Abstract
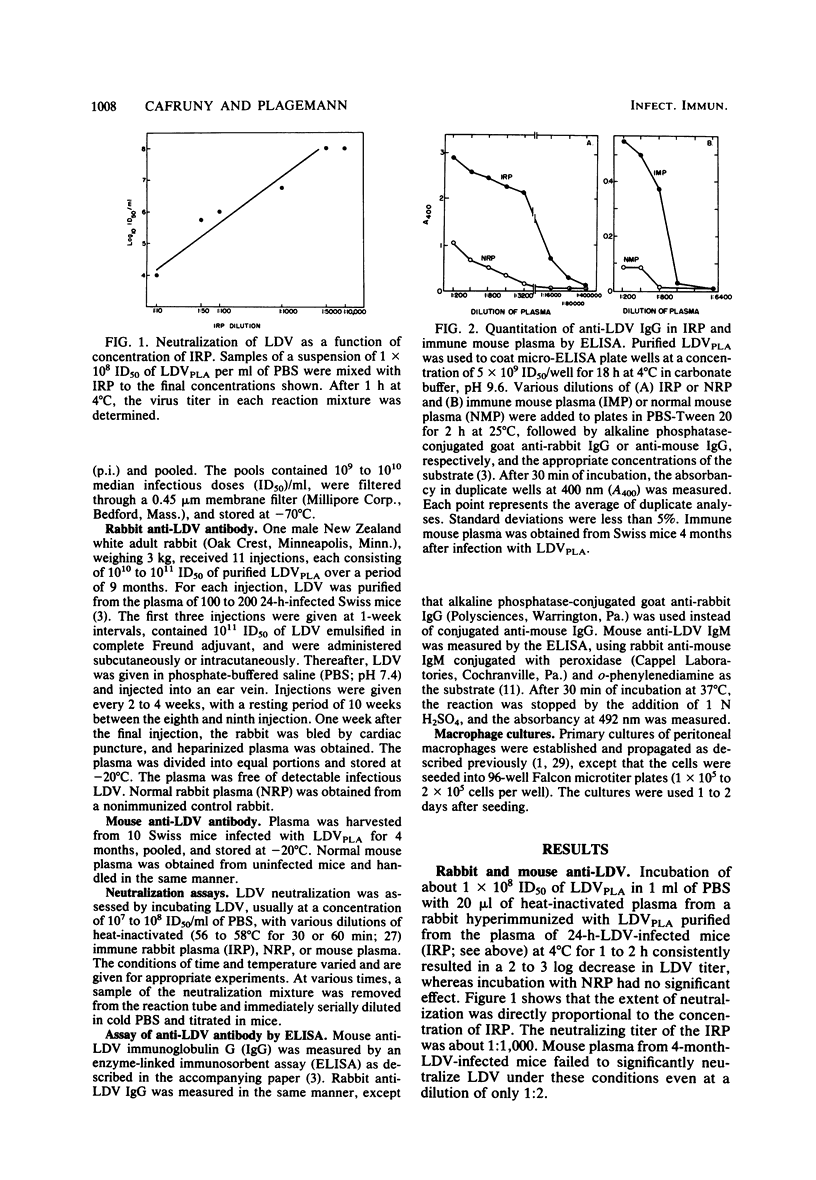
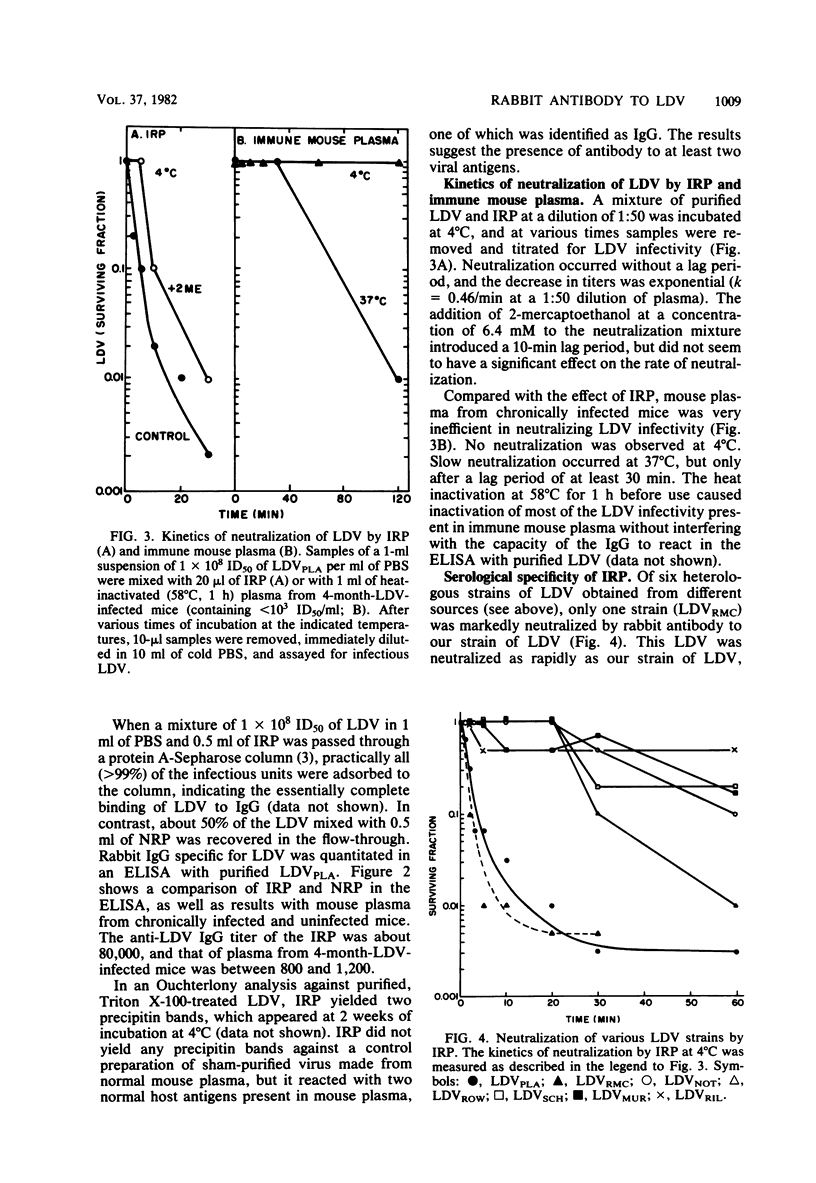
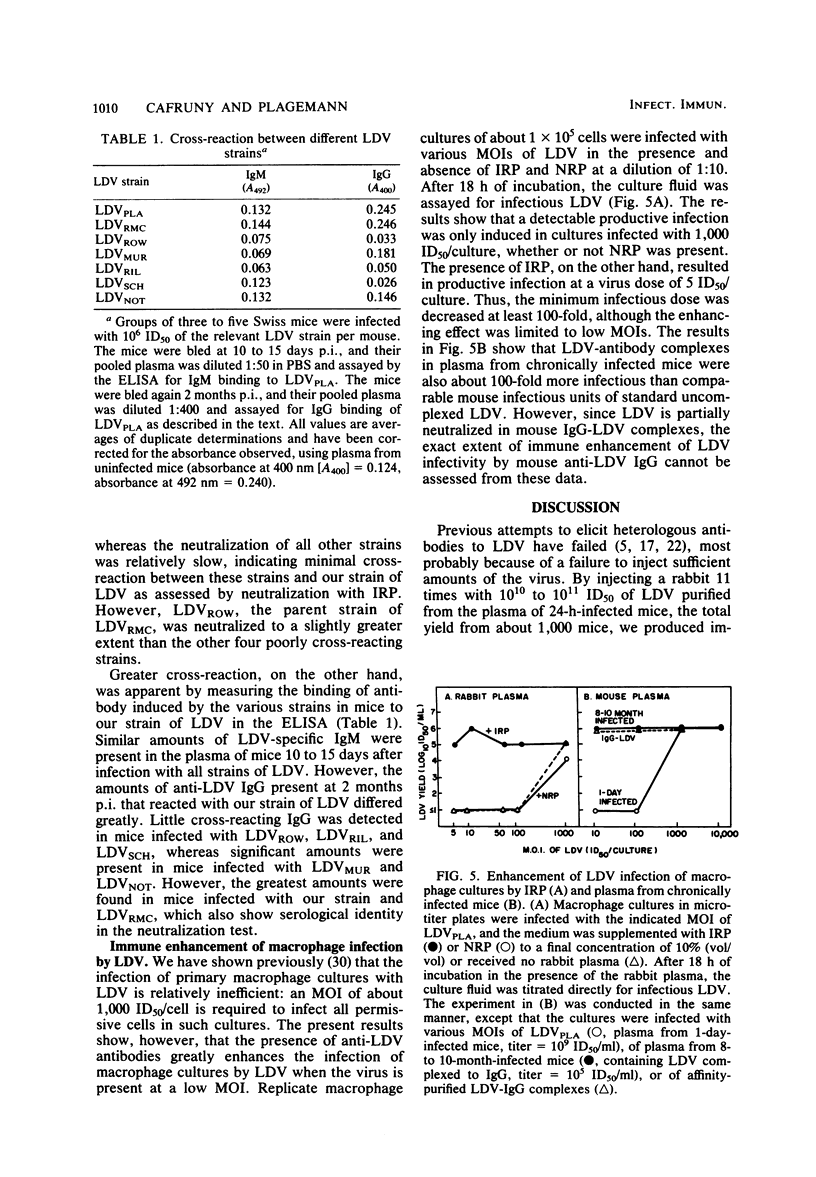
A rabbit was immunized with large amounts of the lactate dehydrogenase-elevating virus (LDV) over a 9-month period. The plasma from this rabbit possessed an anti-LDV IgG titer of 1:80,000 as measured by the enzyme-linked immunosorbent assay (ELISA) method and a neutralizing titer of 1:1,000 for the homologous strain of LDV. LDV neutralization at 4 degrees C followed single-hit kinetics. In contrast, mouse anti-LDV IgG in plasma of chronically LDV-infected mice failed to neutralize LDV at 4 degrees C and neutralization at 37 degrees C was slow, biphasic, and inefficient compared with the neutralization caused by rabbit anti-LDV IgG, even though high levels of anti-LDV IgG were detectable in mouse plasma by the ELISA method. Rabbit anti-LDV IgG neutralized one heterologous strain of LDV as rapidly as it did the homologous strain, but failed to significantly neutralize five other strains of LDV, all of which were originally isolated from different mouse strains bearing transplantable tumors. The results indicate clear serological differences between LDV strains. Cross-reactions between the strains, however, were observed by ELISA, using the antibody induced during persistent infection of mice with each LDV strain. Immunoglobulin M (IgM) from mice infected for 15 days with the various strains bound equally to our LDV strain. IgG obtained from 2-month-infected mice