Abstract
The heterologous expression of laccases is important for their large-scale production and genetic engineering—a prerequisite for industrial application. Pichia pastoris is the preferred expression host for fungal laccases. The recently cloned laccase from the ascomycete Botrytis aclada (BaLac) has been efficiently expressed in P. pastoris under the control of the inducible alcohol oxidase (AOX1) promoter. In this study, we compare these results to the constitutive expression in the same organism using the glyceraldehyde-3-phosphate dehydrogenase (GAP) promoter. The results show that the amounts of BaLac produced with the GAP system (517 mgL-1) and the AOX1 system (495 mgL-1) are comparable. The constitutive expression is, however, faster, and the specific activity of BaLac in the culture supernatant is higher (41.3 Umg-1 GAP, 14.2 Umg-1 AOX1). In microtiter plates, the constitutive expression provides a clear advantage due to easy manipulation (simple medium, no methanol feeding) and fast enzyme production (high-throughput screening assays can already be performed after 48 h).
Keywords: Botrytis aclada, Pichia pastoris, 96-well plate expression, GAP promoter, ascomycete, chloride tolerance, high yield, laccase screening, laccase, constitutive expression
Introduction
Laccases are blue multi-copper oxidoreductases (EC 1.10.3.2) found in fungi and plants, but also in bacteria and insects. They feature a broad substrate spectrum and catalyze the monoelectronic oxidation of phenols and amines in a rather unspecific binding site by a T1 copper center. Four electrons are subsequently transferred via a cysteine to the trinuclear T2/T3 copper center, which binds an oxygen molecule that is stepwise reduced to water by a four-electron reduction.1 The biological functions of laccases include stress defense, lignin degradation and morphogenesis by catalyzing polymerization and depolymerization reactions.2 Laccases are also regarded as promising biocatalysts for a large variety of biocatalytic applications, including bleaching, dye decolorization and bioremediation, and could replace chemical process steps as an environmentally benign alternative. Another emerging field is the application of laccase in biosensors for the detection of phenols and catecholamines in environmental or biological samples, as well as a biofuel cell cathode catalyst for the generation of electricity. By combining biosensors and biofuel cells miniaturized, implantable sensor-transmitter systems for continuous biomedical monitoring can be realized. The inhibition of laccase by chloride reduces the practical usability of these highly promising enzymes. The high NaCl concentration in blood (140 mM) is enough to inhibit the activity of most laccases by > 80%.3 For applications in biosensors, biofuel cells and biocatalyst is a chloride-tolerant laccase is of great value.
The laccase gene from the plant pathogenic ascomycete B. aclada (BaLac) was isolated and heterologously expressed in Pichia pastoris under the control of the inducible alcohol oxidase promoter (AOX1). It was selected from a screening of ascomycetes for chloride tolerant laccases and found to be the most chloride-tolerant fungal laccase identified to date, with an I50 of 1.4 M NaCl.3 Recombinant BaLac is glycosylated, has a very low isoelectric point, 2.4, and a high substrate affinity. Compared to the median KM values of laccases for ABTS, 2.6-DMP and guaiacol (39, 405 and 420 ∝M, respectively),4 the KM values for BaLac were 2.9, 19.2 and 73.3 ∝M, respectively.3 Only very few laccases from ascomycete fungi have been expressed recombinantly,5-7 all of them with relatively low yields. In contrast, the expression of the BaLac gene in P. pastoris resulted in 51,000 UL-1 (495 mgL-1) in the culture supernatant.3 This is the highest yield of a laccase recombinantly produced in yeast—an obvious benefit for possible industrial applications.
A further improvement of the physical and catalytic properties of BaLac for synthetic or analytic applications may be achieved by enzyme engineering. An essential prerequisite for the establishment of a high-throughput screening assay to identify beneficial mutations in site-saturation or directed evolution libraries is a fast, easy and high level expression of BaLac variants. P. pastoris has been successfully used for functional heterologous expression and secretion of a large number of proteins.8 The advantages of this host are the simplicity of the genetic manipulation and the availability of the strong methanol-inducible alcohol oxidase (AOX1) promoter and the constitutive glyceraldehyde-3-phosphate dehydrogenase (GAP) promoter. Most proteins were expressed in P. pastoris using the AOX1 promoter to drive expression. A significant failing of this promoter is that biomass production is separated from expression owing to its strong inhibition by glucose, and it additionally requires continuous induction with methanol.8 In contrast, the constitutive GAP promoter allows simultaneous growth and expression. There is no clear indication as to which promoter yields the higher product levels. Comparisons of expression levels using both promoters were reported to be similar; however, with remarkably shorter fermentation times with the GAP promoter.9 Others achieved higher yields using either the AOX1 promoter10 or the GAP promoter.11
Herein, we report on the constitutive expression of BaLac in P. pastoris using the GAP promoter and the application of this expression system to establish the robust and simple expression of BaLac for high-throughput screenings of enzyme variants. Additionally, the suitability of the constitutive expression system for a large-scale production of BaLac was investigated.
