Abstract
Phosporothioate oligonucleotides represent an important class of therapeutic oligonucleotides, in which none-bridging oxygen atoms of the phosphate groups are replaced by sulfur. These oligonucleotides are designed to treat disease by modulating gene expression of an affected individual. As the development and application of these therapeutical oligonucleotides require analytical support, the development, validation, and application of an assay for the quantitative analysis of a phosporothioate oligonucleotide in rat plasma is described. The method employs ion-pair reversed-phase chromatography on a monolithic capillary column with acetonitrile gradients in cyclohexyldimethylammonium acetate for separation and high-resolution tandem mass spectrometry for detection of nucleic acids. Chromatographic parameters (i.e. column temperature, mobile phase composition) as well as mass spectrometric parameters (i.e. spray voltage, gas flow, and capillary position, scan mode) have been optimized for sensitive oligonucleotide quantification. Furthermore, a solid-phase extraction method was developed which enabled processing of 10 μl of plasma. The five-point calibration curve showed linearity over the range of concentrations from 100 to 1,000 nM of the oligonucleotide. The limit of detection was 50 nM. The intra- and inter-day precision and accuracies were always better than 10.2 %. Using this assay, we performed a pharmacokinetic study of the phosporothioate oligonucleotide in rat treated with a single intravenous dose of 0.39 μmol/kg. The assay sensitivity was sufficient to study the early phase elimination of the oligonucleotide. Small amounts of the oligonucleotide were detectable up to 3 h after dosing.
KEY WORDS: liquid chromatography, mass spectrometry, pharmacokinetic, therapeutic oligonucleotide
INTRODUCTION
Therapeutic oligonucleotides are short nucleic acid molecules designed to treat disease by modulating gene expression of an affected individual (1). Currently, there are two DNA therapeutics approved by the US Food and Drug Administration. Fomivirsen is an antisense oligonucleotide for the treatment of cytomegalovirus (2). Pegaptanib is an aptamer for the treatment of neo-vascular age-related macular degeneration (3). Many more oligonucleotide therapeutics have been or are currently being used in human clinical trials to treat a wide range of diseases, including cancer, infectious disease, neurodegenerative disorders, and cardiovascular disorders (4).
Virtually all methods available to manipulate gene expression rely on some type of nucleotide sequence recognition for targeting specificity, but differ as to where and how they perturb the flow of genetic information (1,5). Anti-gene as well as anti-mRNA strategies have been developed. Despite considerable success of the anti-gene approaches, anti-mRNA approaches are more attractive, because mRNA, unlike the DNA of a given gene is accessible to attack while being transcribed, processed, transported, or translated. A commonly applied anti-mRNA strategy is the antisense strategy, which is based on hybridization of the mRNA of the targeted gene with a reverse complementary nucleic acid therapeutic. Stable mRNA–antisense duplexes can interfere with splicing, block translation, or lead to the destruction of the mRNA by binding of endogenous nucleases.
Therapeutic oligonucleotides are routinely modified to improve bioavailability, to enhance stability against degradation by exonucleases and endonucleases, and to increase the binding affinity to the targeted mRNA molecule (6,7). Backbone-modified oligonucleotides are referred as antisense agents of the first generation. The substitution of an oxygen of the phosphate by sulfur is an easy way to improve performance. Such oligonucleotides are well absorbed from parenteral sites, distribute broadly to all peripheral tissues, do not cross the blood–brain barrier, and are eliminated primarily by slow metabolism. Most of the antisense oligonucleotides currently tested in various phases of clinical trials belong to this group of molecules. More recently introduced modifications include 2′-O-Me modification, 2′-F-substitution, methylphosphonate modification, locked nucleic acids, as well as peptide nucleic acids.
For successful drug development and application, reliable quantitative methods are obligatory that enable purity testing of drug products, therapeutic drug monitoring as well as the determination of pharmacokinetic and pharmacodynamic parameters. Commonly applied techniques for qualitative and quantitative analysis of therapeutic oligonucleotides include immunoassays, quantitative polymerase chain reaction, electrophoresis, liquid chromatography (LC), mass spectrometry (MS), and combinations thereof (4,8–13). Particularly immunoassays provide very low detection limits. These methods, however, often cannot distinguish full-length oligonucleotides from their metabolites giving rise to overestimation of the parent drug. LC/MS techniques provide the most accurate and most thorough characterization of oligonucleotides particularly useful for metabolite profiling. Several methods for oligonucleotide quantitation in biological matrixes using LC/MS with solid-phase extraction (SPE) or phenol/chloroform liquid–liquid extraction have been reported in literature (14–21). Most of the reported assays encountered problems either with MS (e.g. multiple charging, cation adduction), with LC (e.g. oligonucleotide affinity to exposed silica, low retention, low separation efficiency, carryover effects) or with sample preparation (e.g. analyte oxidation, high protein binding, nonspecific binding of analyte to containers). Although some of these problems have already been addressed and solved, the development of a reliable and robust LC/MS method for the quantitative analysis of oligonucleotides still seems to be more of an art than routine work.
We herein report on the development, validation, and application of a LC/MS method for the quantification of a phosphorothioate oligonucleotide in rat plasma. The core technology represents a well-established LC/MS assay dedicated to the characterization of genetic variability which uses ion-pair reversed-phase chromatography on a monolithic capillary column with acetonitrile (ACN) gradients in cyclohexyldimethylammonium acetate (CycHDMAA) for separation and electrospray ionization (ESI) time-of-flight MS for detection and characterization of nucleic acids (22–24). Development steps taken to adapt the method for oligonucleotide quantification will be described. Impact of chromatographic as well as mass spectrometric parameters on separation efficiency and detection sensitivity will be discussed. Furthermore, an extraction method will be presented that allows processing of only 10 μl of plasma enabling the application of the assay for pharmacokinetic studies of a model phosphorothioate oligonucleotide in rats.
EXPERIMENTAL SECTION
Reagents
ACN (HPLC gradient grade) was obtained from Fisher Scientific (Wholen, Switzerland). Ascorbic acid was obtained from Sigma Aldrich (St. Louis, MO, USA). A 1.0 M stock solution of CycHDMAA was prepared by titration of cyclohexyldimethylamine (CycHDMA, Fluka, Buchs, Switzerland) with acetic acid (Fluka) at 5 °C until pH 8.4 was reached. A 1.0 M stock solution of triethylammonium acetate (TEAA) was prepared by titration of triethylamine (Fluka) with acetic acid (Fluka) at 5 °C until pH 8.4 was reached. For preparation of all solutions, HPLC grade water (Sigma Aldrich) was used. The following oligonucleotides were purchased from Microsynth (Balgach, Switzerland): a 24-mer deoxythymidine oligonucleotide ((dT)24), 16-mer phosphorothioate oligonucleotide (PTO) with the sequence 5′-CTG ATA AGA TTT TCA T-3′. The PTO has been used in previous studies to test thiolated particles as an adjuvant for the nasal delivery of antisense oligonucleotides (25,26).