also cross-reacted, but to a varying extent which partly correlated with the specificity detected by neutralization. Both rabbit and mouse anti-LDV IgG enhanced the infectivity of LDV at a low multiplicity of infection for primary cultures of peritoneal mouse macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton-Darnell M., Collins J. K., Plagemann P. G. Lactate dehydrogenase-elevating virus replication, maturation, and viral RNA synthesis in primary mouse macrophage cultures. Virology. 1975 May;65(1):187–195. doi: 10.1016/0042-6822(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Brinton-Darnell M., Plagemann P. G. Structure and chemical-physical characteristics of lactate dehydrogenase-elevating virus and its RNA. J Virol. 1975 Aug;16(2):420–433. doi: 10.1128/jvi.16.2.420-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Du Buy H. G., Johnson M. L. Some properties of the lactic dehydrogenase agent of mice. J Exp Med. 1965 Sep 1;122(3):587–600. doi: 10.1084/jem.122.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977 Feb 24;265(5596):739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol. 1981 Nov;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Kliks S. C., Halstead S. B. An explanation for enhanced virus plaque formation in chick embryo cells. Nature. 1980 Jun 12;285(5765):504–505. doi: 10.1038/285504a0. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Michaelides M. C., Schlesinger S. Structural proteins of lactic dehydrogenase virus. Virology. 1973 Sep;55(1):211–217. doi: 10.1016/s0042-6822(73)81023-2. [DOI] [PubMed] [Google Scholar]
