Abstract
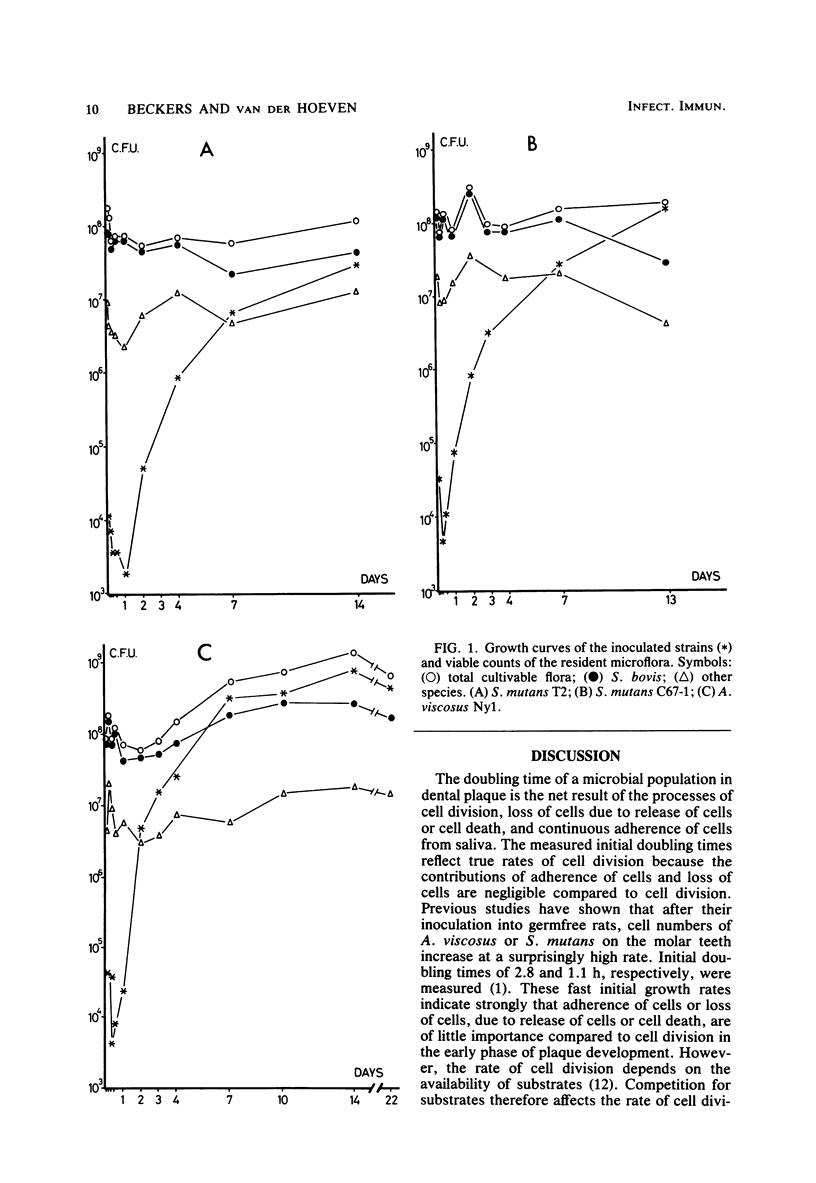
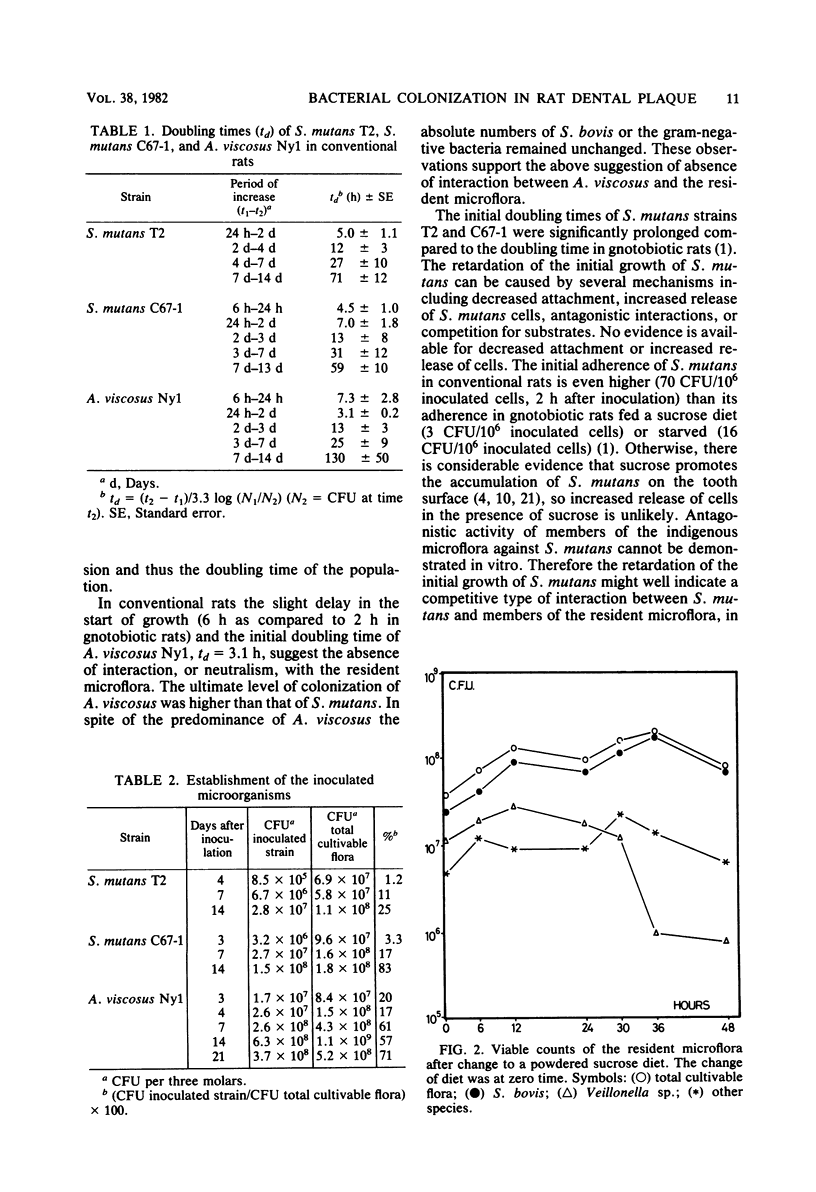
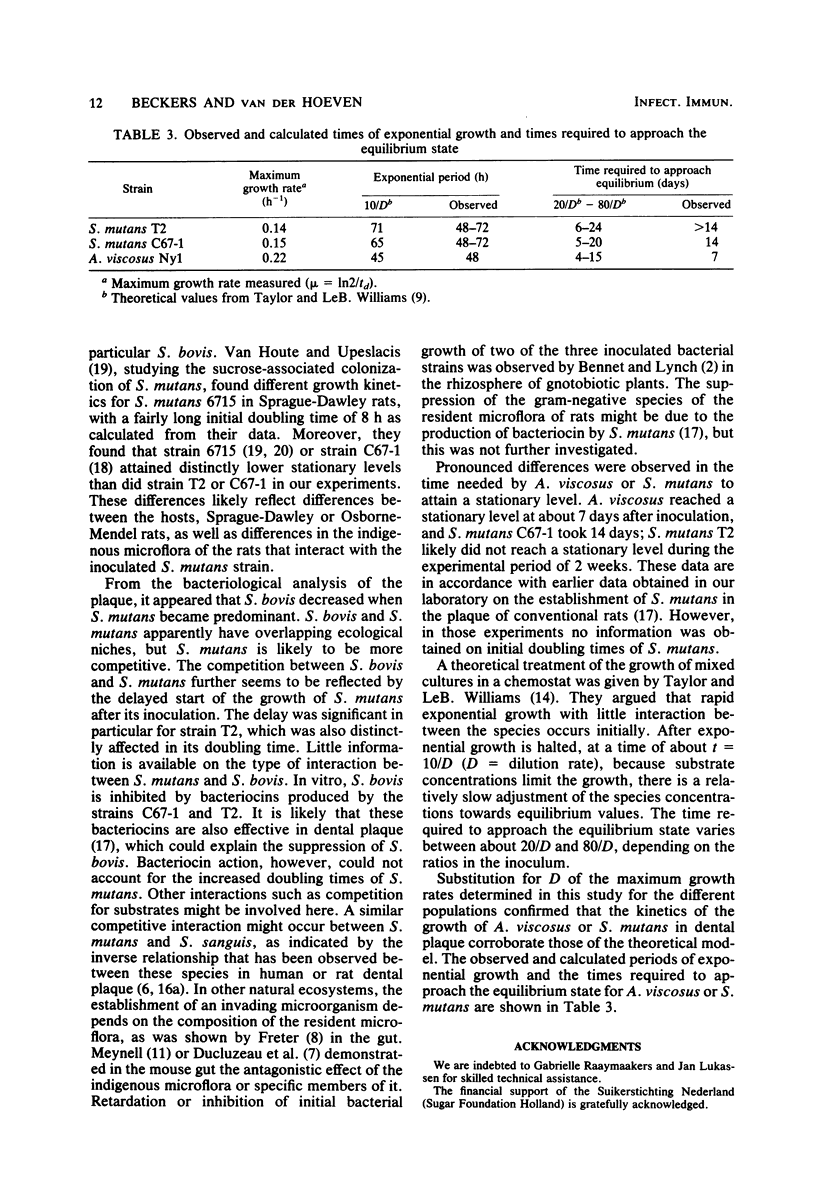
The resident oral microflora of conventional Osborne-Mendel rats was challenged with Actinomyces viscosus or Streptococcus mutans strains. The adherence of the inoculated organism to the tooth surface and the subsequent growth were studied by means of viable counts determination. The initial growth rate of S. mutans in conventional rats was lower than in mono-associated gnotobiotic rats (doubling time, td = 5 h versus td = 1.1 h). The delayed start of growth and the low initial growth rate indicated that a competitive interaction between S. mutans and the resident microflora occurred. The initial growth rate of A. viscosus in conventional rats (td = 3.1 h) was approximately the same as that in gnotobiotic rats (td = 2.8 h). The start of growth of A. viscosus was only slightly delayed compared with the start in gnotobiotic rats. These results suggest a neutralistic relationship between A. viscosus and the resident microflora. A. viscosus reached a stationary level about 7 days after inoculation, whereas the S. mutans strains did not reach stationary levels until 2 weeks after inoculation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckers H. J., van der Hoeven J. S. Growth rates of Actinomyces viscosus and Streptococcus mutans during early colonization of tooth surfaces in gnotobiotic rats. Infect Immun. 