Background: ATP synthase converts a transmembrane gradient of protons or Na+ into chemical energy.
Results: Molecular dynamics simulations mapped a water half-channel as part of the proton path.
Conclusion: The half-channel is inclined against the sense of rotation of the c-ring, minimizing futile proton flow.
Significance: The geometry of the proton path is crucial in understanding the energy coupling of ATP synthase.
Keywords: Computational Biology, F1Fo-ATPase, Membrane Energetics, Proton Transport, Structural Biology, Energy Conversion, Proton Transfer, Rotary Molecular Motor, Water Channel
Abstract
The rotation of F1Fo-ATP synthase is powered by the proton motive force across the energy-transducing membrane. The protein complex functions like a turbine; the proton flow drives the rotation of the c-ring of the transmembrane Fo domain, which is coupled to the ATP-producing F1 domain. The hairpin-structured c-protomers transport the protons by reversible protonation/deprotonation of a conserved Asp/Glu at the outer transmembrane helix (TMH). An open question is the proton transfer pathway through the membrane at atomic resolution. The protons are thought to be transferred via two half-channels to and from the conserved cAsp/Glu in the middle of the membrane. By molecular dynamics simulations of c-ring structures in a lipid bilayer, we mapped a water channel as one of the half-channels. We also analyzed the suppressor mutant cP24D/E61G in which the functional carboxylate is shifted to the inner TMH of the c-protomers. Current models concentrating on the “locked” and “open” conformations of the conserved carboxylate side chain are unable to explain the molecular function of this mutant. Our molecular dynamics simulations revealed an extended water channel with additional water molecules bridging the distance of the outer to the inner TMH. We suggest that the geometry of the water channel is an important feature for the molecular function of the membrane part of F1Fo-ATP synthase. The inclination of the proton pathway isolates the two half-channels and may contribute to a favorable clockwise rotation in ATP synthesis mode.
Introduction
ATP synthases from bacteria, mitochondria, and chloroplasts produce ATP, the universal fuel in biological cells. To synthesize the high energy triphosphate from ADP and inorganic phosphate, the enzymes use a transmembrane proton gradient (1–4) and convert electrochemical into chemical energy. F-type ATP synthases consist of a transmembrane Fo domain and an extramembranous F1 domain. Fo from chloroplasts comprises a rotating c-ring and a stator domain formed by subunits a, b, and b′. Although the stoichiometry of the transmembrane stator domain is fixed in all organisms, the c-ring has 8 subunits in bovine mitochondria (5), 10 subunits in yeast mitochondria (1), 14 subunits in chloroplasts (6, 7), and 10–15 subunits in bacteria (8–15). The chloroplast F1 domain comprises subunits γ and ϵ, which rotate with the c-ring, and the nonrotating catalytic domain α3β3, which is connected to the stator domain of Fo through the δ subunit (see Fig. 1A). Light-driven proton pumps in chloroplasts and phototropic bacteria or respiratory-chain enzymes in mitochondria and aerobic bacteria generate a proton gradient across the membrane, resulting in a positively charged p-side3 and a negatively charged n-side (p-side and n-side refer to lumen and stroma in chloroplasts, intermembrane space and matrix in mitochondria, and periplasm and cytoplasm in bacteria, respectively). The resulting proton motive force drives the c8–15γϵ rotor, which induces conformational changes in the β subunits of the stator and, hence, provides the energy to produce ATP. Some fermenting bacteria use sodium ions instead of protons to drive ATP synthesis (16).
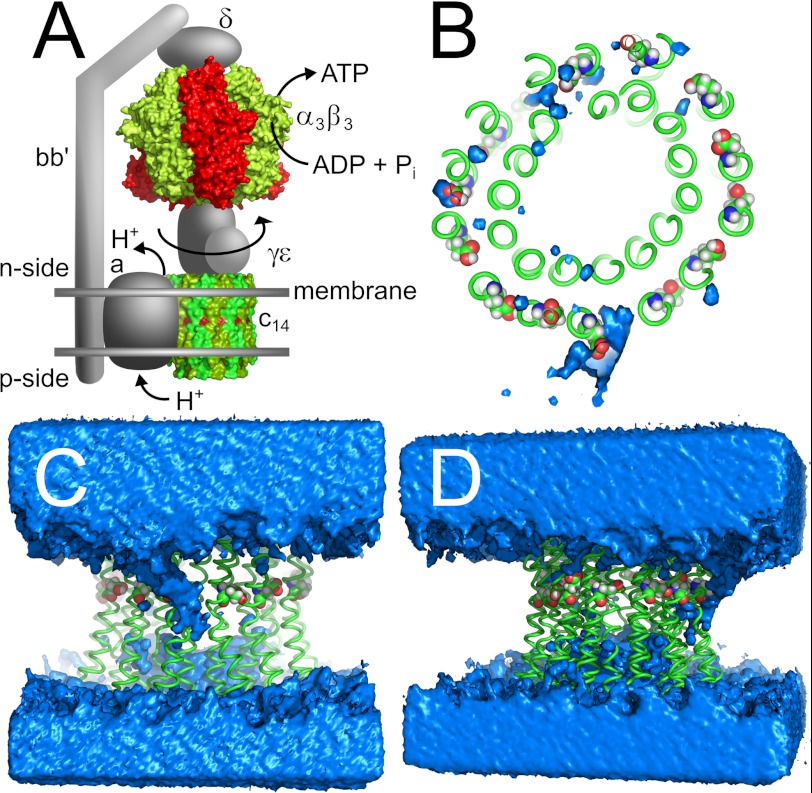
FIGURE 1.
Chloroplast ATP synthase. A, current model using the x-ray structures of α3β3 (PDB code 1KMH) (67) and c14-ring (PDB code 2W5J) (7). Protons flow from the p-side (lumen) through subunit a to the n-side (stroma) and drive the rotation of subunits c14γϵ. This rotation induces ATP synthesis in the β subunits. Subunits b and b′ provide a stator stalk between subunits a and α3β3δ. B, top view; C, front view; D, side view depicting the water channel found in the MD simulation of the 13protonated system. Blue, water density at a contour level of 50 waters/Å3 during the course of the simulation. Green, backbone of c14-ring. Red, oxygen atoms of conserved Glu61. The lipid bilayer is omitted for clarity. A, C, and D, top, n-side, and bottom, p-side.