Pharmacokinetic Study in Rats
The protocol for the in vivo studies on animals was approved by the Animal Ethical Committee of Vienna (Austria) and adheres to the Principles of Laboratory Animal Care. In vivo studies were performed on male Sprague–Dawley rats weighing 200–250 g. Three rats were used. Rats were fasted 2 h before administration and then for additional 2 h during the experiments but had free access to water. Intravenous solutions of the PTO (dose, 0.39 μmol/kg) were injected into a tail vein. About 150 μl of blood samples was taken via the tail vein at 0 (predose), 1, 15, 45, 90, 180 min after the administration of the oligonucleotide. After centrifugation, plasma was collected and stored at −20 °C till further analysis.
Preparation of Standard and Quality Control Samples
For calibrators, stock solutions containing 528 μM of the PTO or 250 μM of (dT)24 were prepared in water and stored at −20 °C until use.
The working standard of PTO (10 μM) was prepared daily by independent dilutions of the stock solutions with water. The working solution of (dT)24 was prepared by diluting the stock solution with water to a final concentration of 10 μM. Calibration samples were prepared by spiking 10 μl of pooled plasma with 4.0 μl (dT)24 working solution and aliquots of the working calibrator solutions (100, 250, 500, 750, 1,000 nM).
Quality control (QC) samples were prepared in a similar manner starting with independently prepared stock solutions. Ten micro-liter of pooled plasma samples (1.0 ml) were spiked with 4.0 μl (dT)24 working solution as well as aliquots of the PTO working solution to obtain concentration levels of 250 and 750 nM. QC samples were stored at −20 °C until use.
Sample Preparation
Before use plasma samples were allowed to equilibrate to room temperature. Ten micro-liter of plasma sample was mixed with 11 μl of water and 25 μl of 1 M TEAA. To each sample, 4.0 μl (dT)24 working solution was added yielding a final concentration of 4.0 μM. Next, plasma samples were vortexed and extracted. SPE was accomplished on a reversed-phase polymeric sorbent (Oasis HLB μElution Plate, 2 mg sorbent, 30 μm particle size, Waters, Milford, MA, USA). Prior to use, cartridges were conditioned with 1 ml ACN and equilibrated with 1 ml 0.1 M TEAA. After loading, columns were washed with 0.5 ml 0.1 M TEAA. Columns were eluted with 100 μl of water/ACN (1/1). To reduce the ACN content in the eluates, the volume was reduced to 40 μl using a stream of nitrogen. Samples were immediately analyzed after the addition of 10 μl 5 mM aqueous ascorbic acid solution.
Liquid Chromatography-Mass Spectrometry
A fully integrated capillary HPLC system (Ultimate system, LC-Packings, Amsterdam, the Netherlands) was used for all chromatographic experiments. A Famos microautosampler (LC-Packings) equipped with a 2 μl loop was used for sample injection. The 50 × 0.2 mm inner diameter (i.d.) monolithic capillary column was prepared according to the published protocol (27). The flow rate was set to 2.0 μl/min. Column temperatures of 70 °C were applied. Mobile phases for chromatographic separation were prepared by diluting the stock solution of CycHDMAA to 1–50 mM with water and ACN. Different amounts of ascorbic acid (0–2.5 mM) were added to the mobile phase and the sample solvent. After injection, the column was washed with a 1–50 mM aqueous solution of CycHDMAA containing 5 % ACN for 3.0 min. Separation of the oligonucleotides was accomplished with a gradient of 5–95 % ACN in 1–50 mM CycHDMAA within 7–10 min. The eluting nucleic acids were detected online by ESI-MS, which was performed on a quadrupole–quadrupole–time-of-flight instrument (QSTAR XL, AB Sciex, Foster City, CA, USA) equipped with a modified TurboIonSpray source (22,28). Optimization of instrumental parameters and mass calibration were performed in the negative ion mode by infusion of 5 μM solutions of (dT)24 or the PTO in 25 mM aqueous CycHDMAA containing 50 % ACN at a flow rate of 2.0 μL/min. Cations present in the oligonucleotide solutions were removed by on-line cation-exchange using a 20 × 0.50 mm i.d. cation-exchange microcolumn packed with 38–75 μm Dowex 50 WX8 particles (Serva, Heidelberg, Germany) (29). The spray voltage was set to 4.0 kV. Gas flows of 5 arbitrary units (nebulizer gas) and 35 arbitrary units (turbo gas) were employed. The temperature of the turbo gas was adjusted to 200 °C. The accumulation time was set to 1 s. Mass spectra in MS mode were typically recorded in the range between 900 and 2,000. For selected ion monitoring (SIM) experiments, the 4-times negatively charged ion of (dT)24 and the 3-times negatively charged ion of the PTO were scanned. For tandem mass spectrometric experiments, the 4-times negatively charged ion of (dT)24 and the 3-times negatively charged ion of the PTO were selected. The collision gas (N2) flow was set to 5 arbitrary units, and collision voltages of −60 V for (dT)24 and −40 V for the PTO were applied. Full tandem mass spectra were recorded in the range between 100 and 2,500. Selected reaction monitoring (SRM) was performed using the precursor-to-product ion transitions 1,808 > 625 for (dT)24 and 1,702 > 722 for the PTO. Chromatograms and mass spectra were recorded on a personal computer operating with the Analyst QS software (1.0, service pack 8 and Bioanalyst extension, AB Sciex).
Method Validation
The following validation parameters were evaluated for the developed LC/MS/MS method: selectivity, linearity, accuracy and precision, limit of quantification, recovery and matrix effects, and carryover.
Selectivity was evaluated by analyzing independent blank plasma samples. To demonstrate the lack of response in blank matrix, plasma samples from three donors were processed with and without the addition of (dT)24.
Calibration was evaluated by analyzing three replicates of spiked plasma samples representing 100, 250, 500, 750, 1,000 nM solutions of the PTO. The obtained data were used to build a calibration curve by taking the peak area ratio for the PTO to (dT)24versus the concentration of the sample. An un-weighted linear, least squares regression model was chosen to fit the calibration curve with the accuracy (bias) and precision data in the required acceptances limits (bias: ±15 % and ±20 % near the lower limit of quantification (LLOQ); precision: 15 % and 20 % near the LLOQ).
