Abstract
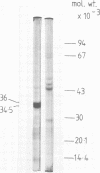
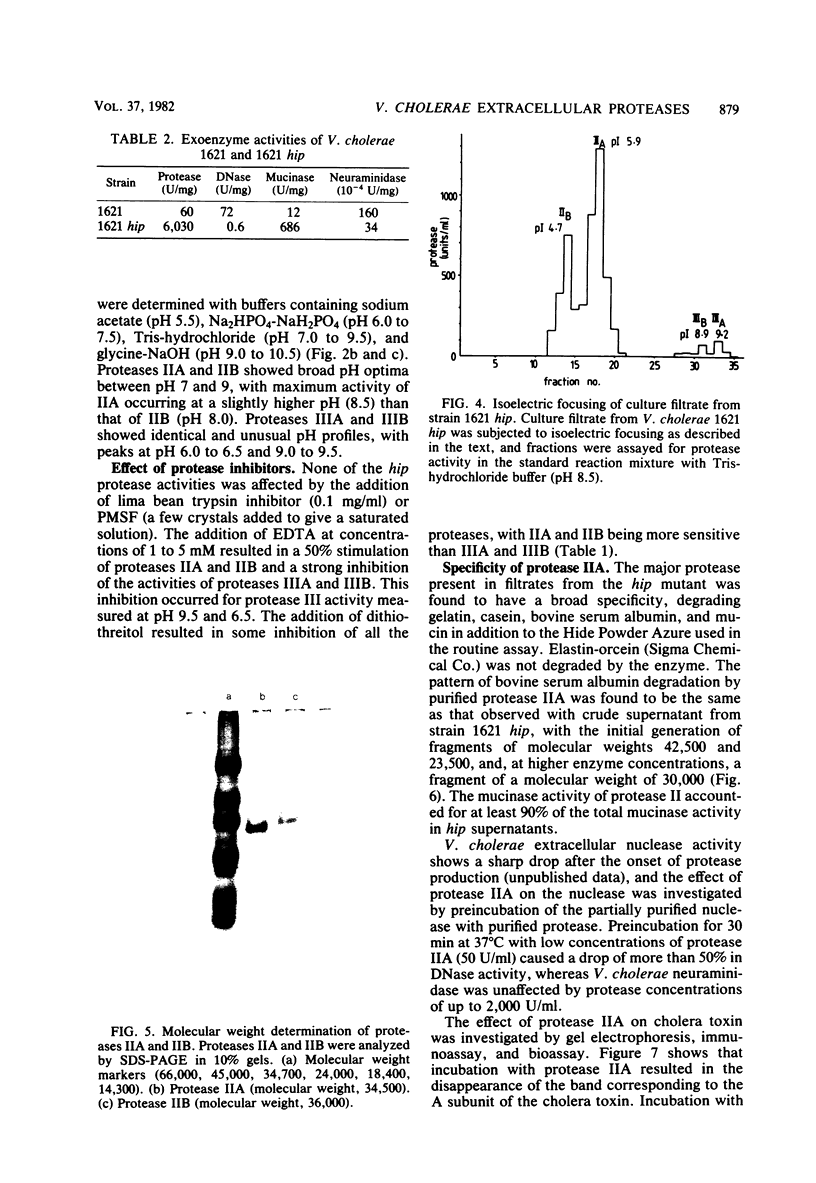
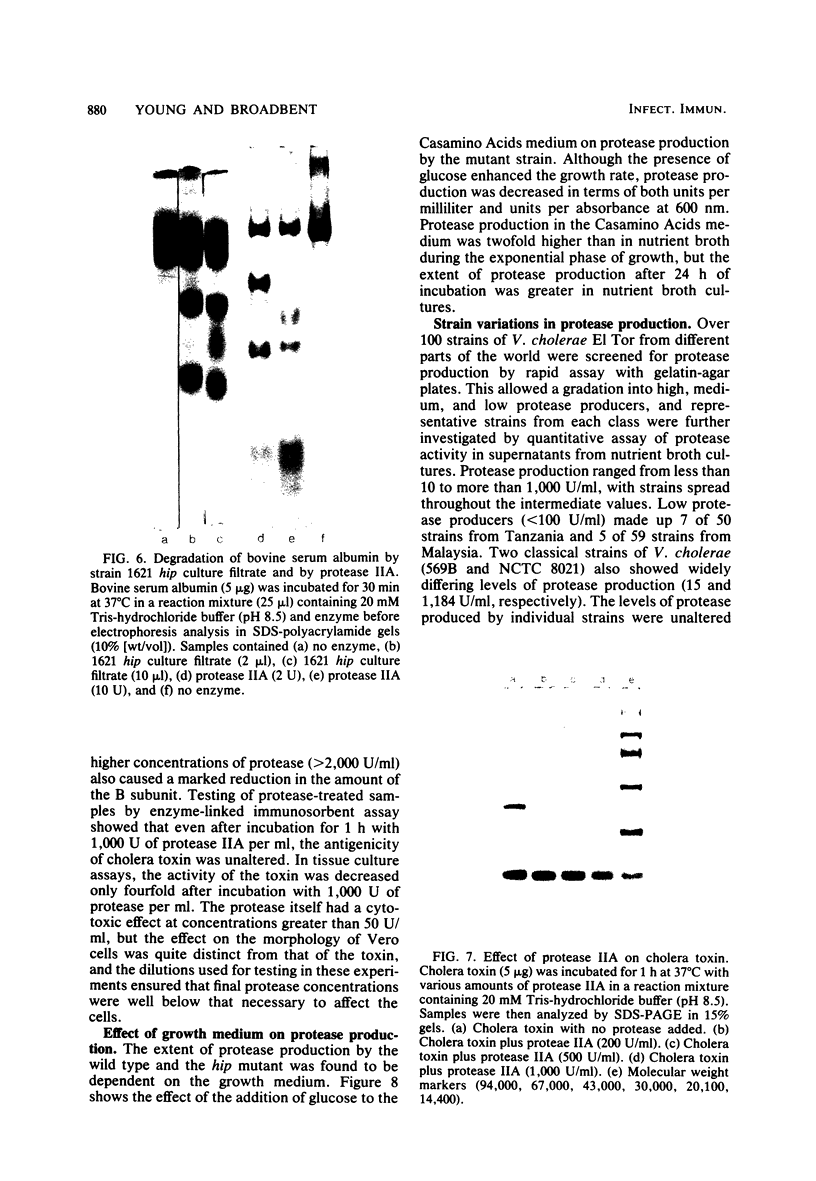
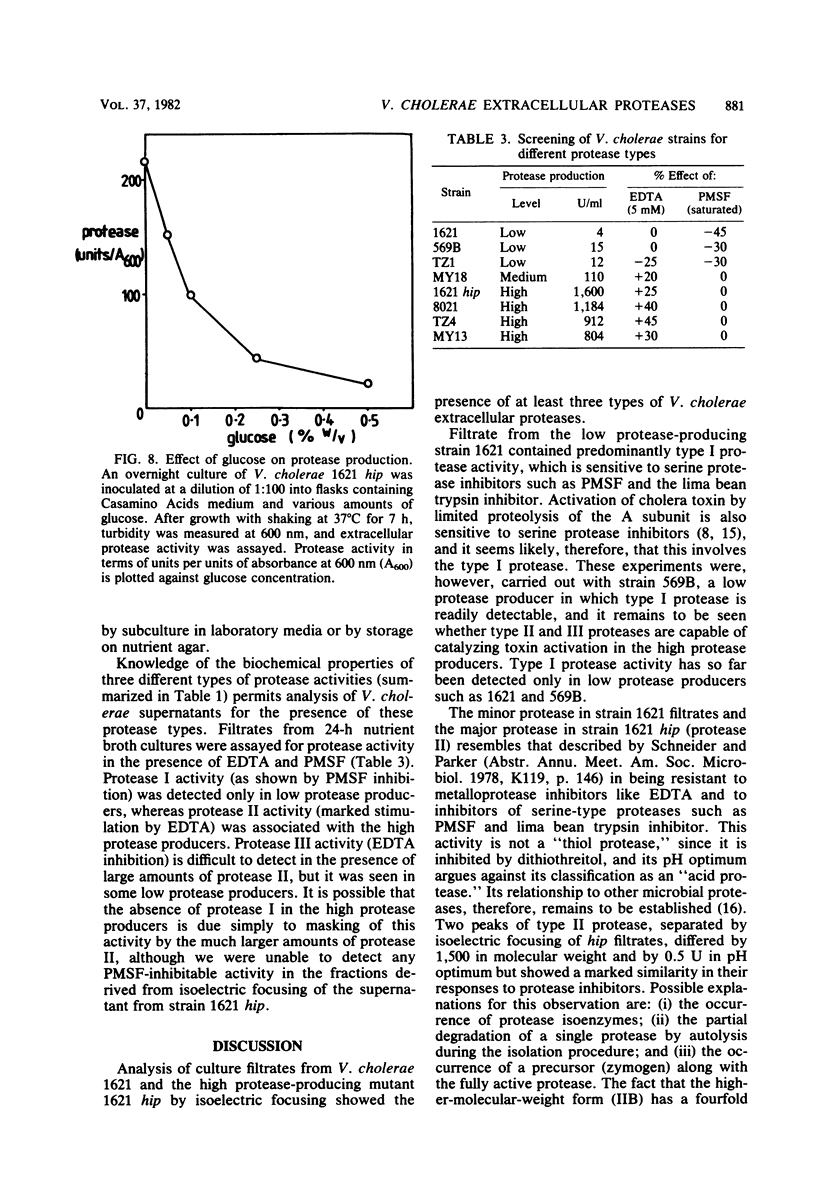
Isoelectric focusing of culture supernatants from Vibrio cholerae El Tor 1621 and high protease-producing mutant strain 1621 hip revealed the presence of three different types of extracellular protease. Type I protease was the major activity in the wild-type strain and was inhibited by phenylmethylsulfonyl fluoride and by the lima bean trypsin inhibitor. Type II protease was present in the wild type and was the major activity in the high protease-producing mutant. It was resistant to inhibitors of metalloproteases and serine proteases. Two peaks of type II protease differed by 1.2 pI units in isoelectric point and by 1,500 in molecular weight. Type II protease had broad specificity, acted as a mucinase, and caused degradation of some other V. cholerae extracellular proteins, including DNase and cholera toxin. Type III protease was EDTA inhibitable and was detected only in the high protease producer. Possible roles of extracellular proteases as virulence factors in cholera pathogenesis are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselski V. S., Medina R. A., Parker C. D. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1979 Apr;24(1):111–116. doi: 10.1128/iai.24.1.111-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle H. K., Sandvik O. Comparative electrophoretic and serological analyses of Vibrio comma and Aeromonas liquefaciens proteinases. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):686–690. doi: 10.1111/j.1699-0463.1971.tb00097.x. [DOI] [PubMed] [Google Scholar]
