Abstract
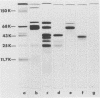
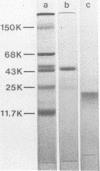
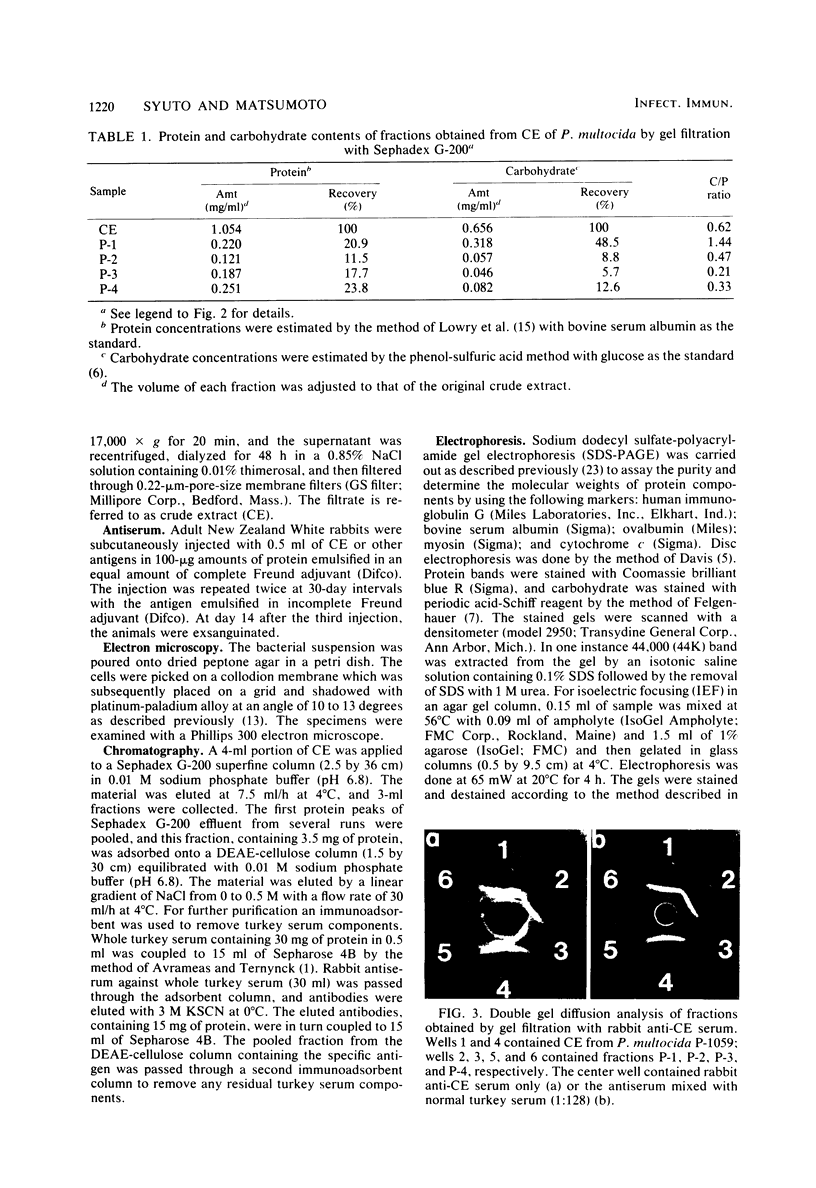
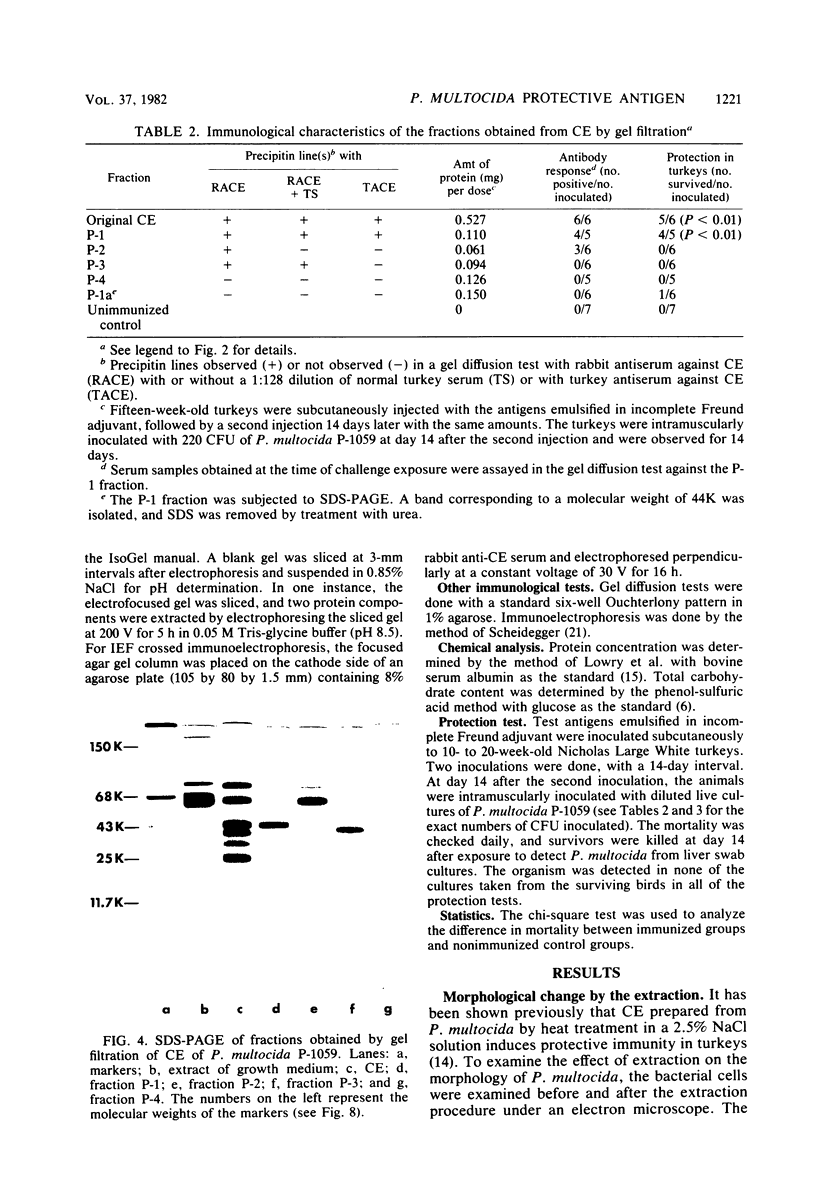
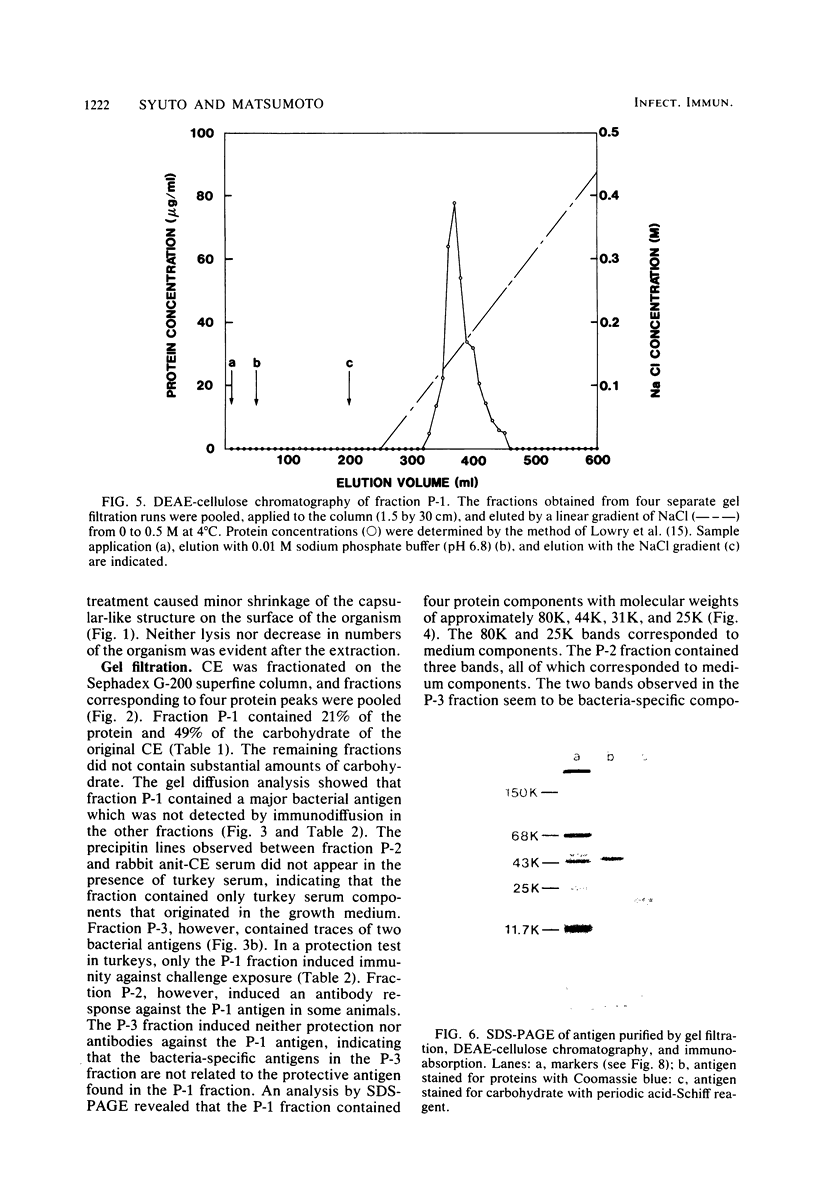
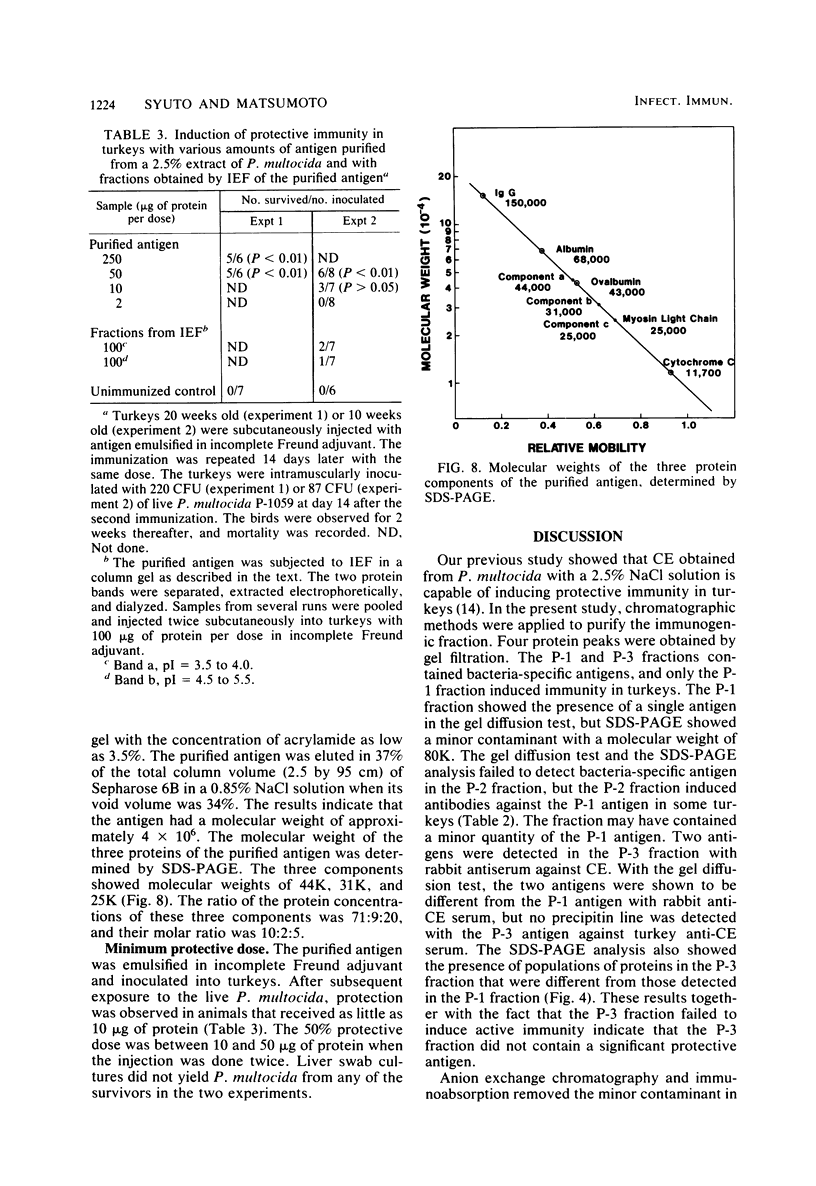
It has been shown previously that soluble material extracted from Pasteurella multocida P-1059 by a 2.5% NaCl solution protects turkeys from generalized septicemia at a subsequent challenge exposure to the organism. In the present study, a protective antigen was purified from the crude soluble material by chromatographic methods. Four protein peaks were obtained by gel filtration with Sephadex G-200. The protective antigen was detected only in the first peak fraction, which contained a substantial amount of carbohydrate. The peak 1 fraction was adsorbed onto DEAE-cellulose and eluted by a linear gradient of NaCl. Fractions corresponding to a single protein peak were pooled and passed through an immunoadsorbent column to remove any possible serum component originating from the growth medium. The purified antigen had a carbohydrate/protein ratio of 1.5 and formed a single precipitin line with rabbit antiserum