- NOTKINS A. L., GREENFIELD R. E., MARSHALL D., BANE L. Multiple enzyme changes in the plasma of normal and tumor-bearing mice following infection with the lactic dehydrogenase agent. J Exp Med. 1963 Feb 1;117:185–195. doi: 10.1084/jem.117.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOTKINS A. L. LACTIC DEHYDROGENASE VIRUS. Bacteriol Rev. 1965 Jun;29:143–160. doi: 10.1128/br.29.2.143-160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki J. F., Pease L. R., Murphy W. H. Etiologic role of lactic dehydrogenase virus infection in an age-dependent neuroparalytic disease in C58 mice. Virology. 1980 May;103(1):259–264. doi: 10.1016/0042-6822(80)90147-6. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mage M., Ashe W. K., Mahar S. Neutralization of sensitized lactic dehydrogenase virus by anti-gammglobulin. J Immunol. 1968 Feb;100(2):314–320. [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J. S., Gordon S., Unkeless J. C., Porterfield J. S. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature. 1981 Jan 15;289(5794):189–191. doi: 10.1038/289189a0. [DOI] [PubMed] [Google Scholar]
- RILEY V., LOVELESS J. D., FITZMAURICE M. A., SILER W. M. MECHANISM OF LACTATE DEHYDROGENASE (LDH) ELEVATION IN VIRUS-INFECTED HOSTS. Life Sci. 1965 Feb;4:487–507. doi: 10.1016/0024-3205(65)90098-6. [DOI] [PubMed] [Google Scholar]