Accuracy and precision of the method were assessed by analyzing QC samples (250 and 750 nM PTO) on five consecutive days. The accuracy was determined by calculating the relative error. For acceptance QC should fall within ±15 % of nominal LLOQ. The inter-day precision was specified as relative standard deviations (RSD). For acceptance, RSD values should not exceed 15 % LLOQ.
The quantitative assessment of matrix effects (ME) and extraction recoveries (ER) was performed according to the method proposed by Matuszewski et al. (30). The concentration of the PTO was 1,000 nM; the concentration of (dT)24 was 4.0 μM. Samples included non-matrix-prepared samples, post-extraction spiked samples, and pre-extraction spiked samples. In each case, three replicates were analyzed. ER were determined by comparing pre-extraction spiked samples and post-extraction spiked samples. ME were tested by comparing post-extraction spiked samples and non-matrix-prepared samples.
For evaluation of carryover effects, the calibrator with the highest concentration and a blank sample were analyzed in two consecutive LC/MS/MS runs. For acceptance, the peak areas of the blank sample should not exceed 20 % of the peak areas obtained at the LLOQ.
RESULTS AND DISCUSSION
Quality Control of the PTO
Phosporothioate oligonucleotides are synthetic oligonucleotides, in which one none-bridging oxygen atom is replaced by sulfur. They are usually produced with a modified phosphoramidite method of solid-phase synthesis (31,32). During every cycle of synthesis the oxidation of the internucleotide O-Me phosphite is replaced by sulfurization. The sulfurization step can be programmed into automated solid-phase oligonucleotide synthesizers allowing the synthesis of therapeutic oligonucleotides from nanomole to millimole scale.
Efficiency of automated solid-phase synthesis of oligodeoxynucleotide sequences is usually excellent. Nevertheless, contamination of the target sequence with truncated sequences, partially deprotected sequences as well as partially desulfurized species can be observed (28,33–35). Therefore, prior to use, we decided to check the quality of the purchased oligonucleotide with MS. In Fig. 1, mass spectra obtained from quality control experiments of two different batches of synthesis are shown. In both cases, signals corresponding to the 3-times negatively charged molecular ion of the target sequence were detected. Due to the high resolution of the time-of-flight mass analyzer (m/∆m > 15,000, m is the measured mass and ∆m the full peak width at half-maximum), isotopic patterns were resolved. In the first batch, a considerable amount of side-products were detected (Fig. 1a). These species represented partially desulfurized oligonucleotides, which are commonly observed impurities of synthetic phosporothioate oligonucleotides (32,36). The other batch hardly contained any desulfurization product (Fig. 1b) and was, therefore, used as sample for the in vivo experiment and as standard for quantitative analysis, respectively.
Fig. 1.
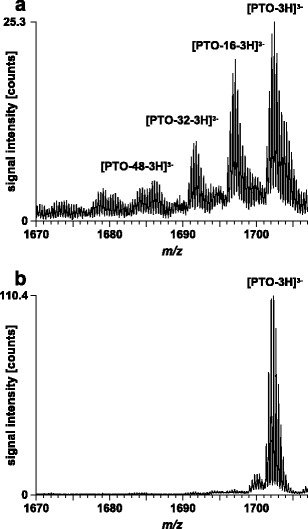
Quality control of two PTO batches by direct infusion ESI-MS. Solvent, 25 mM CycHDMAA, pH 8.4, 50 % ACN; flow rate, 2.0 μl/min; scan mode, MS; acquisition time, 1.0 min; sample, PTO at a concentration of 20 μM
Method Development
For quantitative analysis of the PTO in rat plasma an LC/MS method was developed. Liquid chromatography was used to purify and fractionate the nucleic acids. LC is particularly useful to remove cations, which is a prime requisite to obtain mass spectra of high quality from nucleic acids. MS is used as sensitive and specific detector. Depending on the scan mode applied, nucleotide composition and/or sequence information can be obtained allowing the characterization of targeted as well as altered nucleic acid molecules. Thus, in the context of therapeutic oligonucleotide analysis MS can be applied for drug monitoring and metabolite identification.
In the experimental setup developed, ion-pair reversed-phase chromatography was applied for chromatographic separation. Monolithic capillary columns (50 × 0.2 mm i.d.) comprising a single piece of a continous styrene-divinylbenzene copolymer were used as stationary phase. These chromatographic columns are known to exhibit high mechanical and chemical stability as well as excellent chromatographic performance (27,37,38). The capillary format is particularly useful to improve the sensitivity of the LC/MS method. Thus, by keeping the injected volume constant, lower limits of detection in terms of sample concentration can be reached. Theoretically, chromatographic columns with i.d. of 200 μm offer a gain of detection sensitivity of 40,000 in comparison to classical 4 mm columns. CycHDMAA was selected as ion-pair reagent (23). CycHDMA exhibits a comparably higher affinity to the stationary phase than butyldimethylamine or triethylamine. Thus, with CycHDMAA a significant higher content of ACN in the mobile phase is necessary to elute nucleic acids from the chromatographic column. This is advantageous because the higher content of organic solvent can improve desolvation efficiency during ESI leading to improved ionization efficiency and improved detection sensitivity (39,40). Other chromatographic parameters were optimized during method development.
Chromatographic separations were performed at 70 °C because elevated temperatures give rise to improved separation efficiency and have a positive effect on desalting efficiency (41). Phosphorothioate oligonucleotides can undergo partial desulfurization getting in contact with oxidizing agents (42–44). To prevent unwanted PTO oxidation, we decided to add the potent antioxidant ascorbic acid to the mobile phase as well as to the sample solution.
The impact of the addition of various amounts of ascorbic acid to the mobile phase on the detectability of different charge states of the PTO is shown in Fig. 2a. Peak areas extracted from selected ion chromatograms were used to compare detection sensitivities. Up to 100 μM ascorbic acid only marginal effects were observed. The peak area of [PTO-3H]3− remained almost constant; the decrease of the [PTO-4H]4− peak area was less than 10 %. Higher ascorbic acid concentrations, however, led to a more then 30 % reduction of peak area and should be avoided. In all cases, the 4-times negatively charged ion was more affected than the 3-times negatively charged ion. The addition of ascorbic acid seems to induce “charge state reduction” (45,46), which partially compensated the negative effect of ascorbic acid addition on ionization efficiency for [PTO-3H]3−. Both effects, charge state reduction and ion suppression, can be explained by a decrease of pH as well as an increase of ionic strength in the mobile phase due to the addition of ascorbic acid.
Fig. 2.