Constitutive Expression of Botrytis aclada Laccase in Microtiter Plates
Directed evolution or semi-rational design requires the production of thousands of mutated enzyme variants followed by a fast and reliable high-throughput screening. Expression strategies for high-throughput screenings based on 96-well deep well plates are established for expression in P. pastoris under control of the AOX1 promoter.12 They rely on methanol feeding, which is labor-intensive and limits the amount of plates that can be handled by a single person. Furthermore, due to a batch phase before induction, the cultivation needs a minimum of four to six days until sufficient amounts of recombinant enzyme are secreted for a screening assay.
Therefore, a short cultivation time without the need for additional handling would simplify the production of enzyme variants and increase the number of 96-well plates that can be screened. We therefore studied the use of the alternative expression vector pGAPZA (Invitrogen) which allows constitutive expression using the GAP promoter instead of the AOX1 promoter. The BaLac cDNA with its native signal sequence was amplified with the primers 5BAPml1 (5'-ATA CAC GTG CAA GAT GAA GTA TTT CAC AGT CTT TAC TGC-3') and 3BaApa1 (5'-ATA GGG CCC TTA AAT TCC AGA ATC GTC CTC AGC-3') and cloned into the pGAPZA vector between the restriction sites PmlI and ApaI. To simplify cloning of mutant libraries by allowing time-saving single step digestion, these restriction sites were exchanged to NdeI and NotI by site-directed mutagenesis using the QuikChangeTM Site-Directed Mutagenesis Kit PCR (Stratagene) and the primers 5NdeBAInt (5'-TAT TTC GAA CAT ATG AAG TAT TTC ACA GTC TTT ACT G-3') and 3NdeBAInt (5'-ATA TGT TCG AAA TAG TTG TTC AAT TGA TTG-3') for the NdeI site and 5NotBAint (5'-AGC GGC CGC ACA AAA ACT CAT CTC AGA AGA GG-3') and 3NotBAint (5'-TGT GCG GCC GCT TAA ATT CCA GAA TCG TCC TCA G-3') for the NotI site. The resulting plasmid was transformed into electrocompetent P. pastoris X33 cells (Invitrogen) and the resulting colonies were used to inoculate microtiter plates filled with 200 ∝L medium by picking with sterile toothpicks. To keep the cultivation as simple as possible YPD medium (1% yeast extract, 2% peptone, 1% D-glucose) was used. To avoid a strong drop of pH it was buffered with a 100 mM potassium phosphate buffer, pH 5.5. The 96-well plates were incubated at 25°C on a shaker at 400 rpm for 48 hours. The influence of the glucose concentration on the enzyme yield was investigated by varying the concentration between 0.2% and 1%. The glucose concentration was limited to these low concentrations to guarantee the depletion of the carbon source within cultivation time and therefore minimize the variation in enzyme activity caused by unequal growth. Laccase activity in the harvested supernatants was determined in a photometric activity assay using 1 mM ABTS [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)] in 50 mM citrate buffer, pH 5 at 420 nm (∑420 = 36,000 M-1cm-1). One unit was defined as the amount of enzyme needed to generate 1 ∑mol of the ABTS cation radical per minute at 25°C. The highest average activity of 0.109 ± 0.012 UmL-1 was measured in wells filled with YDP medium with 1% glucose (Table 1). An activity above 0.1 UmL-1 is enough for subsequent screening assays on laccase activity, and a standard deviation ≤15% is acceptable for microtiter plate screening assays. These results can be compared to the yield of 0.34 ± 0.08 UmL-1 reached in the expression of B. aclada laccase in P. pastoris under control of the AOX1 promoter in 96-well plates after six days (unpublished results), which was performed according to Weis et al. The constitutively expressed BaLac activity could already be assayed after two days and handling was simpler without the necessity for methanol feeding. The proposed fast and simple constitutive expression is therefore suitable for high-throughput screening assays.