Subunits a and c of the transmembrane Fo domain are directly involved in proton transport. Transport from the p-side to the middle of the membrane where protons bind to a conserved carboxylate of subunit c (Glu61 in chloroplasts) is facilitated by an intrinsic channel or proton wire in subunit a (p-side half-channel) (17–22). Protonation of the essential carboxylate in the c-subunit drives clockwise rotation of the entire c-ring (as seen from the p-side) (23). The step size of the c-ring corresponds to one subunit of c, indicating a proton motive force-driven Brownian ratchet mechanism (24, 25). The protons are then released from the proton-binding site on subunit c to the n-side (17, 26). An aqueous path at the interface between subunits a and c was inferred by mutagenesis and labeling studies (18, 19, 21, 27–29), which may function as the n-side half of the proton channel. F1Fo-ATP synthases can also catalyze the reverse reaction driven by ATP hydrolysis and pump protons from the n-side to the p-side of the membrane via the same half-channels, resulting in a counterclockwise rotation of the c-ring rotor (23).
Preventing a nonproductive shortcut between the p-side and the n-side half-channels is an essential prerequisite for the transformation of proton flux into mechanical energy (26). A conserved positively charged side chain in subunit a (Arg193 in chloroplasts and Arg210 in Escherichia coli) isolates the two half-channels by preventing any protonated (and thus uncharged) Glu61 of subunit c to pass the positively charged guanidinium group of the aArg193 side chain (30–35). Thus, aArg193 prevents the futile counterclockwise rotation in ATP synthesis mode.
However, the structure of the a/c interface and how the protein complex maintains isolation between the half-channels, i.e. how the nonrotating shortcut of protons is prevented, has remained elusive so far. Although it is established that the n-side half-channel is water-accessible, the pathway of the protons has not yet been identified. Here we resolve this pathway at atomic resolution by MD simulations of the structure of the c14 rotor ring of the chloroplast ATP synthase (7) in a lipid bilayer surrounded by water. The carboxylate cGlu61 of one of the protomers was deprotonated, thus mimicking the protomer facing the a subunit in the intact Fo domain. We identified a water-accessible region inside the lipid bilayer at the periphery of the c-ring rotor. This water channel runs from the n-side of the membrane to the deprotonated carboxylate cGlu61 in the middle of the membrane. The aqueous n-side half-channel is not parallel to the axis of the c-ring rotor but rather inclined, which may enhance the isolation between the half-channels and may contribute to a favorable clockwise rotation in ATP synthesis mode. The MD results also give a plausible explanation of the peculiar properties of the double mutant cP24D/E61G, which, in E. coli, is fully functional despite lacking the essential Asp61 side chain (36); the water channel now reaches deep between two c-protomers, thus enabling the repositioning of the reversibly protonated carboxyl group of the suppressor mutant.
EXPERIMENTAL PROCEDURES
Generation of c14 Rotor Ring/Lipid Bilayer Starting Structures
Starting structures for MD simulations of the c14 rotor ring in a membrane environment were generated from the crystal structure of the c-ring from spinach chloroplasts (Protein Data Bank (PDB) code 2w5j) (7). It comprises 14 identical c-subunits, each of which forms a hairpin structure consisting of two TMHs. Missing amino acids and side chains were added manually in the most plausible rotamer conformations and refined as described (7). The c-ring structure was oriented with respect to a lipid bilayer according to information provided by the Orientations of Proteins in Membranes database (37). The protein structure was then embedded in a bilayer of 288 1,2-dioleoyl-sn-glycero-3-phosphorylcholine lipids, which itself was embedded in 20 Å layers of water molecules above and below. The pre-equilibrated configuration of the lipid bilayer and the water layers was taken from Rosso and Gould (38). Ninety-nine lipids that were sterically overlapping with the protein structure were removed. The center of the c-ring was filled with 12 lipids to model the lipid plug seen in isolated rings (39).
From this system, the following starting structures were generated. I) All but one of the Glu61 residues of the subunits c were protonated (hitherto referred to as “13protonated”). II) All Glu61 residues were protonated (“14protonated”). III) All Glu61 residues were deprotonated (“0protonated”). IV) The double mutant cP24D/E61G was generated by pruning the side chain of Glu61 and replacing Pro24 with the most plausible rotamer of Asp, as provided by Coot (40). In this system, all but one of the Asp24 residues were then protonated (double mutant). To reach electroneutrality for each of these starting structures, sodium ions were added by the leap program of the Amber package (41) as required. This resulted in system sizes of ∼78,000 atoms.
Molecular Dynamics Simulations
MD simulations were performed with the Amber 10 suite of programs (41). For the protein and ions, the force field by Cornell et al. (42) was used with modifications suggested by Simmerling et al. (43). TIP3P was used as a water model (44). For the lipids, force field parameters and charges derived by Rosso and Gould (38) were used, which are based on the General Amber Force Field (45) and the RESP procedure (46), respectively. These parameters have been shown to yield area per lipid values, peak distances, and lipid volumes that converge around values close to experimental ones when simulations were performed under the condition of an isobaric-isothermal ensemble (NPT) with anisotropic pressure control (38). That way, a restraining of the area as in a constant area isobaric-isothermal (NPAT) ensemble can be avoided, which may lead to simulation artifacts (47).
Accordingly, after minimization of the systems for 2000 steps with the protein atom positions restrained, canonical ensemble (NVT) MD was carried out for 50 ps, during which the system was heated from 100 to 300 K, applying harmonic restraints with force constants of 5 kcal mol−1 Å−2 to the protein atoms. Subsequent NPT-MD was used for 150 ps to adjust the density. After gradually reducing the force constants of the harmonic restraints on solute atom positions to zero during 250 ps of NPT-MD, the following 20 ns of NPT-MD at 300 K were used to further equilibrate the system. Equilibration times of this length were shown to be necessary for typical simulation studies involving lipid bilayers (48). Finally, the following trajectories of 30 ns length generated by NPT-MD were used as production runs with conformations extracted every 20 ps. This resulted in ∼250 ns of total simulation time.
Throughout the simulations, the particle mesh Ewald method (49) was used to treat long-range electrostatic interactions, and bond lengths involving bonds to hydrogen atoms were constrained using SHAKE (50). The time step for all MD simulations was 2 fs, with a direct space, nonbonded cutoff of 8 Å. The temperature was controlled using the Berendsen thermostat (51) with a time constant of 10 ps, and the Berendsen barostat (51) was used for anisotropic pressure control with a time constant of 2 ps.