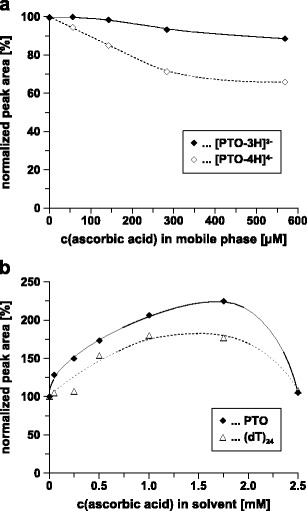
Impact of the addition of ascorbic acid a to the mobile phase and b to the sample solvent on the detectability of oligonucleotides. Column, continuous styrene–divinylbenzene copolymer, 50 × 0.20 mm i.d.; mobile phase, a 25 mM CycHDMAA, pH 8.4, 0–570 μM ascorbic acid, b 25 mM CycHDMAA, pH 8.4, 100 % ACN, 0–570 μM ascorbic acid; linear gradient, 5 % B for 3 min, 5–95 % B in 7 min; flow rate, 2 μl/min; temperature, 70 °C; scan mode, MS; sample, PTO, 2 pmol, or (dT)24, 5 pmol, with 0–2.5 mM ascorbic acid
The impact of the addition of various amounts of ascorbic acid to the sample solvent on detection sensitivity of the PTO and (dT)24 is shown in Fig. 2b. For both oligonucleotides a more than 80 % gain of the peak area was obtained with 1.0–1.75 mM ascorbic acid. Somehow, ascorbic acid seems to “protect” the oligonucleotides during their transfer from the sample vial to the chromatographic column.
The concentration of the ion-pair reagent in the mobile phase was the final parameter of the chromatographic system that was optimized. From the chromatographic point of view, rather high concentrations of an amphiphilic ammonium ion are recommended. Such mobile phases, however, are incompatible with mass spectrometric detection. The most practical way to improve ionization efficiency is based on the reduction of the ion-pair reagent concentration in the solvent down to 10–50 mM (40,41,47). Low-concentration solvents usually allow high sensitive detection at a moderate loss of chromatographic performance. To assess the impact of the CycHDMAA concentration on mass spectrometric detection, solvents containing 1–50 mM of the ion-pair reagent were tested. The results are summarized in Fig. 3. Best performance was obtained with 10 mM CycHDMAA. This observation is in full accordance with results obtained for LC/MS analysis of polymerase chain reaction products (41).
Fig. 3.
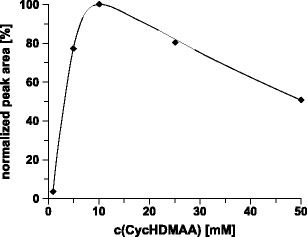
Impact of the CycHDMAA concentration on the detectability of the PTO. Column, continuous styrene-divinylbenzene copolymer, 50 × 0.20 mm i.d.; mobile phase, (A) 1.0–50 mM CycHDMAA, pH 8.4, 100 μM ascorbic acid, (B) 1.0–50 mM CycHDMAA, pH 8.4, 100 % ACN, 100 μM ascorbic acid; linear gradient, 5 % B for 3 min, 5–95 % B in 7 min; flow rate, 2 μl/min; temperature, 70 °C; scan mode, MS; sample, PTO, 2 pmol with 1.0 mM ascorbic acid
A very important parameter for successful mass spectrometric analysis of nucleic acids is tuning of the instrument. Typically, instrumental settings obtained with factory protocols based on the direct infusion of suitable compounds (i.e. caffeine, small peptides, polyethylene glycol) differ significantly from those obtained by fine tuning using oligonucleotides as tuning substances (35,48). For instance, parameters to maintain a stable electrospray (spray voltage, gas flow, and capillary position) strongly depend on the composition of the electrosprayed solvent, and thus, the tuning procedure needs to be performed with the tuning substance dissolved in the mobile phase used later for chromatographic separation. Therefore, we decided to use a 5 μM solution of the PTO in 25 mM aqueous CycHDMAA containing 50 % ACN for instrument tuning. A mass spectrum obtained with optimized settings is depicted in Fig. 4. The 3-times negatively charged ion showed the highest abundance. Besides the PTO, low-abundant signals arising from nucleobase loss as well as CycHDMA adduct formation were detected. Both species represent artifacts produced during the ESI process. Base loss is generated by unintended collision induced decomposition of the oligonucleotide during formation and transfer of the ions to the mass analyzer. The CycHDMA adducts are formed during chromatography. They are usually cleaved in the gas phase by the same processes that are responsible for base loss. Accordingly, instrumental parameters need to be optimized in a way that minimal base loss and maximum decomposition of ammonium ion adducts are observed. With a properly tuned instrument base loss signals as well as adduct signals will exhibit relative intensities of less than 5–10 % and thus will hardly impair detection sensitivity.
Fig. 4.
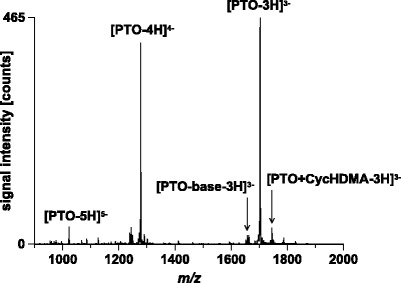
Mass spectrum of the PTO obtained by direct infusion ESI-MS. Solvent, 25 mM CycHDMAA, pH 8.4, 50 % ACN; flow rate, 2.0 μl/min; scan mode, MS; acquisition time, 1.0 min; sample, PTO at a concentration of 5 μM
The scan mode is another mass spectrometric parameter that needs to be carefully selected to ensure high selectivity and low detection limits. Three modes of operation enabled by the quadrupole–quadrupole–time-of-flight instrument were tested. For SIM experiments, the 3-times negatively charged ion of the PTO was scanned. For full scan tandem mass spectrometry (MS/MS) experiments, the 3-times negatively charged ion of the PTO was selected for fragmentation. SRM was performed using the precursor-to-product ion transition 1,702 > 722 for the PTO. Reconstructed ion chromatograms obtained from the analysis of 250 fmol PTO with the three different scan modes are shown in Fig. 5. Best performance in terms of signal intensity as well as signal-to-noise ratio was obtained with the full scan MS/MS mode. A drawback of the full scan MS/MS mode is that all compounds sharing the precursor ion mass-to-charge ratio (m/z) with the PTO will give rise to peaks in the chromatogram. Such a compound was present in the sample analyzed (Fig. 5b). The impurity, however, was distinguishable from the PTO based on the retention time, the exact precursor ion mass as well as the fragmentation pattern. The two compounds did not share the precursor-to-product ion transition 1,702 > 722 used for SRM of the PTO (Fig. 5c). Thus, SRM provided best selectivity. Nevertheless, as full scan MS/MS seems to provide better detection sensitivity than SRM, full scan MS/MS was selected as scan mode for the quantitative assay. If improved selectivity would have been needed to identify an impurity, full scan MS/MS data could be converted into SRM data by extracting ion traces of selected fragment ions.