against the crude material in gel diffusion and immunoelectrophoresis analyses. The antigen produced antibodies in rabbits and turkeys which formed a single precipitin line against the crude material. Upon sodium dodecyl sulfate-polyacryl-amide gel electrophoresis, the purified antigen showed three protein bands, corresponding to molecular weights of 44,000, 31,000, and 25,000, and one carbohydrate band. The carbohydrate band did not correspond to any of the three protein bands. Upon isoelectric focusing gel analysis, the purified antigen showed two bands (pI = 3.5 to 4.0 and 4.5 to 5.5), but the two bands were antigenically identical by isoelectric focusing crossed immunoelectrophoresis. The 50% protective dose of the purified antigen was between 10 and 50 μg of protein in trials where two doses were given at 14-day intervals to 10- to 20-week-old turkeys.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Baba T. Immunogenic activity of a ribosomal fraction obtained from Pasteurella multocida. Infect Immun. 1977 Jan;15(1):1–6. doi: 10.1128/iai.15.1.1-6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. W., Derieux W. T. Immunologic response of turkeys to an avirulent Pasteurella multocida vaccine in the drinking water. Poult Sci. 1972 Mar;51(2):408–416. doi: 10.3382/ps.0510408. [DOI] [PubMed] [Google Scholar]
- Carter G. R. Pasteurellosis: Pasteurella multocida and Pasteurella hemolytica. Adv Vet Sci. 1967;11:321–379. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. Quantitation and specific detection methods after disc electrophoresis of serum proteins. Clin Chim Acta. 1970 Feb;27(2):305–312. doi: 10.1016/0009-8981(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Ganfield D. J., Rebers P. A., Heddleston K. L. Immunogenic and toxic properties of a purified lipopolysaccharide-protein complex from Pasteurella multocida. Infect Immun. 1976 Oct;14(4):990–999. doi: 10.1128/iai.14.4.990-999.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt G., Moffat R., Mukkur T. K. Fowl cholera: immunization of chickens with potassium thiocyanate (KSCN) extract of Pasteurella multocida serotype 3. Avian Dis. 1977 Oct-Dec;21(4):543–548. [PubMed] [Google Scholar]