Analysis of the Production Runs
The production runs were analyzed with ptraj of the Amber suite of programs (41). The “watershell” command was used for analyzing the first and second solvation shells with respect to the oxygen atoms of Glu61 (13protonated, 14protonated, 0protonated) or of Asp24 (double mutant) using lower and upper distances of 3.4 and 5.0 Å, respectively. Water densities were determined with the “grid” command using a cubic grid that encompasses the whole simulation cell with a spacing of 1 Å in each direction. Depicted contour surfaces encompass regions with at least 50 counts of water molecules over the 1,500 snapshots analyzed. Prior to calculating root mean square deviations (r.m.s.d.) and an average structure, the c-ring structure was superimposed with respect to all Cα atoms. A snapshot with a minimal Cα atom r.m.s.d. to the average structure was then taken as the representative structure depicted in Figs. 1 and 2. χ1 and χ2 angles of residues Glu61 were determined as the torsion angles involving atoms N-Cα-Cβ-Cγ and Cα-Cβ-Cγ-Cδ, respectively. The kink of the outer helices of the c-ring was determined as the angle of the point triple (center of mass of Cα atoms of residues 48–50; Cα atom of residue 61; center of mass of Cα atoms of residues 74–76). The S.E. was determined as the S.D. divided by the square root of the number of independent snapshots. The number of independent snapshots was determined from the time correlation function for the respective analysis. The correlation time was 200 ps in the case of the watershell analysis and 2.2 ns in the case of the analysis of the χ1 and χ2 torsion angles of Glu61.
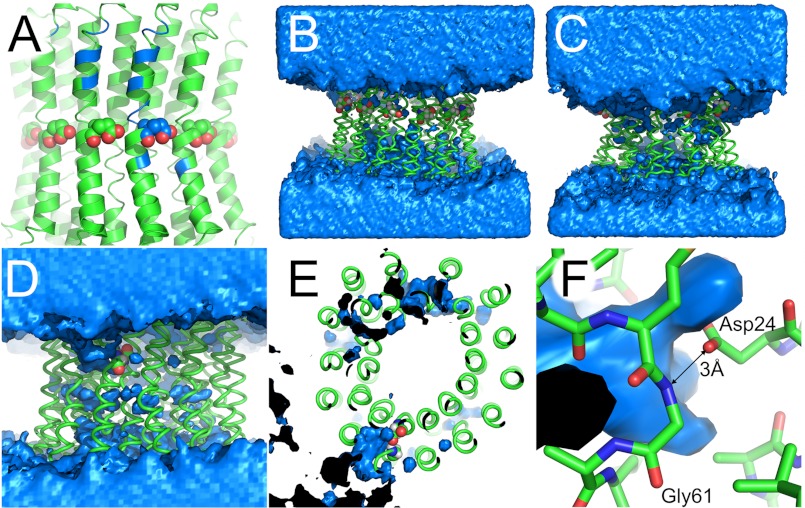
FIGURE 2.
The water channel in different simulations. The lipid bilayer is omitted for clarity. A, map of the water channel from Fig. 1 on the starting structure. Blue, main amino acids in contact with the water channel (see Table 2 for a list). B and C, comparison of water distribution in control MD simulations. B, c-ring with Glu61 of all 14 protomers protonated (uncharged). C, c-ring with all Glu61 deprotonated. Although the protonated ring (B) does not show a distinct water channel, a water ring is drawn toward the charged center of the c-ring (C). D–F, water channel in suppressor mutant cP24D/E61G. D, front view. The water channel developed from the top (n-side) to the one charged Asp24. This channel is inclined to the vertical membrane normal and runs, as depicted here, from the top left to the center. E, top view. The water channel reaches as far as the carboxylate group of Asp24. F, close-up view. The carboxylate group of Asp24 establishes a hydrogen bond with the amide nitrogen of Gly61 with a distance of 3 Å. Gln28 is hidden for clarity. A–D and F, top, n-side; bottom, p-side. A–C, spheres show Glu61 of c-protomers. D–F, spheres show cAsp24. B–F, blue, water density at a contour level of 50 waters/Å3 over the course of the simulation. Black, inside of water density.
To analyze possible conformational changes of the c-ring, an elastic network model analysis was performed with the ElNémo webserver (52) using default parameters. Molecule figures were prepared with PyMOL (Schrödinger, New York, NY).
RESULTS
We performed MD simulations of 50 ns length of the chloroplast c14-ring in an aqueous lipid bilayer with Na+ counter ions, with the last 30 ns taken for analysis. Although the conserved Glu61 side chain from 13 protomers was protonated, the carboxyl group of one Glu61 was deprotonated and thus charged (13protonated). This situation reflects the physiological condition of the c-ring in active ATP synthase where aArg189 serves as counter ion. The water density was determined by counting the presence of water molecules within a cubic grid of 1 Å spacing. Fig. 1 shows an MD snapshot with a minimal r.m.s.d. to the average protein structure together with the water density as seen from the top, front, and side (Fig. 1, B, C, and D, respectively). Although no water was present inside the 1,2-dioleoyl-sn-glycero-3-phosphorylcholine membrane at the start of the calculation, a water channel developed during the simulation originating from the n-side and reaching to the middle of the membrane bilayer, enclosing the deprotonated Glu61 with its tip (Table 1).
TABLE 1.
Number of water molecules around reversibly protonated amino acid side chains
Average ± S.E. of the number of water molecules within 3.4 and 5 Å over the MD trajectories from 20 to 50 ns.
| Name | Protonation state | Amino acid | 3.4 Å shell | 5 Å shell |
|---|---|---|---|---|
| 13protonated | 13 neutral +1 charged | Deprotonated Glu61 | 5.41 ± 0.20 | 8.16 ± 0.38 |
| Protonated Glu61 | 0.28 ± 0.01 | 0.92 ± 0.21 | ||
| 14protonated | 14 uncharged | Protonated Glu61 | 0.90 ± 0.03 | 0.58 ± 0.21 |
| 0protonated | 14 charged | Deprotonated Glu61 | 5.12 ± 0.05 | 10.97 ± 0.10 |
| Double mutant | P24D/E61G (13 + 1) | Deprotonated Asp24 | 3.10 ± 0.12 | 4.28 ± 0.35 |
| Protonated Asp24 | 0.44 ± 0.19 | 1.23 ± 0.41 |
We mapped the wetted surface on the c-ring conformation with a minimal r.m.s.d. to the average structure (Fig. 2A). The main amino acids in contact with the water channel are listed in Table 2. This water channel is in agreement with experimental data that implied a water-accessible region in the interface between subunits a and c (18, 19, 21, 27–29). We also performed an MD simulation of a c-ring with Glu61 of all 14 protomers protonated (14protonated) and of a completely deprotonated c-ring (0protonated). No water channel developed with the protonated (uncharged) c-ring (Fig. 2B; Table 1). In contrast, with the fully deprotonated and therefore charged c-ring, the water encompassed almost completely the n-side half of the c-ring (Fig. 2C; Table 1).
