Abstract
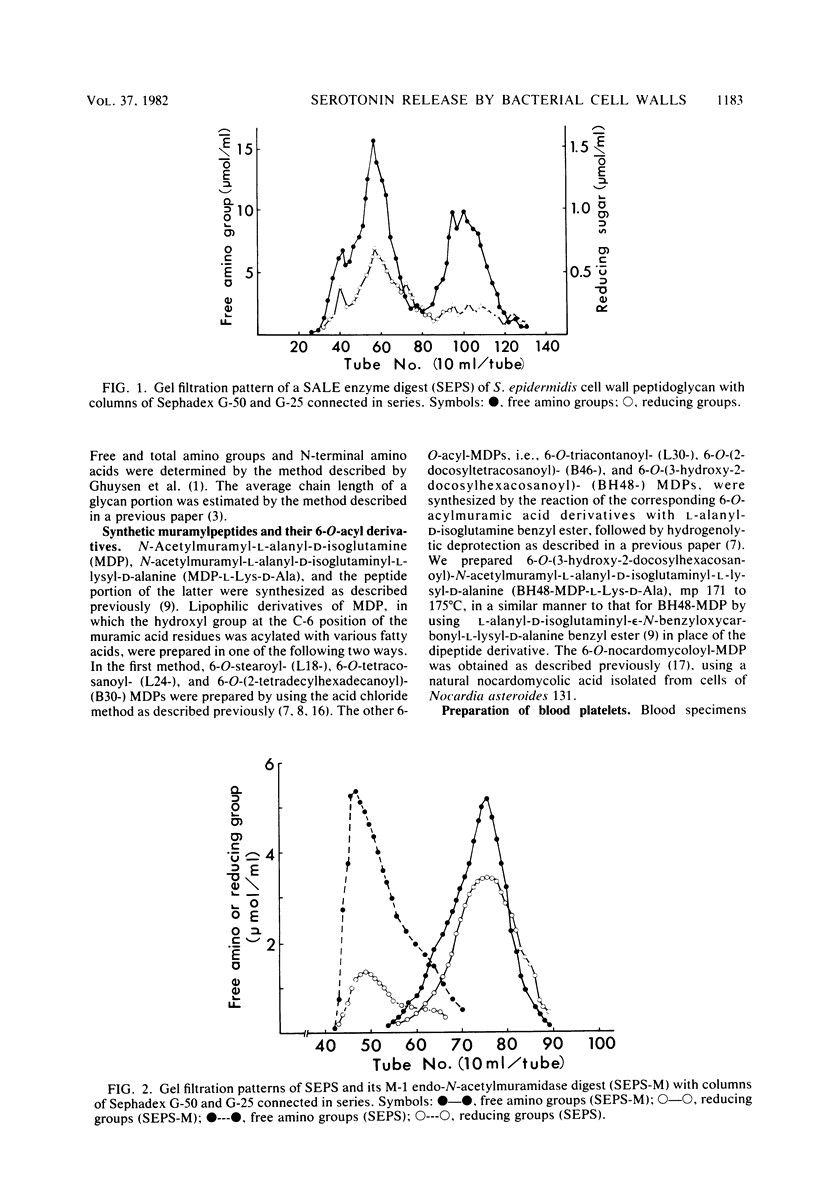
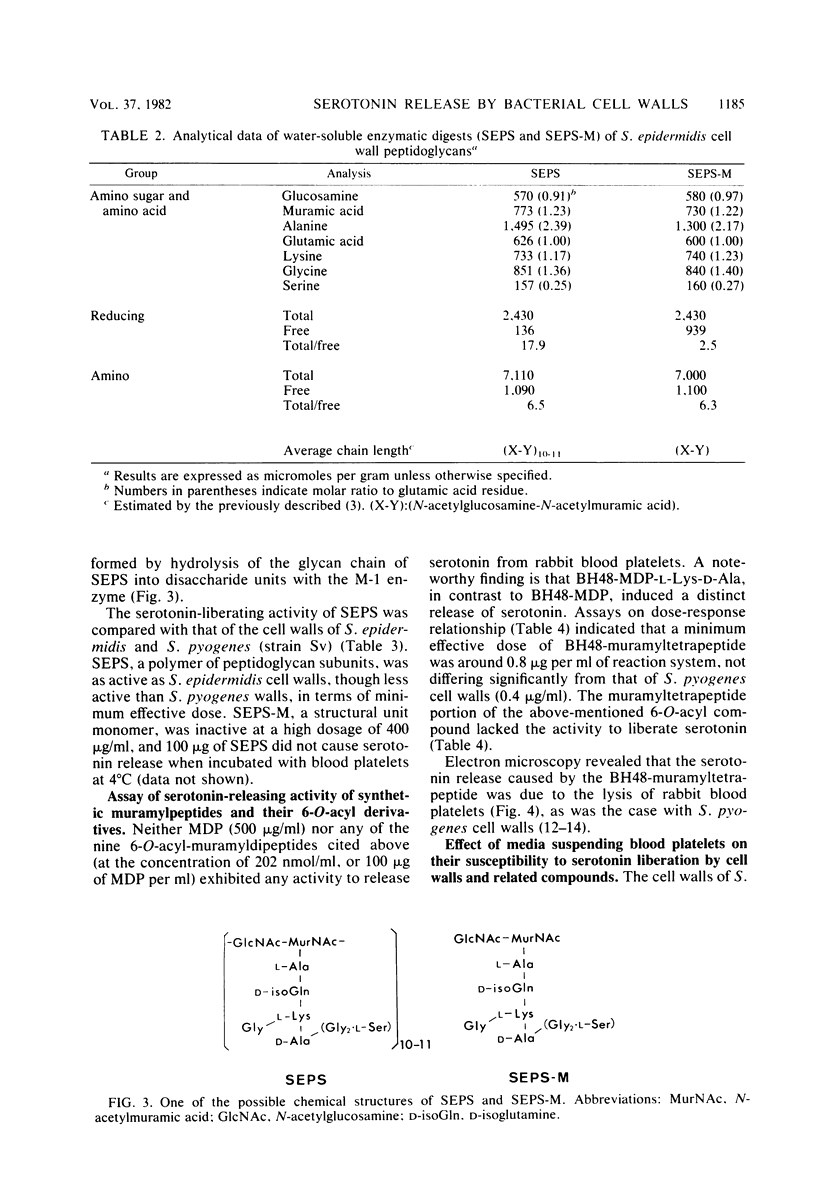
A study was made on the activity of various bacterial cell walls and peptidoglycans to liberate serotonin from rabbit blood platelets. All of the test cell walls or peptidoglycans prepared from 27 strains of 21 bacterial species were shown to cause a marked release of serotonin, regardless of differences in types of peptidoglycan and non-peptidoglycan moieties and in some biological properties. The assay made with the water-soluble "digests" of Staphylococcus epidermidis cell wall peptidoglycans, which were prepared by use of appropriate enzymes, revealed that a polymer of peptidoglycan subunits (a disaccharide-stempeptide) was definitely active in the release of serotonin, but a structural unit monomer was inactive. Among a variety of synthetic muramylpeptides and their 6-O-acyl derivatives, only 6-O-(3-hydroxy-2-docosylhexacosanoyl)-N-acetylmuramyl-L-alanyl-D-isoglutaminyl- L-lysyl-D-alanine was found to hold a strong serotonin-liberating activity.