Table 1. Average Botrytis aclada laccase activity after cultivation in buffered YPD medium (0.2; 0.4; 0.7 and 1% glucose) for 48 hours.
| Glucose concentration [%] |
Activity [U mL-1] |
|---|---|
| 0.2 |
0.013 ± 0.006 |
| 0.4 |
0.027 ± 0.012 |
| 0.7 |
0.034 ± 0.008 |
| 1 | 0.109 ± 0.012 |
Fermentation of Pichia pastoris (pGAPZA) in a 5 L Bioreactor
Following the expression in 96-well plates a large-scale fermentation of BaLac was done in a 5 L bioreactor (MBR, Wetzikon, Switzerland) to compare the constitutive expression to the inducible expression with the AOX1 promotor.3 The batch phase of the fermentation started with an initial volume of 2.5 L medium for 21.5 h followed by a 75 h glycerol fed-batch phase according to modified “Pichia Fermentation Process Guidelines” (Invitrogen). The medium was supplemented with 0.1 mM CuSO4 and inoculation was done with a 250 ml overnight preculture. The stirrer speed was set to 800 rpm while the air flow was increased from initially 3 Lmin-1 to 5 Lmin-1 throughout the fed-batch phase. The 50% glycerol feed medium contained 12 mlL-1 Pichia trace metal (PTM1) salts and the feed rate was adjusted to keep dissolved oxygen concentration at 4%. To limit the growth rate and increase oxygen saturation, the temperature was gradually decreased from 30 to 25°C after the batch phase, from 25°C to 23°C after 45.5 h, and from 23 to 20°C after 69.25 h. Antifoam was injected manually, as required. Samples were analyzed for wet biomass, laccase activity and total protein concentration as described in Kittl et al.
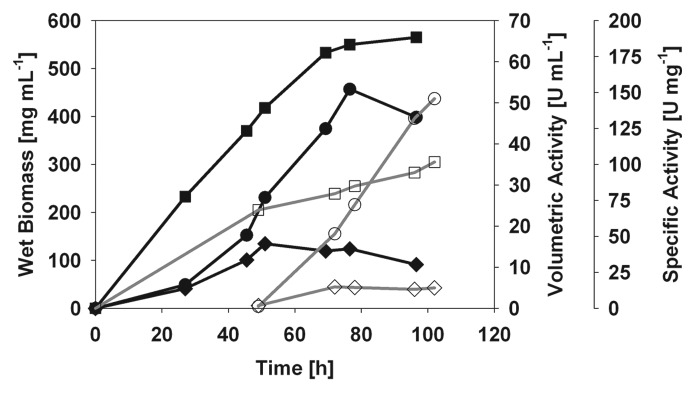
The advantage of the constitutive expression is obviously that the target protein is produced throughout the whole fermentation. At the time when the AOX1 fermentation was induced and BaLac production started, already more than 23 UmL-1 of laccase activity were measured in the culture supernatant of the GAP fermentation (Fig. 1). The maximum activity of 53.3 UmL-1 was reached after only 76 hours with a very high specific activity (> 40 Umg-1). At this time, the wet biomass exceeded 550 gL-1, and oxygen limitation slowed down the growth of the culture causing cell lysis. This was followed by a degradation of BaLac by proteases. Switching from air to pure oxygen would have kept the culture growing, and a higher target protein yield could have been achieved by an elongated fermentation time. In any case, this is, to the best of our knowledge, the highest target protein yield of a recombinant laccase produced in yeast.
Figure 1.
Fermentation of a P. pastoris clone (GAP promoter) constitutively expressing BaLac [wet biomass (filled square), volumetric activity (filled circle), specific activity (filled diamond)] in comparison to a fermentation of a P. pastoris clone expressing BaLac under control of the inducible AOX1 promoter [wet biomass (empty square), volumetric activity (empty circle), specific activity (empty diamond), data taken from Kittl et al.].
In recent literature, the GAP promoter was rarely used for laccase production and gave relatively low expression yields (Table 2). However, in our study, the maximum volumetric activities in the supernatants of both fermentations employing the AOX1 promoter3 and the GAP promoter were similar, while the GAP fermentation was faster than the fermentation using methanol induction. Additionally, 25% of the protein in the supernatant of the GAP fermentation was BaLac compared to only 8% in the AOX1 fermentation (according to the specific activity3). The downside of the GAP system is the higher biomass production, which decreases the volume of supernatant per liter fermentation medium by 34%. Consequently, the target protein yield of the fermentation decreases by the same percentage.