TABLE 2.
Main amino acids in contact with the water channel
Listed are amino acids close to the water channel as mapped in Fig 2A. The c-protomer with the deprotonated Glu61 is denoted n. During ATP synthesis, the protomers to the left (in the orientation of Fig. 1A) are the next to be rotated into proton release position and are thus numbered n−1 and n−2. Accordingly, the protomer to the right is denoted n+1.
| Protomer | Amino acid |
|---|---|
| n−2 | Glu46 |
| n−1 | Glu46, Gly47, Arg50, Gly51, Leu54 |
| n | Glu46, Gly47, Lys48, Arg50, Gly51, Leu55, Ala58, Phe59, Met60, Glu61, Ile65 |
| n+1 | Tyr66 |
Suppressor Mutant cP24D/E61G
We also performed an MD simulation of the suppressor mutant cP24D/E61G, in which the carboxyl group of one Asp24 was deprotonated and thus charged. In this mutant, the function of the reversibly protonated carboxyl group of conserved Glu61 (Asp61 in E. coli) is transferred from the outer cTMH2 to Asp24, which is located on the adjacent inner cTMH1 (36). Fig. 2, D–F, show the resulting water density maps over the last 30 ns of the trajectory together with the closest-to-average conformation of the c-ring. As with the wild type c-ring, a water channel developed and reached as far as the carboxylate group of Asp24 (Table 1).
Structural Changes of the c-Ring
Regarding possible structural changes of subunit c upon deprotonation, we do not observe significant differences between the protomer with deprotonated Glu61 and the other, protonated protomers for the 13protonated system. In fact, the overall Cα atom r.m.s.d. remains stable below 4 Å over the trajectory, and the Cα atom r.m.s.d. of almost all TMHs remains below 3 Å. Furthermore, the kink of TMH2 of subunit c, which gives the c-ring the characteristic hourglass shape, is essentially the same; the kink angle of the helix with deprotonated Glu61 is 158.7° ± 1.0° (S.E.), whereas the average of 11 protonated helices is 159.7° ± 0.8°. In the latter case, the two protomers adjacent to the helix with the deprotonated Glu61 have been excluded to prevent a possible influence of the deprotonated protomer. The circular shape of the c-ring got distorted during the MD simulations (Fig. 1B). The degree and direction of distortion are consistent with results from an elastic network model analysis. This analysis reveals that the first four low frequency modes lead to a deformation of the c-ring structure perpendicular to the axis of the c-ring rotor; the first two of these modes preferentially distort the p-side of the c-ring, the last two distort the n-side (data not shown). In each case, the distorted structures take on an oval shape. A similar distortion, although to a lesser degree, has been observed in electron micrographs from Ilyobacter tartaricus (53). Finally, visual inspection of the trajectories showed no swiveling motion of the helices (data not shown).
Glu61 Side Chain Conformation
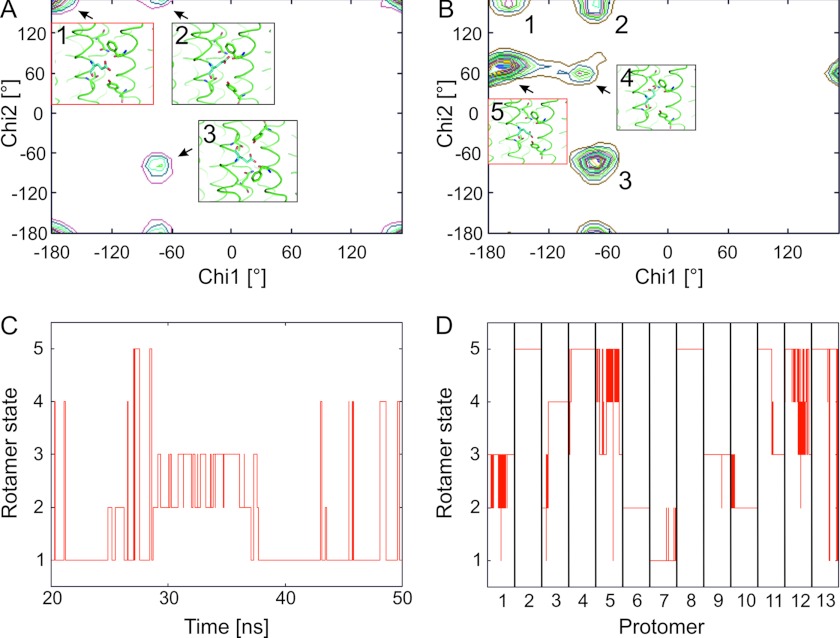
Proton binding of Glu61 is linked to a conformational change of this amino acid (7, 54, 55). We analyzed the conformations of Glu61 according to the torsion angles χ1 and χ2 and classified the conformations as plus, trans, and minus according to Lovell et al. (56). Torsion angle χ1 of the deprotonated Glu61 adopts values of around −65° (minus) and around −177° (trans), whereas torsion angle χ2 is mostly trans (Fig. 3A). Overall, the all-trans rotamer is mostly populated where the side chain points to the solvent. This finding agrees with the observation of an extended conformation of Glu59 in the deprotonated c-ring from yeast (55). The protonated side chains have similar χ1 torsion angles with minus and trans conformations and similar χ2 torsion angles in minus and trans conformations. However, the plus conformation of χ2 is populated, too (Fig. 3B). Overall, this leads to the trans/plus rotamer being most abundant, with the side chain pointing inward toward Gln28. On multiple occasions during the MD simulation, transitions between rotamer states of Glu61 occur (Fig. 3, C and D) for both the deprotonated Glu61 and the protonated ones, showing a pronounced mobility of these side chains.
FIGURE 3.
