genet 4: 457–469. [DOI] [PubMed] [Google Scholar]
- 33. Wilson DJ, McVean G (2006) Estimating diversifying selection and functional constraint in the presence of recombination. Genetics 172: 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, et al. (2011) CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 39: D225–D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tajima F (1989) Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fraser RSS, Loughlin SAR (1980) Resistance to Tobacco Mosaic Virus in Tomato: Effects of the Tm-1 Gene on Virus Multiplication. J Gen Virol 48: 87–96. [Google Scholar]
- 37. Komoda K, Naito S, Ishikawa M (2004) Replication of plant RNA virus genomes in a cell-free extract of evacuolated plant protoplasts. Proc Natl Acad Sci U S A 101: 1863–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Komoda K, Mawatari N, Hagiwara-Komoda Y, Naito S, Ishikawa M (2007) Identification of a Ribonucleoprotein Intermediate of Tomato Mosaic Virus RNA Replication Complex Formation. J Virol 81: 2584–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pelham J, Fletcher JT, Hawkins JH (1970) The establishment of a new strain of tobacco mosaic virus resulting from the use of resistant varieties of tomato. Ann Appl Biol 65: 293–297. [Google Scholar]
- 40.Fraser RSS, Gerwitz A (1987) The genetics of resistance and virulence in plant virus disease. In: Day PR, Jellis GJ, editors. Genetics and Plant Pathogenesis. Oxford: Blackwell. pp. 33–44.
- 41. Ishibashi K, Meshi T, Ishikawa M (2011) Gaining Replicability in a Nonhost Compromises the Silencing Suppression Activity of Tobacco Mild Green Mosaic Virus in a Host. J Virol 85: 1893–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charron C, Nicolaï M, Gallois J-L, Robaglia C, Moury B, et al. (2008) Natural variation and functional analyses provide evidence for co-evolution between plant eIF4E and potyviral VPg. Plant J 54: 56–68. [DOI] [PubMed] [Google Scholar]
- 43. Cavatorta JR, Savage AE, Yeam I, Gray SM, Jahn MM (2008) Positive Darwinian Selection at Single Amino Acid Sites Conferring Plant Virus Resistance. J Mol Evol 67: 551–559. [DOI] [PubMed] [Google Scholar]
- 44. Hofinger BJ, Russell JR, Bass CG, Baldwin T, Dos Reis M, et al. (2011) An exceptionally high nucleotide and haplotype diversity and a signature of positive selection for the eIF4E resistance gene in barley are revealed by allele mining and phylogenetic analyses of natural populations. Mol Ecol 20: 3653–3668. [DOI] [PubMed] [Google Scholar]
- 45. Salvaudon L, Giraud T, Shykoff JA (2008) Genetic diversity in natural populations: a fundamental component of plant–microbe interactions. Curr Opin Plant Biol 11: 135–143. [DOI] [PubMed] [Google Scholar]
- 46. Brown JKM, Tellier A (2011) Plant-parasite coevolution: bridging the gap between genetics and ecology. Annu Rev Phytopathol 49: 345–367. [DOI] [PubMed] [Google Scholar]
- 47. Bergelson J, Dwyer G, Emerson J (2001) Models and data on plant-enemy coevolution. Annu Rev Genet 35: 469–499. [DOI] [PubMed] [Google Scholar]
- 48. Fraile A, Pagán I, Anastasio G, Sáez E, García-Arenal F (2011) Rapid Genetic Diversification and High Fitness Penalties Associated with Pathogenicity Evolution in a Plant Virus. Mol Biol Evol 28: 1425–1437. [DOI] [PubMed] [Google Scholar]
- 49. Kubota K, Tsuda S, Tamai A, Meshi T (2003) Tomato Mosaic Virus Replication Protein Suppresses Virus-Targeted Posttranscriptional Gene Silencing. J Virol 77: 11016–11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamamoto H, Watanabe Y, Kamada H, Okada Y (1997) A Single Amino Acid Substitution in the Virus-Encoded Replicase of Tomato Mosaic Tobamovirus Alters Host Specificity. Mol Plant Microbe Interact 10: 1015–1018. [Google Scholar]
- 51.Ishibashi K, Komoda K, Ishikawa M (2006) In Vitro Translation and Replication of Tobamovirus RNA in a Cell-Free Extract of Evacuolated Tobacco BY-2 Protoplasts. In: Nagata T, Matsuoka K, Inzé D, editors. Tobacco BY-2 Cells: From Cellular Dynamics to Omics: Springer Berlin Heidelberg. pp. 183–194.
- 52. Hagiwara Y, Komoda K, Yamanaka T, Tamai A, Meshi T, et al. (2003) Subcellular localization of host and viral proteins associated with tobamovirus RNA replication. EMBO J 22: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 54. Watanabe Y, Meshi T, Okada Y (1987) Infection of tobacco protoplasts with in vitro transcribed tobacco mosaic virus RNA using an improved electroporation method. FEBS Lett 219: 65–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid sequences under positive selection in the Tm-1 protein of S. habrochaites . 48 amino acid sequences of the Tm-1 protein from 24 S. habrochaites accessions were aligned. The positively selected region (79–112) is indicated. Identical amino acid residues to those of Tm-1GCR237 are indicated by dots. a and b indicate two sequences obtained from a single plant. The same sequence is represented twice as both a and b when the plant had no sequence heterogeneity in the indicated region.
(TIF)
Amino acid sequence alignments of the Tm-1 protein from LT1-resistant S. habrochaites . Deduced amino acid sequences of the Tm-1 protein from three S. habrochaites plant individuals showing the LT1-resistant phenotypes (PI251304, PI365904, PI365906), GCR237 (LT1-susceptible but ToMV-L-resistant), and GCR26 (susceptible to both ToMV-L and LT1) are compared. Common changes in LT1-resistant S. habrochaites are highlighted.
(TIF)
Estimation of relative fitness of a virus variant to the other co-inoculated virus variant in plants. A model developed for estimation of relative fitness of a virus variant to a co-inoculated virus is schematically shown. The ratios of exclusive infections by one of the two variants and coinfection by the two variants were calculated by this model using different parameter sets, and were compared with the frequencies of exclusive infections and coinfections that were experimentally observed to estimate most likely parameter values for r, λ 1, λ 2, and λ 3. The most-likely estimates and their standard errors or standard deviations are also shown in a table. See Text S1 for detailed procedures.
(TIF)
Pyrosequencing examinations of the proportion of viral strains accumulated in co-inoculated protoplasts.
(DOCX)
Estimation of relative fitness of ToMV derivatives in co-inoculated tomato plants.
(DOCX)