Fig. 5.
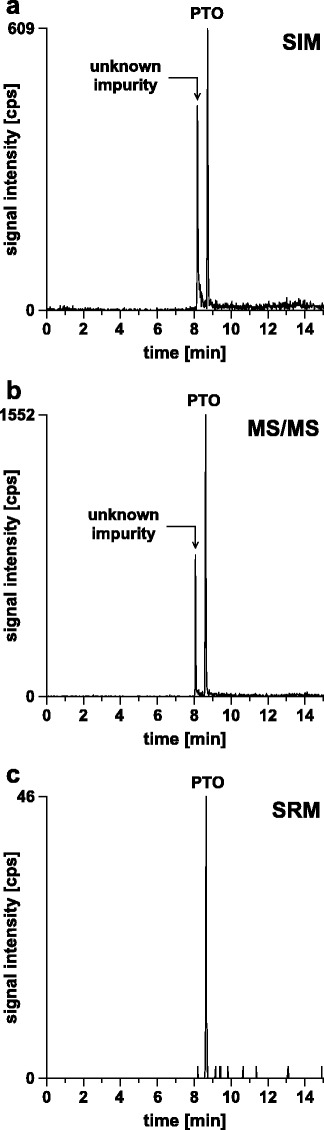
Reconstructed ion chromatograms of the PTO obtained with a SIM, b full scan MS/MS, and c SRM. Column, continuous styrene–divinylbenzene copolymer, 50 × 0.20 mm i.d.; mobile phase, (A) 25 mM CycHDMAA, pH 8.4, 100 μM ascorbic acid, (B) 25 mM CycHDMAA, pH 8.4, 100 % ACN, 100 μM ascorbic acid; linear gradient, 5 % B for 3 min, 5–95 % B in 7 min; flow rate, 2 μl/min; temperature, 70 °C; scan modes, a SIM, b MS/MS, c SRM; sample, PTO, 250 fmol, and (dT)24, 8 pmol
For processing of plasma samples, an SPE method was developed. SPE was accomplished on a reversed-phase polymeric sorbent. Binding of the nucleic acids to the extraction material was accomplished by ion pairing with TEAA. For elution, mixtures of water and ACN (1:1) were used. This solvent was found to provide good recoveries and seems to allow good separation of nucleic acids from more hydrophobic matrix components. We observed severe sample loss by evaporation to dryness (20). Thus, the ACN content was reduced by partial evaporation using a stream of nitrogen to prepare the eluat for injection into the LC/MS system. To evaluate the performance of the extraction method applied, ER and ME were determined.
ER values were assessed by comparing the signal response of post-extraction spiked samples and pre-extraction spiked samples using three different plasma lots. The determined extraction recoveries were 30 % for (dT)24 and 48 % for the PTO. The obtained ER values are below the minimum value of 50 % recommended for quantification of small bioorganic molecules (49). With respect to SPE of oligonucleotides, ER seem to be quite excellent (13).
ME values were determined by comparing the signal response of post-extraction spiked samples and non-matrix-prepared samples. The determined changes of peak areas cause of matrix-related ionization effects were 10 % for (dT)24 and 21 % for the PTO. Obviously, the matrix causes a moderate enhancement of ionization efficiency.
In Fig. 6a, representative reconstructed ion chromatograms of (dT)24 and the PTO obtained from the analysis of a processed plasma sample are depicted. Total run time was 15 min. Resolution of the two species almost down to baseline was achieved. The oligonucleotides eluted at 8.6 and 8.9 min, respectively.
Fig. 6.
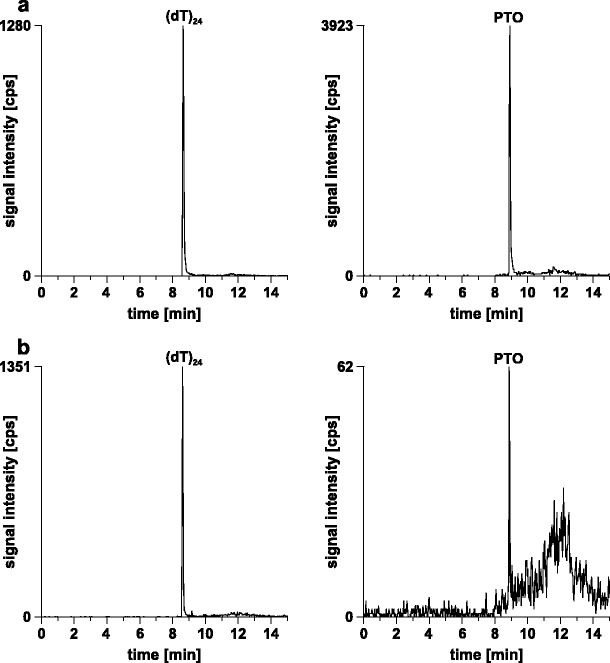
Reconstructed ion chromatograms of two plasma samples processed after the addition of 4 μM (dT)24 as well as a 1,000 nM or b 50 nM PTO, respectively. Column, continuous styrene–divinylbenzene copolymer, 50 × 0.20 mm i.d.; mobile phase, (A) 10 mM CycHDMAA, pH 8.4, 100 μM ascorbic acid, (B) 10 mM CycHDMAA, pH 8.4, 100 % ACN, 100 μM ascorbic acid; linear gradient, 5 % B for 3 min, 5–95 % B in 7 min; flow rate, 2 μl/min; temperature, 70 °C; scan mode, MS/MS; sample, processed plasma samples
Method Validation
To evaluate the usability of the developed LC/MS/MS method for the quantification of the PTO in rat plasma, the following validation parameters were determined: selectivity, linearity, accuracy, and precision, limit of quantification, recovery, and matrix effects (see above), and carryover.
To assess the selectivity of the assay, processed plasma samples from three different sources were surveyed in full scan MS mode and MS/MS mode for interfering peaks from matrix compounds. In both modes, no peaks interfering with the PTO or (dT)24 were detected.
Calibration curves were found to be linear over a concentration range of 100–1,000 nM. The mean correlation coefficient (r2) was >0.99. The average error of back calculated concentration values was comprised in the range of ±8.1 %. The RSD was always smaller than 10.2 %. Accordingly, the lowest calibrator level was defined as LLOQ. The rather narrow dynamic range of the developed LC/MS/MS can be explained by the use of a rather old fashioned time-of-flight instrument (50). Such an instrument uses a detection system that is sensitive to saturation effects. This problem, however, has been overcome recently. Thus, a more modern instrument would have offered increased linearity and sensitivity.