- FRETER R. The serologic character of cholera Vibrio mucinase. J Infect Dis. 1955 Nov-Dec;97(3):238–245. doi: 10.1093/infdis/97.3.238. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Rappaport R. S. Origin of the enzymatically active A1 fragment of cholera toxin. J Infect Dis. 1979 Jun;139(6):674–680. doi: 10.1093/infdis/139.6.674. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Hsieh H., Liu P. V. Serological identities of proteases and alkaline phosphatases of the so-called nonagglutinable (NAG) vibrios and those of Vibrio cholerae. J Infect Dis. 1970 Mar;121(3):251–259. doi: 10.1093/infdis/121.3.251. [DOI] [PubMed] [Google Scholar]
- Kusama H., Craig J. P. Production of Biologically Active Substances by Two Strains of Vibrio cholerae. Infect Immun. 1970 Jan;1(1):80–87. doi: 10.1128/iai.1.1.80-87.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyerly D., Gray L., Kreger A. Characterization of rabbit corneal damage produced by Serratia keratitis and by a serratia protease. Infect Immun. 1981 Sep;33(3):927–932. doi: 10.1128/iai.33.3.927-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti M., Sur P., Chatterjee S. N. Polyacrylamide gel electrophoresis and infrared spectroscopy of vibrio biotypes. Can J Microbiol. 1981 Oct;27(10):1048–1052. doi: 10.1139/m81-163. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Ogg J. E., Shrestha M. B., Poudayl L. Phage-induced changes in Vibrio cholerae: serotype and biotype conversions. Infect Immun. 1978 Jan;19(1):231–238. doi: 10.1128/iai.19.1.231-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Bucher D., Kilbourne E. D. Applications of a synthetic neuraminidase substrate. Appl Microbiol. 1973 Feb;25(2):195–201. doi: 10.1128/am.25.2.195-201.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Huda S., Neogi P. K., Daniel R. R., Spira W. M. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980 Jan;11(1):35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvik O., Dahle H. K. Enzymoserological relationships between Vibrio comma and other gram-negative organisms. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):285–290. doi: 10.1111/j.1699-0463.1971.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Isolation and characterization of protease-deficient mutants of vibrio cholerae. J Infect Dis. 1978 Aug;138(2):143–151. doi: 10.1093/infdis/138.2.143. [DOI] [PubMed] [Google Scholar]
- Schneider D. R., Sigel S. P., Parker C. D. Characterization of Vibrio cholerae protease activities with peptide digest analysis. J Clin Microbiol. 1981 Jan;13(1):80–84. doi: 10.1128/jcm.13.1.80-84.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J. I., Stavric S., Konowalchuk J. Assay of Escherichia coli heat-labile enterotoxin with vero cells. Infect Immun. 1977 May;16(2):617–622. doi: 10.1128/iai.16.2.617-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]