- HOUWINK A. L., van ITERSON W. Electron microscopical observations on bacterial cytology; a study on flagellation. Biochim Biophys Acta. 1950 Mar;5(1):10–44. doi: 10.1016/0006-3002(50)90144-2. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Kodama H., Matsumoto M., Snow L. M. Immunogenicity of capsular antigens of Pasteurella multocida in turkeys. Am J Vet Res. 1981 Oct;42(10):1838–1841. [PubMed] [Google Scholar]
- Krivastava K. K., Foster J. W. Characterization of an immunogenic fraction of Pasteurella multocida culture filtrates. Can J Microbiol. 1977 Feb;23(2):197–201. doi: 10.1139/m77-028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maheswaran S. K., Johnson G. H., Pomeroy B. S. Studies on Pasteurella multocida. I. The capsular polysaccharides from turkey isolates. Avian Dis. 1973 Oct-Dec;17(4):705–716. [PubMed] [Google Scholar]
- Nagy L. K., Penn C. W. Protection of cattle against experimental haemorrhagic septicaemia by the capsular antigens of Pasteurella multocida, types B and E. Res Vet Sci. 1976 May;20(3):249–253. [PubMed] [Google Scholar]
- Rimler R. B., Rebers P. A., Rhoades K. R. Fowl cholera: cross-protection induced by Pasteurella multocida separated from infected turkey blood. Avian Dis. 1979 Jul-Sep;23(3):730–741. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]