Table 2. Recombinant expression of fungal laccases in Pichia pastoris.
| Laccase source | Expression yield | Promoter | Reference |
|---|---|---|---|
|
Botrytis acladaa |
53,300 UL-1, 517 mgL-1 |
GAP |
This work |
|
Botrytis acladaa |
51,000 UL-1, 495 mgL-1 |
AOX1 |
3 |
|
Ganoderma fornicatumb |
3,460 UL-1 |
AOX1 |
13 |
|
Pleurotus sajor-cajub |
2,100 U L-1, 4.85 mgL-1 |
AOX1 |
14 |
|
Polyporus grammocephalus TR16b |
320.8 UL-1 |
AOX1 |
15 |
|
Pycnoporus cinnabarinusb |
8 mgL-1 |
AOX1 |
16 |
|
Rigidoporus microsporusb (syn. Fomes lignosus) |
9,030 UL-1 |
GAP |
17 |
| Trametes spp laccase Bb |
32,000 UL-1, 31.6 mgL-1 |
AOX1 |
18 |
| Trametes spp b |
239,000 UL-1, 136 mgL-1 |
AOX1 |
19 |
| Trametes spp AH28-2b |
5,440 UL-1, 4 mgL-1 |
AOX1 |
20 |
|
Trametes trogiib |
2,520 UL-1, 17 mgL-1 |
AOX1 |
21 |
|
Trametes versicolorb |
140,000 UL-1 |
AOX1 |
22 |
|
Trametes versicolorb |
2.8 UL-1 |
GAP |
23 |
| Trametes sp 48424b | 104.5 UL-1 | AOX1 | 24 |
a Ascomycete; bBasidiomycete. Yields are given as reported or recalculated from data provided in the references.
Conclusions
In our recently published work,3 the laccase of the ascomycete B. aclada was heterologously expressed in P. pastoris under control of the AOX1 promoter at extraordinarily high yields. Characterization presented a highly interesting enzyme showing almost no inhibition by chloride and extremely high affinities to phenolic substrates. To further improve the enzyme’s applicability by expanding the pH range or its stability-directed evolution is the method of choice. In the current study we developed a fast and easy high-throughput expression method—a prerequisite for a successful directed evolution experiment—based on the constitutive expression with the GAP promoter, which delivers sufficient enzyme for any high-throughput assay within 48 hours. This 96-well plate based expression can easily be automated with picking and pipetting robots to increase the number of plates that can be screened in a round of evolution. The expression of B. aclada laccase with the constitutive GAP promoter is also an interesting alternative to expression with the AOX1 promoter for large-scale production because of comparable yields in the supernatant, higher specific activity and a shorter fermentation time. Therefore, it was shown that GAP controlled constitutive expression in P. pastoris has significant potential as an expression system for high-throughput mutagenesis approaches and also for the production of improved variants for purification and characterization.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF) (grant TRP218).
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/20037
References
- 1.Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–26. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Gianfreda L, Xu F, Bollag JM. Laccases: a useful group of oxidoreductive enzymes. Bioremediat J. 1999;3:1–25. doi: 10.1080/10889869991219163. [DOI] [Google Scholar]
- 3.Kittl R, Mueangtoom K, Gonaus C, Khazaneh ST, Sygmund C, Haltrich D, et al. A chloride tolerant laccase from the plant pathogen ascomycete Botrytis aclada expressed at high levels in Pichia pastoris. J Biotechnol. 2012;157:304–14. doi: 10.1016/j.jbiotec.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Baldrian P. Fungal laccases - occurrence and properties. FEMS Microbiol Rev. 2006;30:215–42. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- 5.Andberg M, Hakulinen N, Auer S, Saloheimo M, Koivula A, Rouvinen J, et al. Essential role of the C-terminus in Melanocarpus albomyces laccase for enzyme production, catalytic properties and structure. FEBS J. 2009;276:6285–300. doi: 10.1111/j.1742-4658.2009.07336.x. [DOI] [PubMed] [Google Scholar]
- 6.Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, et al. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol. 1997;63:3151–7. doi: 10.1128/aem.63.8.3151-3157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JM, Park SM, Kim DH. Heterologous expression of a tannic acid-inducible laccase3 of Cryphonectria parasitica in Saccharomyces cerevisiae. BMC Biotechnol. 2010;10:18. doi: 10.1186/1472-6750-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24:45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 9.Menéndez C, Hernández L, Banguela A, País J. Functional production and secretion of the Gluconacetobacter diazotrophicus fructose-releasing exo-levanase (LsdB) in Pichia pastoris Enzym Microb Tech 2004; 34:446-52. [Google Scholar]
