Abstract
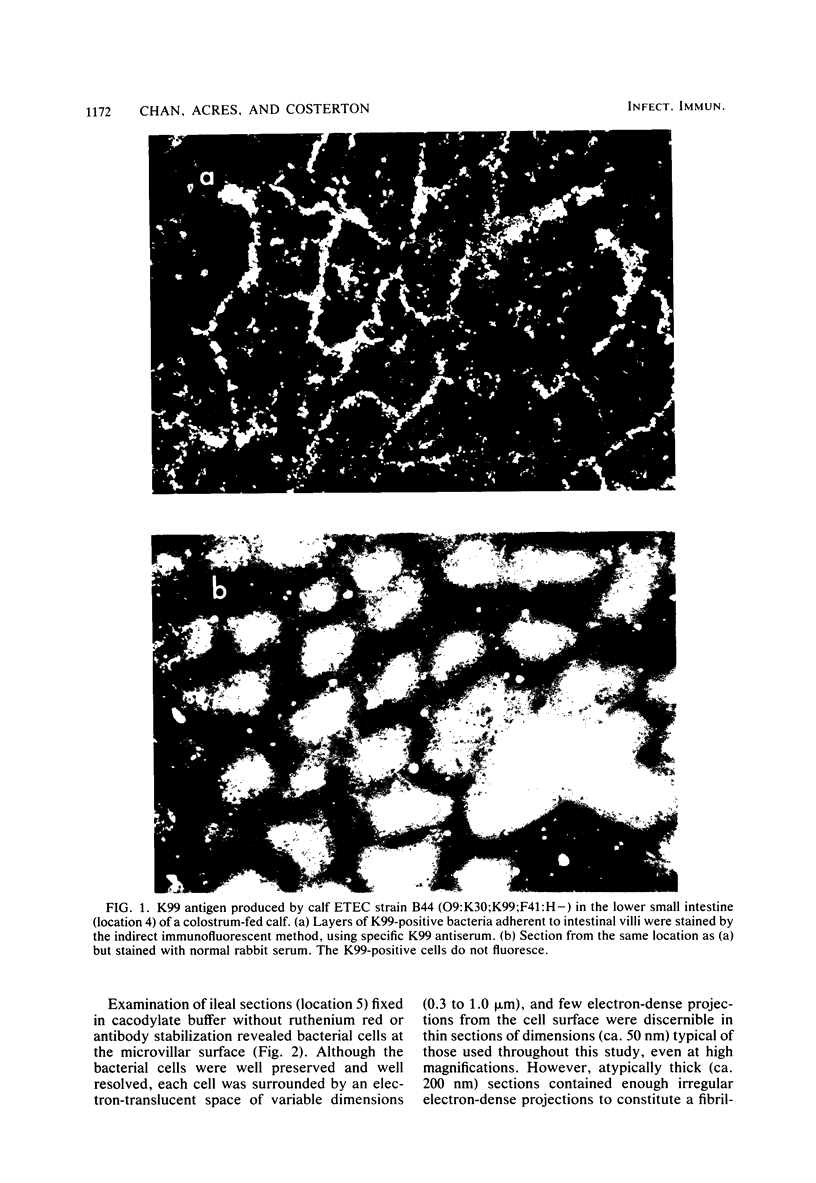
The attachment of enterotoxigenic Escherichia coli (ETEC) strain B44 (O9:K30:K99:F41:H-) to the ileal epithelium of newborn colostrum-fed calves was studied by electron microscopy. Stabilization of the bacterial glycocalyx (K30) and pili (K99) by fixation of tissue sections in specific antibody and staining with ruthenium red were used so that the bacterial surface structures could be clearly visualized and their spatial relationship to the intestinal brush border defined. When sections of ileum from infected calves were neither fixed in antibody nor stained with ruthenium red, the ETEC cells colonizing the small intestine were separated from each other and from the brush border by an electron-translucent halo; neither the glycocalyx nor the pili could be clearly resolved. When ruthenium red staining was used, the halo was partially filled by a net of electron-dense fibers composed of pili and condensed glycocalyx which extended to the brush border. Tissue sections reacted with anti-K30 antibody before staining with ruthenium red revealed microcolonies of ETEC surrounded by a discrete electron-dense glycocalyx 0.3 to 1.0 micrometers thick and in tight contact with the epithelial cell surface. When ileal tissue was treated with K99 antibody, the K99 pili were visible as discrete fibers extending from the bacterial cell surface through the glycocalyx. We discuss the role of these cell surface components in pathogenic adhesion and in the formation of protected microcolonies at the surface of the infected ileal epithelium.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy J. E., Acres S. D. Enterotoxigenic colibacillosis in colostrum-fed calves: pathologic changes. Am J Vet Res. 1979 Oct;40(10):1391–1397. [PubMed] [Google Scholar]
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A. K., Friebolin H., Stirm S. Primary structure of the Escherichia coli serotype K30 capsular polysaccharide. J Bacteriol. 1980 Feb;141(2):971–972. doi: 10.1128/jb.141.2.971-972.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Deneke C. F., Thorne G. M., Gorbach S. L. Serotypes of attachment pili of enterotoxigenic Escherichia coli isolated from humans. Infect Immun. 1981 Jun;32(3):1254–1260. doi: 10.1128/iai.32.3.1254-1260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Detection of enterotoxigenicity and attachment factors in Escherichia coli strains of human, porcine and bovine origin; a comparative study. Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):245–257. [PubMed] [Google Scholar]