On the basis of a signal-to-noise level above 3:1 (Fig. 6b), the limit of detection (LOD) was found to be 50 nM. Lower LOD values (∼0.5 nM) have been reported for another assay (21). Thus, the developed method seems to be two orders of magnitude less sensitive than the most sensitive assay available. If for instance the differences in processed plasma volume (10 vs. 200 μl) and injection volume (2 vs. 20 μl) are taken into account, then our assay provides nearly the same detection sensitivity as the reported assay. Different strategies can be envisioned to improve the LOD of the presented assay. One strategy would make use of more sensitive mass spectrometric methods. SRM experiments on a triple quadrupole instrument, for instance, are considered to provide lower LOD. Another possible strategy would involve modification of the SPE method applied. In the protocol developed, 10 μl of plasma yielded 50 μl of eluate. As only 2 μl were injected onto the chromatographic column, extended desolvation of the eluate could have been used to increase concentration and to improve detection sensitivity. Furthermore, as 80–100 μl were available it would have been possible to reach lower LOD by increasing the volume of plasma extracted. With the current setup, however, modification of the SPE protocol will result in an undesired shift of the whole calibration range giving rise to a simultaneous decrease of the lower and upper limits of quantification. Accordingly, the implementation of mass spectrometric detection systems that are able to extend the calibration range in both directions would represent the most appropriate approach to increase the capabilities of the developed assay.
Accuracy and precision were assessed by analyzing QC samples at two concentration levels on five consecutive days. The average errors were 3.9 % and −11.3 %, respectively. The inter-day precisions were found to be 5.0 % and 12.2 %, respectively. Accordingly, the method can be rated accurate and precise. Carryover was determined by analyzing a blank sample immediately after the highest concentration level. No carryover was observed.
Pharmacokinetic Study
The developed LC/MS/MS method was applied for quantification of the PTO in three rats after a single intravenous administration of 0.39 μmol/kg. Plasma samples were collected 0 (predose), 1, 15, 45, 90, 180 min after injection of the oligonucleotide. Plasma samples with concentration beyond the upper limit of quantification (1,000 nM) were diluted with the corresponding predose sample to enable accurate quantification. The mean plasma concentration versus time profile is depicted in Fig. 7. A two-compartment model with first order elimination was used to fit the data. The PTO has α and β half-lives of 3.8 and 69 min, respectively. The mean Cmax was found to be 5.6 μM. The area under the curve (AUC0 − ∞) determined by the trapezoidal rule was 110 μM min. The mean total body clearance was 3.5 ml/kg/min. The apparent volume of the central compartment (Vc) was 64.8 ml/kg and the appearant volume in the β-phase (Vβ) was 310 ml/kg. Approximately 180 min post-dose, plasma PTO levels reached the LLOQ of the assay.
Fig. 7.
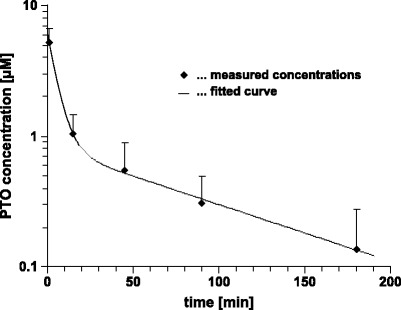
Plasma concentration time profiles of PTO following intravenous administration of 0.39 μmol/kg in rat
The obtained pharmacokinetic data on intravenous administration of the PTO will be of importance in a subsequent study on the use of thiolated particles as an adjuvant for the nasal delivery of antisense oligonucleotides (25,26). The goal of this study will be to assess the permeation-enhancing properties of synthesized particles as well as to determine the nasal bioavailability of the PTO in rats.
Besides quantification of the PTO, we were also interested in identifying its metabolites. For this purpose, a plasma sample collected 15 min after PTO administration was processed without internal standard addition and analyzed by LC/MS (Fig. 8). The obtained mass spectrometric information enabled the identification of five metabolites (3′ N-1 to 3′ N-5) which were produced by subsequent cleavage of nucleotides from the 3′-end of the PTO. Thus, based on these results, metabolism of the PTO mainly involves 3′-exonuclease cleavage, which is consisted with the metabolism of other antisense oligonucleotides (19).
Fig. 8.
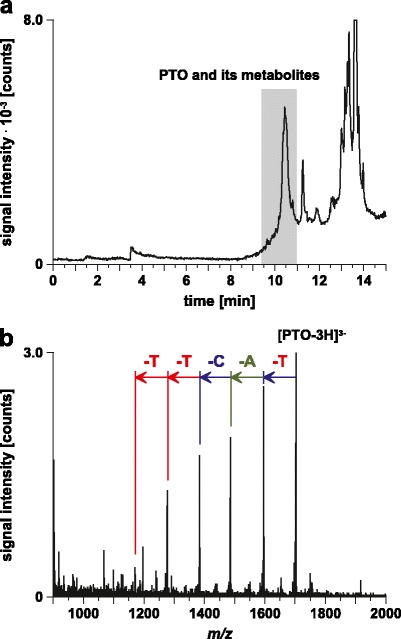
LC/MS analysis of a plasma sample collected 15 min after PTO administration. The plasma sample was processed without the internal standard. Column, continuous styrene-divinylbenzene copolymer, 50 × 0.20 mm i.d.; mobile phase, (A) 10 mM CycHDMAA, pH 8.4, 100 μM ascorbic acid, (B) 10 mM CycHDMAA, pH 8.4, 100 % ACN, 100 μM ascorbic acid; linear gradient, 5 % B for 3 min, 5–95 % B in 10 min; flow rate, 2 μl/min; temperature, 70 °C; scan mode, MS; sample, processed plasma sample
CONCLUSIONS
Due to the offered selectivity, LC/MS is regarded as proper tool for the analysis of therapeutic oligonucleotides. Reports on routine analysis, however, are rare. It seems as if the development of robust, reliable, and sensitive methods for the quantification of oligonucleotides in biological samples is still a challenging task. To provide guidance for proper choice of experimental parameters for extraction, chromatography, and mass spectrometry, individual steps necessary for the successful development of a quantitative LC/MS/MS method have been described herein. The presented setup uses ion-pair reversed-phase chromatography on a monolithic capillary column with ACN gradients in CycHDMAA for separation and high-resolution MS/MS for detection of nucleic acids. The SPE method allowed processing of 10 μl of plasma, which enabled application of the assay to a pharmacokinetic study in rat. The sensitivity was sufficient to study the early phase elimination of the oligonucleotide; small amounts of the oligonucleotide were detectable up to 3 h after dosing.