- 10.Boer H, Teeri TT, Koivula A. Characterization of Trichoderma reesei cellobiohydrolase Cel7A secreted from Pichia pastoris using two different promoters. Biotechnol Bioeng. 2000;69:486–94. doi: 10.1002/1097-0290(20000905)69:5<486::AID-BIT3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Vassileva A, Chugh DA, Swaminathan S, Khanna N. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter. J Biotechnol. 2001;88:21–35. doi: 10.1016/S0168-1656(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 12.Weis R, Luiten R, Skranc W, Schwab H, Wubbolts M, Glieder A. Reliable high-throughput screening with Pichia pastoris by limiting yeast cell death phenomena. FEMS Yeast Res. 2004;5:179–89. doi: 10.1016/j.femsyr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Huang WT, Tai R, Hseu RS, Huang CT. Overexpression and characterization of a thermostable, pH-stable and organic solvent-tolerant Ganoderma fornicatum laccase in Pichia pastoris. Process Biochem. 2011;46:1469–74. doi: 10.1016/j.procbio.2011.03.020. [DOI] [Google Scholar]
- 14.Soden DM, O’Callaghan J, Dobson ADW. Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology. 2002;148:4003–14. doi: 10.1099/00221287-148-12-4003. [DOI] [PubMed] [Google Scholar]
- 15.Huang SJ, Liu ZM, Huang XL, Guo LQ, Lin JF. Molecular cloning and characterization of a novel laccase gene from a white-rot fungus Polyporus grammocephalus TR16 and expression in Pichia pastoris. Lett Appl Microbiol. 2011;52:290–7. doi: 10.1111/j.1472-765X.2010.02997.x. [DOI] [PubMed] [Google Scholar]
- 16.Otterbein L, Record E, Longhi S, Asther M, Moukha S. Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem. 2000;267:1619–25. doi: 10.1046/j.1432-1327.2000.01166.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Chao Y, Liu S, Bao H, Qian S. Molecular cloning and characterization of a laccase gene from the basidiomycete Fome lignosus and expression in Pichia pastoris. Appl Microbiol Biotechnol. 2003;63:174–81. doi: 10.1007/s00253-003-1398-0. [DOI] [PubMed] [Google Scholar]
- 18.Li JF, Hong YZ, Xiao YZ, Xu YH, Fang W. High production of laccase B from Trametes sp in Pichia pastoris. World J Microbiol Biotechnol. 2007;23:741–5. doi: 10.1007/s11274-006-9286-2. [DOI] [Google Scholar]
- 19.Cui TJ, Wang XT, Zhou HM, Hong YZ, Xiao YZ, Cui TJ, et al. [High output of a Trametes laccase in Pichia pastoris and characterization of recombinant enzymes] Sheng Wu Gong Cheng Xue Bao. 2007;23:1055–9. doi: 10.1016/S1872-2075(07)60063-6. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y, Xiao Y, Zhou H, Fang W, Zhang M, Wang J, et al. Expression of a laccase cDNA from Trametes sp. AH28-2 in Pichia pastoris and mutagenesis of transformants by nitrogen ion implantation. FEMS Microbiol Lett. 2006;258:96–101. doi: 10.1111/j.1574-6968.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- 21.Colao MC, Lupino S, Garzillo AM, Buonocore V, Ruzzi M. Heterologous expression of lcc1 gene from Trametes trogii in Pichia pastoris and characterization of the recombinant enzyme. Microb Cell Fact. 2006;5:31. doi: 10.1186/1475-2859-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong F, Meinander NQ, Jönsson LJ. Fermentation strategies for improved heterologous expression of laccase in Pichia pastoris. Biotechnol Bioeng. 2002;79:438–49. doi: 10.1002/bit.10297. [DOI] [PubMed] [Google Scholar]
- 23.Bohlin C, Jönsson LJ, Roth R, van Zyl WH. Heterologous expression of Trametes versicolor laccase in Pichia pastoris and Aspergillus niger. Appl Biochem Biotechnol. 2006;129-132:195–214. doi: 10.1385/ABAB:129:1:195. [DOI] [PubMed] [Google Scholar]
- 24.Fan F, Zhuo R, Sun S, Wan X, Jiang M, Zhang X, et al. Cloning and functional analysis of a new laccase gene from Trametes sp. 48424 which had the high yield of laccase and strong ability for decolorizing different dyes. Bioresour Technol. 2011;102:3126–37. doi: 10.1016/j.biortech.2010.10.079. [DOI] [PubMed] [Google Scholar]