Full text
PDF


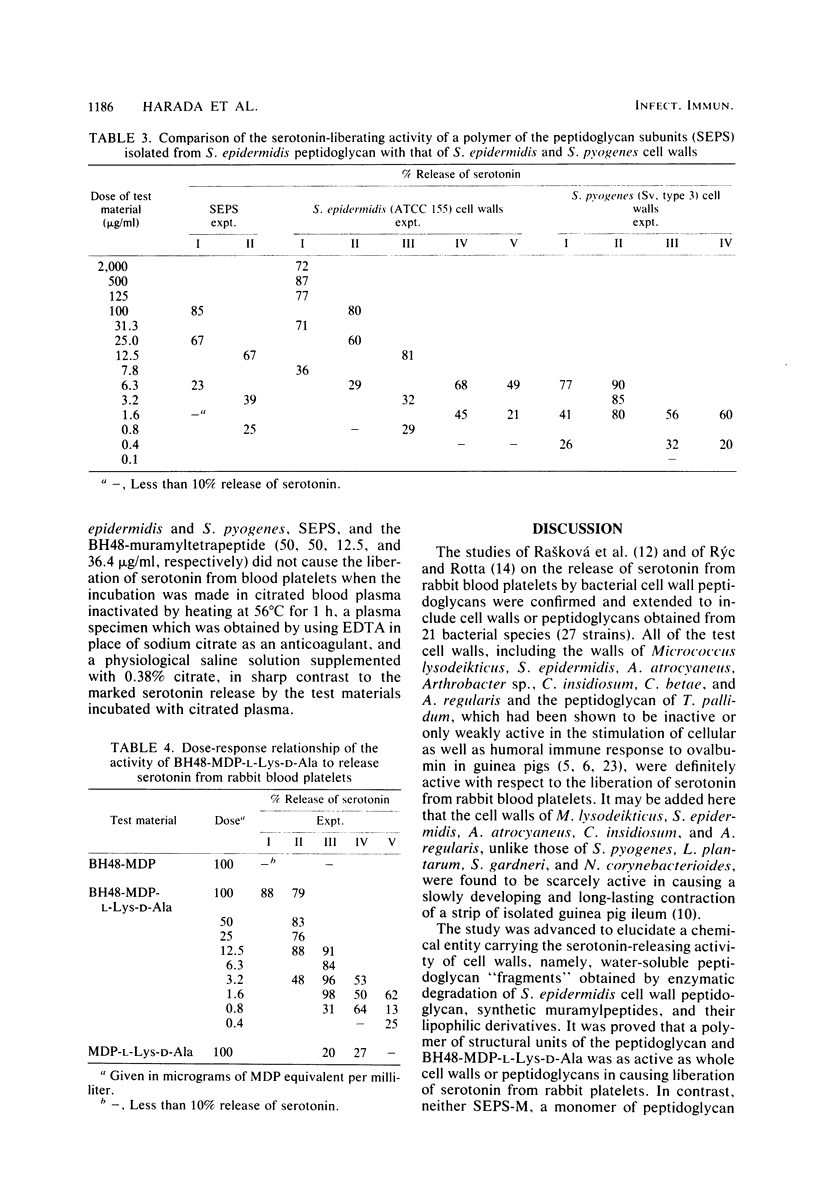
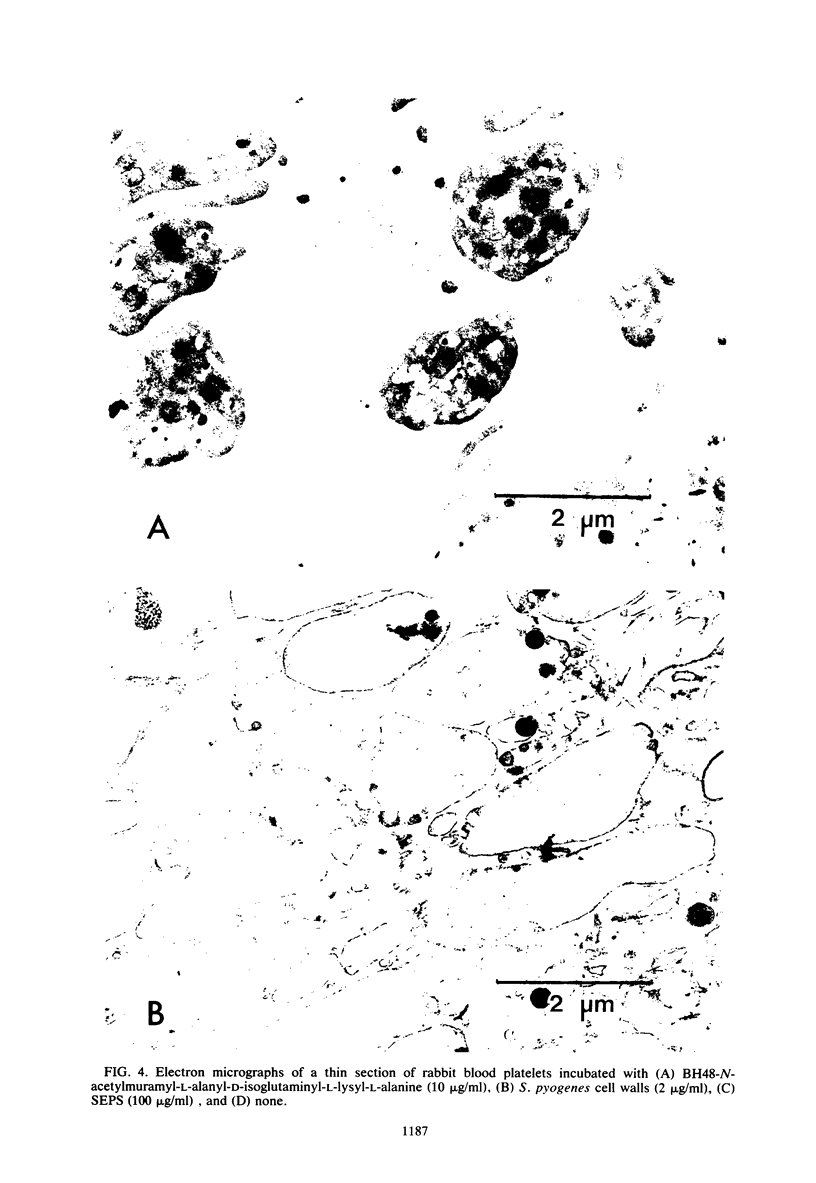
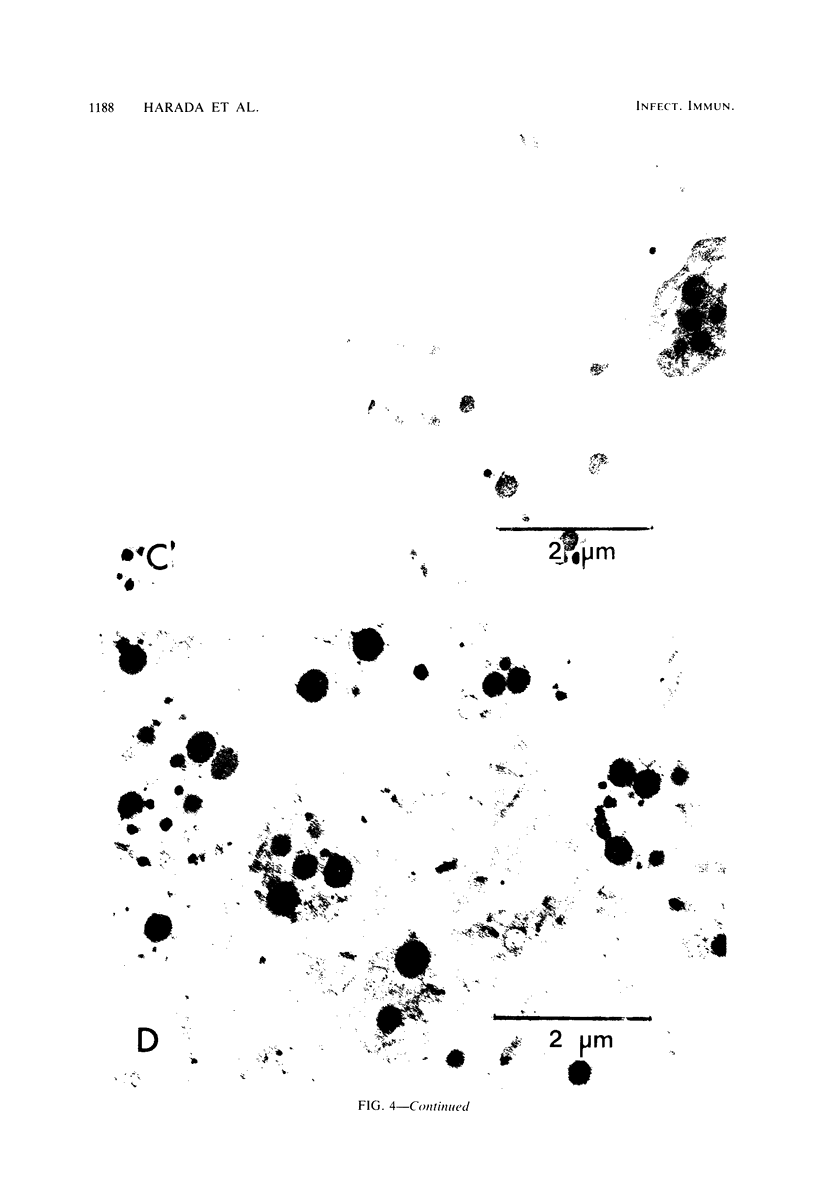


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kato K., Iwata S., Suginaka H., Namba K., Kotani S. Chemical structure of the peptidoglycan of Vibrio parahaemolyticus A55 with special reference to the extent of interpeptide cross-linking. Biken J. 1976 Dec;19(4):139–150. [PubMed] [Google Scholar]
- Kato K., Strominger J. L. Structure of the cell wall of Staphylococcus aureaus. IX. Mechanism of hydrolysis by the L11 enzyme. Biochemistry. 1968 Aug;7(8):2745–2761. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Schleifer K. H. Inabilities as an immunoadjuvant of cell walls of the group B peptidoglycan types and those of arthrobacters. Biken J. 1977 Mar;20(1):1–4. [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Tsujimoto M., Kusumoto S., Shiba T., Kawata S., Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982 Feb;35(2):612–619. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rotta J., Rýc M., Masek K., Zaoral M. Biological activity of synthetic subunits of streptococcus peptidoglycan. I. Pyrogenic and thrombocytolytic activity. Exp Cell Biol. 1979;47(4):258–268. doi: 10.1159/000162944. [DOI] [PubMed] [Google Scholar]
- Rýc M., Rotta J. The thrombocytolytic activity of bacterial peptidoglycans. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):265–272. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Axelrod J., Zweig M. A sensitive and specific fluorescence assay for tissue serotonin. Biochem Pharmacol. 1965 May;14(5):831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- Takada H., Hirachi Y., Hashizume H., Kotani S. Mitogenic activity of cytoplasmic membranes isolated from L-forms of Staphylococcus aureus. Microbiol Immunol. 1980;24(11):1079–1090. doi: 10.1111/j.1348-0421.1980.tb02913.x. [DOI] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Berman M. F. Structures of the cell wall peptidoglycans of Staphylococcus epidermidis Texas 26 and Staphylococcus aureus Copenhagen. I. Chain length and average sequence of cross-bridge peptides. Biochemistry. 1969 May;8(5):2183–2192. doi: 10.1021/bi00833a060. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Structures of the cell wall peptidoglycans of Staphylococcus epidermidis Texas 26 and Staphylococcus aureus Copenhagen. II. Structure of neutral and basic peptides from hydrolysis with the Myxobacter al-1 peptidase. Biochemistry. 1969 May;8(5):2192–2202. doi: 10.1021/bi00833a061. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Ota T., Sagawa H., Kato K., Takada H., Tsujimoto M., Kawasaki A., Ogawa T., Harada K., Kotani S. Chemical and biological properties of a peptidoglycan isolated from Treponema pallidum kazan. Infect Immun. 1981 Feb;31(2):767–774. doi: 10.1128/iai.31.2.767-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]