Rotamer states of the side chain of Glu61 from the MD simulation of the 13protonated system. A and C, states of deprotonated (charged) Glu61 rotamers. B and D, states of protonated (uncharged) Glu61 rotamers. A, distribution of rotamer states according to the χ1/χ2 map. The number of rotamer states is qualitatively indicated by 20 equally spaced contour lines. The insets show the most abundant rotamer states 1–3 with 1 being the most populated one (red frame). B, distribution of rotamer states according to the χ1/χ2 map. The insets show the two rotamer states not observed for the deprotonated Glu61 (see panel A). Rotamer 5 is the most populated one (red frame). C, transitions between rotamer states along the trajectory. States 1–3 correspond to the ones shown in panel A; rotamer states 4 and 5 are lowly populated and, hence, not shown in panel A. D, transitions between rotamer states along the trajectory for each of the 13 protomers. States 1–5 correspond to the ones shown in panel B. Each column represents a trajectory from 20–50 ns.
DISCUSSION
A major challenge in understanding the molecular mechanism of energy coupling in the F1Fo-ATP synthase is to resolve the proton transfer pathway in the transmembrane Fo domain at atomic resolution. Currently, there are only two cryo-electron microscopy structures of Fo subcomplexes available. The first structure is from Thermus thermophilus ATP synthase with 10 Å resolution (57). Here, subunit I (the functional homolog of subunit a) forms two complete half-channels from eight TMHs that are thought to be offset laterally. The other available structure is the ab2c11 Fo subcomplex of the Na+-translocating F-ATP synthase from I. tartaricus with 7 Å resolution (53). Here, subunit a forms one four-helix bundle that probably provides the p-side half-channel. Additionally, up to three more TMHs, which belong to subunit a and perhaps subunit b, can be seen in the electron density. Subunits a of ATP synthases from E. coli or chloroplasts comprise five TMHs (58, 59), and a water channel serves as the n-side half-channel (18, 19, 21, 27–29). Hence, the Fo subcomplexes from E. coli, chloroplasts, and I. tartaricus may share the same architecture. The current model of the active ATP synthase (3) predicts that one protomer of subunit c has the conserved acidic side chain (Glu61 in chloroplast ATP synthase) deprotonated. Its charge is neutralized by aArg189. The remaining c-protomers (13 in chloroplasts) have protonated Glu61 and are embedded in the hydrophobic part of the membrane. To reflect this situation in our MD calculations, our starting model of the spinach chloroplast c-ring rotor was set to an ensemble of 13 protonated c-protomers plus one deprotonated c-protomer. This complex was embedded in a lipid bilayer enclosed by water layers. Although c-protomers are unlikely to be charged outside the a/c interaction, the MD simulation of this setting provides an elegant way to investigate possible structural changes that result from deprotonation of one c-protomer. We predicted earlier that for proton translocating ATP synthases, the conformational differences between protonated and deprotonated protomers are mainly limited to side chain movements of Glu61 (7), as is probably the case with Na+-translocating ATP synthases (60). This model was later confirmed with a high resolution structure of ATP synthase from cyanobacterium Spirulina platensis (54).
Our MD simulations presented here do support the idea that the main conformational changes of a protomer upon deprotonation are limited to the side chain movements of Glu61. The trajectories show no indication of helix swiveling, nor other major changes in helix conformation. Transitions between rotamer states in our simulation reveal a certain mobility of the side chains of both the protonated Glu61 and the deprotonated Glu61. This observed mobility of the side chain in the hydrophobic part of the membrane moderates the stringent implication of the description of a “locked conformation” for protonated Glu61 (60).
The most striking result from our MD simulations is the location of water molecules inside the hydrophobic part of the lipid bilayer forming a water channel. The existence of a water channel has been extensively shown by in vitro experiments using chemical modification of c-rings with Cys substitutions (28, 29). Previous MD simulations also found water molecules drawn into the membrane. However, these simulations used c-rings with all Glu61 deprotonated (equivalent to our 0protonated system) (55, 61), and a complete water ring was formed. Other simulations used c-rings with a single protomer deprotonated (equivalent to our 13protonated system), but the authors missed to observe a distinct water channel (61, 62). The form and direction of the water channel identified here are instructive because the channel is not perpendicular to the membrane but points at an angle of ∼45° from the n-side of the membrane to cGlu61 and beyond. Therefore, the resulting proton wire does not follow the shortest path from cGlu61 to the n-side but points against the rotational sense of the c-ring (in ATP synthesis mode). This orientation isolates the protons arriving from the p-side at the c-ring and the protons leaving from the c-ring to the n-side. As a result, two laterally offset half-channels are formed as proposed (3, 4) and seen in T. thermophilus ATP synthase (57), but with the difference that the n-side half-channel in the case of chloroplast ATP synthase is a hydration layer in the a/c interface as depicted in Fig. 2A. The orientation can also be expected to favor deprotonation of Glu61 on rotating from the position at protomer n−1 to the one at protomer n due to the presence of a higher dielectric environment that better stabilizes the emerging charge than a pure lipid environment. Accordingly, one may speculate that the orientation of the water channel contributes to a favorable clockwise rotation in ATP synthesis mode.
Control Calculations
Two additional MD simulations were performed to test the notion that a water channel is drawn into the hydrophobic part of the membrane if cGlu61 is deprotonated and thus charged. With a fully protonated c-ring, less than 0.6 waters are found within 5 Å of the carboxyl group of Glu61 (Table 1). The fully deprotonated c-ring provides the opposite setting, where nearly 11 waters are found in the 5 Å shell (Table 1). Comparison of the water densities shows that the water did not integrate into the membrane with the fully protonated complex, whereas with the fully deprotonated complex, a complete water ring developed from the n-side to the center of the membrane (Fig. 2, B and C). Thus, these results confirm that the charged Glu61 in the hydrophobic part of the membrane leads to water molecules hydrating the protein/lipid interface.
Implications on an a/c Interface Model
As was argued earlier (62), c-protomers are not likely to be deprotonated outside the a/c interface. Here, we take full advantage of the possibility to simulate a functional state of the c-ring with one deprotonated and 13 protonated protomers in MD calculations. We predict that the resulting water channel shows a negative imprint of the hydrated a/c interface. This prediction is in line with findings from the E. coli subunit a. I) aTMH4 is positioned next to the outer TMH of c (63). II) This helix is kinked at the conserved Gly213, as shown by NMR experiments on site-directed paramagnetic spin-labeled protein (64). III) According to the topology established with vesicles (58) and with a/c fusion proteins (65), aTMH4 is oriented with the N terminus on the n-side and the C terminus on the p-side. IV) The 15 amino acids preceding the conserved Arg210 are inaccessible to surface labeling (66). Therefore, it is feasible that the p-side half of aTMH4, i.e. the first 15 amino acids up to the conserved Arg210 (E. coli numbering), forms a straight helix from the p-side to the middle of the membrane. This part of the helix is probably parallel to the rotational axis of the c-ring. The helix is kinked at Gly213 and probably follows the direction of the water channel to the n-side. A layer of water molecules at the protein-protein interface provides the n-sided proton half-channel.