- Riley V., Lilly F., Huerto E., Bardell D. Transmissible Agent Associated with 26 Types of Experimental Mouse Neoplasms. Science. 1960 Aug 26;132(3426):545–547. doi: 10.1126/science.132.3426.545. [DOI] [PubMed] [Google Scholar]
- Ritzi D. M., Holth M., Smith M. S., Swart W. J., Cafruny W. A., Plagemann G. W., Stueckemann J. A. Replication of lactate dehydrogenase-elevating virus in macrophages. 1. Evidence for cytocidal replication. J Gen Virol. 1982 Apr;59(Pt 2):245–262. doi: 10.1099/0022-1317-59-2-245. [DOI] [PubMed] [Google Scholar]
- Rowson K. E. A new host species for lactic dehydrogenase virus. Experientia. 1980 Sep 15;36(9):1066–1067. doi: 10.1007/BF01965972. [DOI] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W., Bendinelli M. Riley virus neutralizing activity in the plasma of infected mice with persistent viraemia. Virology. 1966 Apr;28(4):775–778. doi: 10.1016/0042-6822(66)90268-6. [DOI] [PubMed] [Google Scholar]
- Stueckemann J. A., Holth M., Swart W. J., Kowalchyk K., Smith M. S., Wolstenholme A. J., Cafruny W. A., Plagemann P. G. Replication of lactate dehydrogenase-elevating virus in macrophages. 2. Mechanism of persistent infection in mice and cell culture. J Gen Virol. 1982 Apr;59(Pt 2):263–272. doi: 10.1099/0022-1317-59-2-263. [DOI] [PubMed] [Google Scholar]
- Tong S. L., Stueckemann J., Plagemann P. G. Autoradiographic method for detection of lactate dehydrogenase-elevating virus-infected cells in primary mouse macrophage cultures. J Virol. 1977 Apr;22(1):219–227. doi: 10.1128/jvi.22.1.219-227.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