The developed LC/MS/MS assay as well as the determined pharmacokinetic data will represent integral parts of a study on the use of thiolated particles as an adjuvant for the nasal delivery of antisense oligonucleotides (25,26). Both will be used to assess the permeation-enhancing properties of synthesized particles as well as to determine the nasal bioavailability of the PTO in rats.
ACKNOWLEDGMENTS
This work was supported by a grant of the Austrian Science Fund (FWF): P 22526-B11.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
REFERENCES
- 1.Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–514. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- 2.Perry CM, Balfour JA. Fomivirsen. Drugs. 1999;57:375–380. doi: 10.2165/00003495-199957030-00010. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MA, Keating GM. Pegaptanib: in exudative age-related macular degeneration. Drugs. 2005;65:1571–1577. doi: 10.2165/00003495-200565110-00010. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis AC, Chen B, Bartlett MG. Chromatographic methods for the determination of therapeutic oligonucleotides. J Chromatogr B. 2011. doi:10.1016/j.jchromb.2011.09.007. [DOI] [PubMed]
- 5.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 7.Dean NM, Bennett CF. Antisense oligonucleotide-based therapeutics for cancer. Oncogene. 2003;22:9087–9096. doi: 10.1038/sj.onc.1207231. [DOI] [PubMed] [Google Scholar]
- 8.Tewary HK, Iversen PL. Qualitative and quantitative measurements of oligonucleotides in gene therapy: part II in vivo models. J Pharm Biomed Anal. 1997;15:1127–1135. doi: 10.1016/S0731-7085(97)01942-0. [DOI] [PubMed] [Google Scholar]
- 9.Yu RZ, Geary RS, Levin AA. Application of novel quantitative bioanalytical methods for pharmacokinetic and pharmacokinetic/pharmacodynamic assessments of antisense oligonucleotides. Curr Opin Drug Discov Devel. 2004;7:195–203. [PubMed] [Google Scholar]
- 10.Lin ZJ, Li W, Dai G. Application of LC-MS for quantitative analysis and metabolite identification of therapeutic oligonucleotides. J Pharm Biomed Anal. 2007;44:330–341. doi: 10.1016/j.jpba.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Bartlett MG. Determination of therapeutic oligonucleotides using capillary gel electrophoresis. Biomed Chromatogr. 2011. doi:10.1002/bmc.1696. [DOI] [PubMed]
- 12.Beverly MB. Applications of mass spectrometry to the study of siRNA. Mass Spectrom Rev. 2011;30:979–998. doi: 10.1002/mas.20260. [DOI] [PubMed] [Google Scholar]
- 13.van Dongen WD, Niessen WM. Bioanalytical LC-MS of therapeutic oligonucleotides. Bioanalysis. 2011;3:541–564. doi: 10.4155/bio.11.8. [DOI] [PubMed] [Google Scholar]
- 14.Gaus HJ, Owens SR, Winniman M, Cooper S, Cummins LL. On-line HPLC electrospray mass spectrometry of phosphorothioate oligonucleotide metabolites. Anal Chem. 1997;69:313–319. doi: 10.1021/ac960557q. [DOI] [PubMed] [Google Scholar]
- 15.Griffey RH, Greig MJ, Gaus HJ, Liu K, Monteith D, Winniman M, et al. Characterization of oligonucleotide metabolism in vivo via liquid chromatography/electrospray tandem mass spectrometry with a quadrupole ion trap mass spectrometer. J Mass Spectrom. 1997;32:305–313. doi: 10.1002/(SICI)1096-9888(199703)32:3<305::AID-JMS482>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad A, Khan S, Ahmad I. Mass spectrometry and enzyme-linked immunosorbent assay methods for the quantitation of liposomal antisense oligonucleotide (LE-rafAON) in human plasma. Methods Enzymol. 2004;387:230–241. doi: 10.1016/S0076-6879(04)87014-9. [DOI] [PubMed] [Google Scholar]
- 17.Murphy AT, Brown-Augsburger P, Yu RZ, Geary RS, Thibodeaux S, Ackermann BL. Development of an ion-pair reverse-phase liquid chromatographic/tandem mass spectrometry method for the determination of an 18-mer phosphorothioate oligonucleotide in mouse liver tissue. Eur J Mass Spectrom. 2005;11:209–215. doi: 10.1255/ejms.674. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JL, Guo W, Zang J, Khan S, Bardin S, Ahmad A, et al. Quantification of raf antisense oligonucleotide (rafAON) in biological matrices by LC-MS/MS to support pharmacokinetics of a liposome-entrapped rafAON formulation. Biomed Chromatogr. 2005;19:272–278. doi: 10.1002/bmc.450. [DOI] [PubMed] [Google Scholar]
- 19.Dai G, Wei X, Liu Z, Liu S, Marcucci G, Chan KK. Characterization and quantification of Bcl-2 antisense G3139 and metabolites in plasma and urine by ion-pair reversed phase HPLC coupled with electrospray ion-trap mass spectrometry. J Chromatogr B. 2005;825:201–213. doi: 10.1016/j.jchromb.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Lin J, Srinivasan K, Kavetskaia O, Duncan JN. Strategies for bioanalysis of an oligonucleotide class macromolecule from rat plasma using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:3416–3424. doi: 10.1021/ac0618674. [DOI] [PubMed] [Google Scholar]
- 21.Deng P, Chen X, Zhang G, Zhong D. Bioanalysis of an oligonucleotide and its metabolites by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:571–579. doi: 10.1016/j.jpba.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Oberacher H, Niederstätter H, Casetta B, Parson W. Detection of DNA sequence variations in homo- and heterozygous samples via molecular mass measurements by electrospray ionization time-of-flight mass spectrometry. Anal Chem. 2005;77:4999–5008. doi: 10.1021/ac050399f. [DOI] [PubMed] [Google Scholar]
- 23.Oberacher H, Niederstatter H, Pitterl F, Parson W. Profiling 627 mitochondrial nucleotides via the analysis of a 23-plex polymerase chain reaction by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Anal Chem. 2006;78:7816–7827. doi: 10.1021/ac061210i. [DOI] [PubMed] [Google Scholar]
- 24.Oberacher H, Parson W. Forensic DNA fingerprinting by liquid chromatography-electrospray ionization mass spectrometry. Biotechniques. 2007;43:vii–xiii. doi: 10.2144/000112581. [DOI] [PubMed] [Google Scholar]