- Guinée P. A., Veldkamp J., Jansen W. H. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977 Feb;15(2):676–678. doi: 10.1128/iai.15.2.676-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad J. J., Gyles C. L. Scanning and transmission electron microscopic study of the small intestine of colostrum-fed calves infected with selected strains of Escherichia coli. Am J Vet Res. 1982 Jan;43(1):41–49. [PubMed] [Google Scholar]
- Hadad J. J., Gyles C. L. The role of K antigens of enteropathogenic Escherichia coli in colonization of the small intestine of calves. Can J Comp Med. 1982 Jan;46(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- Hohmann A., Wilson M. R. Adherence of enteropathogenic Escherichia coli to intestinal epithelium in vivo. Infect Immun. 1975 Oct;12(4):866–880. doi: 10.1128/iai.12.4.866-880.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Moon H. W., Schneider R. A. Distribution and virulence of Escherichia coli in the small intestines of calves with and without diarrhea. Am J Vet Res. 1978 Nov;39(11):1750–1755. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Skartvedt S. M. Etiologic diagnosis of diarrheal disease of calves: frequency and methods for detecting enterotoxin and K99 antigen production by Escherichia cola. Am J Vet Res. 1976 Sep;37(9):1025–1029. [PubMed] [Google Scholar]
- Morris J. A., Thorns C. J., Sojka W. J. Evidence for two adhesive antigens on the K99 reference strain Escherichia coli B41. J Gen Microbiol. 1980 May;118(1):107–113. doi: 10.1099/00221287-118-1-107. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Thorns C., Scott A. C., Sojka W. J., Wells G. A. Adhesion in vitro and in vivo associated with an adhesive antigen (F41) produced by a K99 mutant of the reference strain Escherichia coli B41. Infect Immun. 1982 Jun;36(3):1146–1153. doi: 10.1128/iai.36.3.1146-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L. L., Guinée P. A. Occurrence and characteristics of enterotoxigenic Escherichia coli isolated from calves with diarrhea. Infect Immun. 1976 Apr;13(4):1117–1119. doi: 10.1128/iai.13.4.1117-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Rother U. A., Rother K. O. Interaction of complement components with a serum-resistant strain of Salmonella typhimurium. Infect Immun. 1975 May;11(5):944–948. doi: 10.1128/iai.11.5.944-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]