The proton-providing half-channel on the p-side is thought to be within subunit a, presumably inside a four-helix bundle (19, 20, 53). We predict that the location of this four-helical bundle is offset by one c-protomer from the n-sided water channel. As a result of the model, two c-protomers may be deprotonated simultaneously: one protomer with Glu61 in the water channel, releasing a proton, and one protomer with Glu61 interacting with conserved aArg189 (aArg210 in E. coli). This prediction is in accordance with the observation that in T. thermophilus ATP synthase, two four-helix bundles of subunit I are seen in the interface with the rotor (57).
Double Mutant
We modeled the suppressor mutant cP24D/E61G. In this variant, the reversibly protonated carboxylate is transferred from Glu61 in cTMH2 to position 24 in the adjacent helix of the c hairpin. E. coli cells with this double mutant grow via oxidative phosphorylation (36). The ATP synthase complex with this suppressor mutant actually produces ATP (although slightly less efficiently) and is resistant to dicyclohexylcarbodiimide, a potent inhibitor of wild type c-ring (36). Our MD simulation shows that water is drawn deep into the protein structure to the carboxylate group of deprotonated Asp24 (Fig. 2, D–F). Presumably, here at the interface between cTMH1 and cTMH2 is where the protons are bound. Thus, the water channel allows the protons to be released from a different position than in wild type. Its location in the inner interface of the hairpin-structured c-protomer may render the mutant insensitive to dicyclohexylcarbodiimide. In this simulation, the water channel is oriented in an angle against the rotational sense, too (i.e. in Fig. 2D, from the upper left to the center). The water channel is narrower than with wild type protein (Table 1), which may explain why the mutant is less efficient. Our model may also explain why Gly61 is essential for this double mutant; the side chain of Asp24 is likely to form a hydrogen bond with the nitrogen atom of Gly61 with an O → N distance of ∼3 Å (Fig. 2E), which may be prevented by Cβ of any other amino acid in position 61.
Conclusion
The MD simulations presented here give a convincing picture; the charges of aArg198 and cGlu61 provide a strong driving force for water to move into the a/c interface, which is known to be hydrated at the n-side (18, 19, 21, 27–29). The surface of subunit c, and presumably also of subunit a, orients this water channel at an angle to the membrane normal. This water channel forms a proton path that is partially directed against the rotational sense of the c-ring (in ATP synthesis mode). Therefore, the hydrated proton path on the n-side is laterally offset to the p-side path, which presumably is inside a four-helix bundle in subunit a. This geometry promotes isolation between half-channels minimizing futile proton flow and is suggested to contribute to a favorable clockwise rotation in ATP synthesis mode.
Acknowledgments
We thank the Heinrich Heine University Center for Information and Media Technology for computational support and Elisabeth Stratmann for assisting with the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (GR 1616/5-3) (to G. G.) and by funds of the initiative “Fit for Excellence” at the Heinrich Heine University (to H. G.).
- p-side
- positive side of the membrane
- n-side
- negative side of the membrane
- MD
- molecular dynamics
- NPAT
- constant area isobaric-isothermal ensemble
- NPT
- isobaric-isothermal ensemble
- NVT
- canonical ensemble
- r.m.s.d.
- root mean square deviation(s)
- TMH
- transmembrane helix
- PDB
- Protein Data Bank.
REFERENCES
- 1. Stock D., Leslie A. G., Walker J. E. (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705 [DOI] [PubMed] [Google Scholar]
- 2. von Ballmoos C., Wiedenmann A., Dimroth P. (2009) Essentials for ATP synthesis by F1F0 ATP synthases. Annu. Rev. Biochem. 78, 649–672 [DOI] [PubMed] [Google Scholar]
- 3. Junge W., Sielaff H., Engelbrecht S. (2009) Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature 459, 364–370 [DOI] [PubMed] [Google Scholar]
- 4. Vik S. B., Antonio B. J. (1994) A mechanism of proton translocation by F1F0 ATP synthases suggested by double mutants of the a subunit. J. Biol. Chem. 269, 30364–30369 [PubMed] [Google Scholar]
- 5. Watt I. N., Montgomery M. G., Runswick M. J., Leslie A. G., Walker J. E. (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. U.S.A. 107, 16823–16827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seelert H., Poetsch A., Dencher N. A., Engel A., Stahlberg H., Müller D. J. (2000) Proton-powered turbine of a plant motor. Nature 405, 418–419 [DOI] [PubMed] [Google Scholar]
- 7. Vollmar M., Schlieper D., Winn M., Büchner C., Groth G. (2009) Structure of the c14 rotor ring of the proton translocating chloroplast ATP synthase. J. Biol. Chem. 284, 18228–18235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang W., Hermolin J., Fillingame R. H. (2001) The preferred stoichiometry of c subunits in the rotary motor sector of Escherichia coli ATP synthase is 10. Proc. Natl. Acad. Sci. U.S.A. 98, 4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitome N., Suzuki T., Hayashi S., Yoshida M. (2004) Thermophilic ATP synthase has a decamer c-ring: indication of noninteger 10:3 H+/ATP ratio and permissive elastic coupling. Proc. Natl. Acad. Sci. U.S.A. 101, 12159–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stahlberg H., Müller D. J., Suda K., Fotiadis D., Engel A., Meier T., Matthey U., Dimroth P. (2001) Bacterial Na+-ATP synthase has an undecameric rotor. EMBO Rep. 2, 229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meier T., Matthey U., von Ballmoos C., Vonck J., Krug von Nidda T., Kühlbrandt W., Dimroth P. (2003) Evidence for structural integrity in the undecameric c-rings isolated from sodium ATP synthases. J. Mol. Biol. 325, 389–397 [DOI] [PubMed] [Google Scholar]