- 25.Vetter A, Bernkop-Schnurch A. Nasal delivery of antisense oligonucleotides: in vitro evaluation of a thiomer/glutathione microparticulate delivery system. J Drug Target. 2010;18:303–312. doi: 10.3109/10611860903450031. [DOI] [PubMed] [Google Scholar]
- 26.Vetter A, Martien R, Bernkop-Schnurch A. Thiolated polycarbophil as an adjuvant for permeation enhancement in nasal delivery of antisense oligonucleotides. J Pharm Sci. 2010;99:1427–1439. doi: 10.1002/jps.21887. [DOI] [PubMed] [Google Scholar]
- 27.Premstaller A, Oberacher H, Huber CG. High-performance liquid chromatography-electrospray ionization mass spectrometry of single- and double stranded nucleic acids using monolithic capillary columns. Anal Chem. 2000;72:4386–4393. doi: 10.1021/ac000283d. [DOI] [PubMed] [Google Scholar]
- 28.Oberacher H, Niederstatter H, Parson W. Characterization of synthetic nucleic acids by electrospray ionization quadrupole time-of-flight mass spectrometry. J Mass Spectrom. 2005;40:932–945. doi: 10.1002/jms.870. [DOI] [PubMed] [Google Scholar]
- 29.Huber CG, Buchmeiser MR. On-line cation-exchange for suppression of adduct formation in negative-ion electrospray mass spectrometry of nucleic acids. Anal Chem. 1998;70:5288–5295. doi: 10.1021/ac980791b. [DOI] [PubMed] [Google Scholar]
- 30.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 31.Uhlmann E, Peyman A. Antisense oligonucleotides: a new therapeutic principle. Chem Rev. 1990;90:543–584. doi: 10.1021/cr00102a001. [DOI] [Google Scholar]
- 32.Guga P, Koziolkiewicz M. Phosphorothioate nucleotides and oligonucleotides—recent progress in synthesis and application. Chem Biodivers. 2011;8:1642–1681. doi: 10.1002/cbdv.201100130. [DOI] [PubMed] [Google Scholar]
- 33.Deroussent A, Le Caer J-P, Gouyette A. Electrospray mass spectrometry for the purity of natural and modified oligodeoxynucleotides. Rapid Commun Mass Spectrom. 1995;9:1–4. doi: 10.1002/rcm.1290090102. [DOI] [PubMed] [Google Scholar]
- 34.Gilar M, Fountain KJ, Budman Y, Holyoke JL, Davoudi H, Gebler JC. Characterization of therapeutic oligonucleotides using liquid chromatography with on-line mass spectrometry detection. Oligonucleotides. 2003;13:229–243. doi: 10.1089/154545703322460612. [DOI] [PubMed] [Google Scholar]
- 35.Nikcevic I, Wyrzykiewicz TK, Limbach PA. Detecting low-level synthesis impurities in modified phosphorothioate oligonucleotides using liquid chromatography-high resolution mass spectrometry. Int J Mass Spectrom. 2011;304:98–104. doi: 10.1016/j.ijms.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese CB, Song Q. Avoidance of sulfur loss during ammonia treatment of oligonucleotide phosphorothioates. Nucleic Acids Res. 1997;25:2943–2944. doi: 10.1093/nar/25.14.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberacher H, Huber CG. Capillary monoliths for the analysis of nucleic acids by high-performance liquid chromatography-electrospray ionization mass spectrometry. Trends Anal Chem. 2002;21:166–174. doi: 10.1016/S0165-9936(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 38.Oberacher H, Premstaller A, Huber CG. Characterization of some physical and chromatographic properties of monolithic poly(styrene-co-divinylbenzene) columns. J Chromatogr A. 2004;1030:201–208. doi: 10.1016/j.chroma.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Bleicher K, Bayer E. Various factors influencing the signal intensity of oligonucleotides in electrospray mass spectrometry. Biol Mass Spectrom. 1994;23:320–322. doi: 10.1002/bms.1200230604. [DOI] [PubMed] [Google Scholar]
- 40.Huber CG, Krajete A. Analysis of nucleic acids by capillary ion-pair reversed-phase HPLC coupled to negative ion-electrospray ionization mass spectrometry. Anal Chem. 1999;71:3730–3739. doi: 10.1021/ac990378j. [DOI] [PubMed] [Google Scholar]
- 41.Oberacher H, Parson W, Holzl G, Oefner PJ, Huber CG. Optimized suppression of adducts in polymerase chain reaction products for semi-quantitative SNP genotyping by liquid chromatography-mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1897–1906. doi: 10.1016/j.jasms.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Turney BJ, Cheruvallath ZS, Andrade M, Cole DL, Ravikumar VT. Stability of phosphorothioate oligonucleotides in aqueous ammonia in presence of stainless steel. Nucleosides Nucleotides. 1999;18:89–93. doi: 10.1080/07328319908045596. [DOI] [PubMed] [Google Scholar]
- 43.Kodra JT, Kehler J, Dahl O. Stability of oligodeoxynucleoside phosphorodithioates and phosphorothioates in aqueous ammonia. Nucleic Acids Res. 1995;23:3349–3350. doi: 10.1093/nar/23.16.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krotz AH, Mehta RC, Hardee GE. Peroxide-mediated desulfurization of phosphorothioate oligonucleotides and its prevention. J Pharm Sci. 2005;94:341–352. doi: 10.1002/jps.20235. [DOI] [PubMed] [Google Scholar]
- 45.Cheng X, Gale D, Udseth HR, Smith RD. Charge state reduction of oligonucleotide negative ions from electrospray ionization. Anal Chem. 1995;67:586–593. doi: 10.1021/ac00099a016. [DOI] [Google Scholar]
- 46.Muddiman DC, Cheng X, Udseth HR, Smith RD. Charge-state reduction with improved signal intensity of oligonucleotides in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 1996;7:697–706. doi: 10.1016/1044-0305(96)80516-2. [DOI] [PubMed] [Google Scholar]
- 47.Apffel A, Chakel JA, Fischer S, Lichtenwalter K, Hancock WS. Analysis of oligonucleotides by HPLC-electrospray ionization mass spectrometry. Anal Chem. 1997;69:1320–1325. doi: 10.1021/ac960916h. [DOI] [PubMed] [Google Scholar]
- 48.Oberacher H, Walcher W, Huber CG. Effect of instrument tuning on the detectability of biopolymers in electrospray ionization mass spectrometry. J Mass Spectrom. 2003;38:108–116. doi: 10.1002/jms.419. [DOI] [PubMed] [Google Scholar]
- 49.Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165:216–224. doi: 10.1016/j.forsciint.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Chernushevich IV, Loboda AV, Thomson BA. An introduction to quadrupole-time-of-flight mass spectrometry. J Mass Spectrom. 2001;36:849–865. doi: 10.1002/jms.207. [DOI] [PubMed] [Google Scholar]