1982 Feb;35(2):583–587. doi: 10.1128/iai.35.2.583-587.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON J., EGELBERG J. EFFECT OF DIET ON EARLY PLAQUE FORMATION IN MAN. Odontol Revy. 1965;16:112–125. [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Ducluzeau R., Ladire M., Callut C., Raibaud P., Abrams G. D. Antagonistic effect of extremely oxygen-sensitive clostridia from the microflora of conventional mice and of Escherichia coli against Shigella flexneri in the digestive tract of gnotobiotic mice. Infect Immun. 1977 Aug;17(2):415–424. doi: 10.1128/iai.17.2.415-424.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J Exp Med. 1956 Sep 1;104(3):411–418. doi: 10.1084/jem.104.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Krasse B., Edwardsson S., Svensson I., Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967 Feb;12(2):231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocinogeny and the properties of some bacteriocins of Streptococcus mutans. Arch Oral Biol. 1976;21(2):99–104. doi: 10.1016/0003-9969(76)90079-0. [DOI] [PubMed] [Google Scholar]
- Taylor P. A., leB Williams P. J. Theoretical studies on the coexistence of competing species under continuous-flow conditions. Can J Microbiol. 1975 Jan;21(1):90–98. doi: 10.1139/m75-013. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Edelstein S. Decreased oral colonization of Streptococcus mutans during aging of Sprague-Dawley rats. Infect Immun. 1977 Apr;16(1):203–212. doi: 10.1128/iai.16.1.203-212.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Jordan H. V., Skobe Z., Green D. B. Role of sucrose in colonization of Streptococcus mutans in conventional Sprague-Dawley rats. J Dent Res. 1976 Mar-Apr;55(2):202–215. doi: 10.1177/00220345760550020801. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N. Studies of the mechanism of sucrose-associated colonization of Streptococcus mutans on teeth of conventional rats. J Dent Res. 1976 Mar-Apr;55(2):216–222. doi: 10.1177/00220345760550020901. [DOI] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S. A slime-producing microorganism in dental plaque of rats, selected by glucose feeding. Chemical composition of extracellular slime elaborated by Actinomyces viscosus, strain Nyl. Caries Res. 1974;8(3):193–210. doi: 10.1159/000260109. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., Franken H. C. Production of acids in rat dental plaque with or without Streptococcus mutans. Caries Res. 1982;16(5):375–383. doi: 10.1159/000260623. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., Rogers A. H. Stability of the resident microflora and the bacteriocinogeny of Streptococcus mutans as factors affecting its establishment in specific pathogen-free rats. Infect Immun. 1979 Feb;23(2):206–213. doi: 10.1128/iai.23.2.206-212.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