- 12. Pogoryelov D., Yu J., Meier T., Vonck J., Dimroth P., Muller D. J. (2005) The c15-ring of the Spirulina platensis F-ATP synthase: F1/F0 symmetry mismatch is not obligatory. EMBO Rep. 6, 1040–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meier T., Ferguson S. A., Cook G. M., Dimroth P., Vonck J. (2006) Structural investigations of the membrane-embedded rotor ring of the F-ATPase from Clostridium paradoxum. J. Bacteriol. 188, 7759–7764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meier T., Morgner N., Matthies D., Pogoryelov D., Keis S., Cook G. M., Dimroth P., Brutschy B. (2007) A tridecameric c-ring of the adenosine triphosphate (ATP) synthase from the thermoalkaliphilic Bacillus sp. strain TA2.A1 facilitates ATP synthesis at low electrochemical proton potential. Mol. Microbiol. 65, 1181–1192 [DOI] [PubMed] [Google Scholar]
- 15. Pogoryelov D., Reichen C., Klyszejko A. L., Brunisholz R., Muller D. J., Dimroth P., Meier T. (2007) The oligomeric state of c-rings from cyanobacterial F-ATP synthases varies from 13 to 15. J. Bacteriol. 189, 5895–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimroth P., Wang H., Grabe M., Oster G. (1999) Energy transduction in the sodium F-ATPase of Propionigenium modestum. Proc. Natl. Acad. Sci. U.S.A. 96, 4924–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vik S. B., Patterson A. R., Antonio B. J. (1998) Insertion scanning mutagenesis of subunit a of the F1F0 ATP synthase near His245 and implications on gating of the proton channel. J. Biol. Chem. 273, 16229–16234; Correction (1998) J. Biol. Chem.273, 22159 [DOI] [PubMed] [Google Scholar]
- 18. Angevine C. M., Fillingame R. H. (2003) Aqueous access channels in subunit a of rotary ATP synthase. J. Biol. Chem. 278, 6066–6074 [DOI] [PubMed] [Google Scholar]
- 19. Angevine C. M., Herold K. A., Fillingame R. H. (2003) Aqueous access pathways in subunit a of rotary ATP synthase extend to both sides of the membrane. Proc. Natl. Acad. Sci. U.S.A. 100, 13179–13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwem B. E., Fillingame R. H. (2006) Cross-linking between helices within subunit a of Escherichia coli ATP synthase defines the transmembrane packing of a four-helix bundle. J. Biol. Chem. 281, 37861–37867 [DOI] [PubMed] [Google Scholar]
- 21. Angevine C. M., Herold K. A., Vincent O. D., Fillingame R. H. (2007) Aqueous access pathway in ATP synthase subunit a: reactivity of cysteine substituted into transmembrane helices 1, 3, and 5. J. Biol. Chem. 282, 9001–9007 [DOI] [PubMed] [Google Scholar]
- 22. Dong H., Fillingame R. H. (2010) Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane. J. Biol. Chem. 285, 39811–39818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Börsch M., Diez M., Zimmermann B., Reuter R., Gräber P. (2002) Stepwise rotation of the γ-subunit of EF0F1-ATP synthase observed by intramolecular single-molecule fluorescence resonance energy transfer. FEBS Lett. 527, 147–152 [DOI] [PubMed] [Google Scholar]
- 24. Düser M. G., Zarrabi N., Cipriano D. J., Ernst S., Glick G. D., Dunn S. D., Börsch M. (2009) 36° step size of proton-driven c-ring rotation in FoF1-ATP synthase. EMBO J. 28, 2689–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishmukhametov R., Hornung T., Spetzler D., Frasch W. D. (2010) Direct observation of stepped proteolipid ring rotation in E. coli FoF1-ATP synthase. EMBO J. 29, 3911–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Junge W., Lill H., Engelbrecht S. (1997) ATP synthase: an electrochemical transducer with rotatory mechanics. Trends Biochem. Sci. 22, 420–423 [DOI] [PubMed] [Google Scholar]
- 27. Moore K. J., Angevine C. M., Vincent O. D., Schwem B. E., Fillingame R. H. (2008) The cytoplasmic loops of subunit a of Escherichia coli ATP synthase may participate in the proton translocating mechanism. J. Biol. Chem. 283, 13044–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steed P. R., Fillingame R. H. (2008) Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1F0 ATP synthase. J. Biol. Chem. 283, 12365–12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steed P. R., Fillingame R. H. (2009) Aqueous accessibility to the transmembrane regions of subunit c of the Escherichia coli F1F0 ATP synthase. J. Biol. Chem. 284, 23243–23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cain B. D., Simoni R. D. (1989) Proton translocation by the F1F0-ATPase of Escherichia coli: mutagenic analysis of the a subunit. J. Biol. Chem. 264, 3292–3300 [PubMed] [Google Scholar]
- 31. Hatch L. P., Cox G. B., Howitt S. M. (1995) The essential arginine residue at position 210 in the a subunit of the Escherichia coli ATP synthase can be transferred to position 252 with partial retention of activity. J. Biol. Chem. 270, 29407–29412 [DOI] [PubMed] [Google Scholar]
- 32. Valiyaveetil F. I., Fillingame R. H. (1997) On the role of Arg-210 and Glu-219 of subunit a in proton translocation by the Escherichia coli F0F1-ATP synthase. J. Biol. Chem. 272, 32635–32641 [DOI] [PubMed] [Google Scholar]
- 33. Langemeyer L., Engelbrecht S. (2007) Essential arginine in subunit a and aspartate in subunit c of FoF1 ATP synthase: effect of repositioning with helix 4 of subunit a and helix 2 of subunit c. BBA-Bioenergetics 1767, 998–1005 [DOI] [PubMed] [Google Scholar]
- 34. Ishmukhametov R. R., Pond J. B., Al-Huqail A., Galkin M. A., Vik S. B. (2008) ATP synthesis without R210 of subunit a in the Escherichia coli ATP synthase. Biochim. Biophys. Acta. 1777, 32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitome N., Ono S., Sato H., Suzuki T., Sone N., Yoshida M. (2010) Essential arginine residue of the F0-a subunit in F0F1-ATP synthase has a role to prevent the proton shortcut without c-ring rotation in the F0 proton channel. Biochem. J. 430, 171–177 [DOI] [PubMed] [Google Scholar]
- 36. Miller M. J., Oldenburg M., Fillingame R. H. (1990) The essential carboxyl group in subunit c of the F1F0 ATP synthase can be moved and H+ translocating function retained. Proc. Natl. Acad. Sci. U.S.A. 87, 4900–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lomize M. A., Lomize A. L., Pogozheva I. D., Mosberg H. I. (2006) OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625 [DOI] [PubMed] [Google Scholar]
- 38. Rosso L., Gould I. R. (2008) Structure and dynamics of phospholipid bilayers using recently developed general all-atom force fields. J. Comput. Chem. 29, 24–37 [DOI] [PubMed] [Google Scholar]
- 39. Meier T., Matthey U., Henzen F., Dimroth P., Müller D. J. (2001) The central plug in the reconstituted undecameric c cylinder of a bacterial ATP synthase consists of phospholipids. FEBS Lett. 505, 353–356 [DOI] [PubMed] [Google Scholar]
- 40. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Case D. A., Cheatham T. E., 3rd, Darden T., Gohlke H., Luo R., Merz K. M., Jr., Onufriev A., Simmerling C., Wang B., Woods R. J. (2005) The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cornell W. D., Cieplak P., Bayly C. I., Gould I. R., Merz K. M., Ferguson D. M., Spellmeyer D. C., Fox T., Caldwell J. W., Kollman P. A. (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117, 5179–5197 [Google Scholar]
- 43. Simmerling C., Strockbine B., Roitberg A. E. (2002) All-atom structure prediction and folding simulations of a stable protein. J. Am. Chem. Soc. 124, 11258–11259 [DOI] [PubMed] [Google Scholar]
- 44. Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 [Google Scholar]
- 45. Wang J., Wolf R. M., Caldwell J. W., Kollman P. A., Case D. A. (2004) Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174; Correction (2004) J. Comput. Chem.26, 114 [DOI] [PubMed] [Google Scholar]
- 46. Bayly C. I., Cieplak P., Cornell W. D., Kollman P. A. (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atom charges: the RESP model. J. Phys. Chem. 97, 10269–10280 [Google Scholar]
- 47. Tieleman D. P., Marrink S. J., Berendsen H. J. (1997) A computer perspective of membranes: molecular dynamics studies of lipid bilayer systems. Biochim. Biophys. Acta 1331, 235–270 [DOI] [PubMed] [Google Scholar]
- 48. Anézo C., de Vries A. H., Höltje H.-D., Tieleman D. P., Marrink S.-J. (2003) Methodological issues in lipid bilayer simulations. J. Phys. Chem. B 107, 9424–9433 [Google Scholar]
- 49. Darden T., York D., Pedersen L. (1993) Particle mesh Ewald: an N · log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 [Google Scholar]
- 50. Ryckaert J.-P., Ciccotti G., Berendsen H. J. (1977) Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 [Google Scholar]
- 51. Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J. R. (1984) Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 [Google Scholar]
- 52. Suhre K., Sanejouand Y. H. (2004) ElNémo: a normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res. 32, W610–W614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hakulinen J. K., Klyszejko A. L., Hoffmann J., Eckhardt-Strelau L., Brutschy B., Vonck J., Meier T. (2012) Structural study on the architecture of the bacterial ATP synthase Fo motor. Proc. Natl. Acad. Sci. U.S.A. 109, E2050–E2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pogoryelov D., Yildiz O., Faraldo-Gómez J. D., Meier T. (2009) High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat. Struct. Mol. Biol. 16, 1068–1073 [DOI] [PubMed] [Google Scholar]
- 55. Symersky J., Pagadala V., Osowski D., Krah A., Meier T., Faraldo-Gómez J. D., Mueller D. M. (2012) Structure of the c10-ring of the yeast mitochondrial ATP synthase in the open conformation. Nat. Struct. Mol. Biol. 19, 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lovell S. C., Word J. M., Richardson J. S., Richardson D. C. (2000) The penultimate rotamer library. Proteins 40, 389–408 [PubMed] [Google Scholar]
- 57. Lau W. C., Rubinstein J. L. (2012) Subnanometre-resolution structure of the intact Thermus thermophilus H+-driven ATP synthase. Nature 481, 214–218 [DOI] [PubMed] [Google Scholar]
- 58. Valiyaveetil F. I., Fillingame R. H. (1998) Transmembrane topography of subunit a in the Escherichia coli F1F0 ATP synthase. J. Biol. Chem. 273, 16241–16247 [DOI] [PubMed] [Google Scholar]
- 59. Long J. C., Wang S., Vik S. B. (1998) Membrane topology of subunit a of the F1F0 ATP synthase as determined by labeling of unique cysteine residues. J. Biol. Chem. 273, 16235–16240 [DOI] [PubMed] [Google Scholar]
- 60. Meier T., Polzer P., Diederichs K., Welte W., Dimroth P. (2005) Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus. Science 308, 659–662 [DOI] [PubMed] [Google Scholar]
- 61. Krah A., Pogoryelov D., Meier T., Faraldo-Gómez J. D. (2010) On the structure of the proton-binding site in the Fo rotor of chloroplast ATP synthases. J. Mol. Biol. 395, 20–27 [DOI] [PubMed] [Google Scholar]
- 62. Pogoryelov D., Krah A., Langer J. D., Yildiz Ö., Faraldo-Gómez J. D., Meier T. (2010) Microscopic rotary mechanism of ion translocation in the Fo complex of ATP synthases. Nat. Chem. Biol. 6, 891–899 [DOI] [PubMed] [Google Scholar]
- 63. Jiang W., Fillingame R. H. (1998) Interacting helical faces of subunits a and c in the F1Fo-ATP synthase of Escherichia coli defined by disulfide cross-linking. Proc. Natl. Acad. Sci. U.S.A. 95, 6607–6612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dmitriev O. Y., Freedman K. H., Hermolin J., Fillingame R. H. (2008) Interaction of transmembrane helices in ATP synthase subunit a in solution as revealed by spin label difference NMR. BBA-Bioenergetics 1777, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pierson H. E., Uhlemann E. M., Dmitriev O. Y. (2011) Interaction with monomeric subunit c drives insertion of ATP synthase subunit a into the membrane and primes a-c complex formation. J. Biol. Chem. 286, 38583–38591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang D., Vik S. B. (2003) Close proximity of a cytoplasmic loop of subunit a with c subunits of the ATP synthase from Escherichia coli. J. Biol. Chem. 278, 12319–12324 [DOI] [PubMed] [Google Scholar]
- 67. Groth G. (2002) Structure of spinach chloroplast F1-ATPase complexed with the phytopathogenic inhibitor tentoxin. Proc. Natl. Acad. Sci. U.S.A. 99, 3464–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]