Abstract
Monoclonal antibodies against type C1 toxin produced by Clostridium botulinum type C strain Stockholm (C-ST) were prepared by fusion of BALB/c myeloma cells P3X63-Ag8, with spleen cells from the mice immunized by C-ST toxoid. About 5% of single-cell colonies in wells were found to produce antibodies against the toxin as determined by an enzyme-linked immunosorbent assay (ELISA). Four different hybridoma cell lines, no. 9, 12, 14, and 17, were established, cloned by limiting dilution, and intraperitoneally injected into mice to obtain the ascites fluids containing high-titered antibodies. The reactions of these antibodies to type C1 and D toxins of strains C-ST, D-1873, and D-South African (D-SA) were observed by both neutralization and ELISA tests. Three monoclonal antibodies, no. 9, 14, and 17, reacted with C-ST toxin, but only no. 17 highly neutralized the toxin. These antibodies did not react with type D toxins. On the contrary, no. 12 reacted with toxins of both C-ST and D-SA (but not of D-1873) and commonly neutralized these two toxins. This indicates that there is a common antigenic part between C-ST and D-SA toxin molecules which participates in the toxin-neutralizing reaction. The neutralization profiles of C-ST toxin by no. 12 and 17 antibodies were different in a time-to-death test of mice. The mechanisms of neutralization by no. 12 and 17 may be different.
Full text
PDF

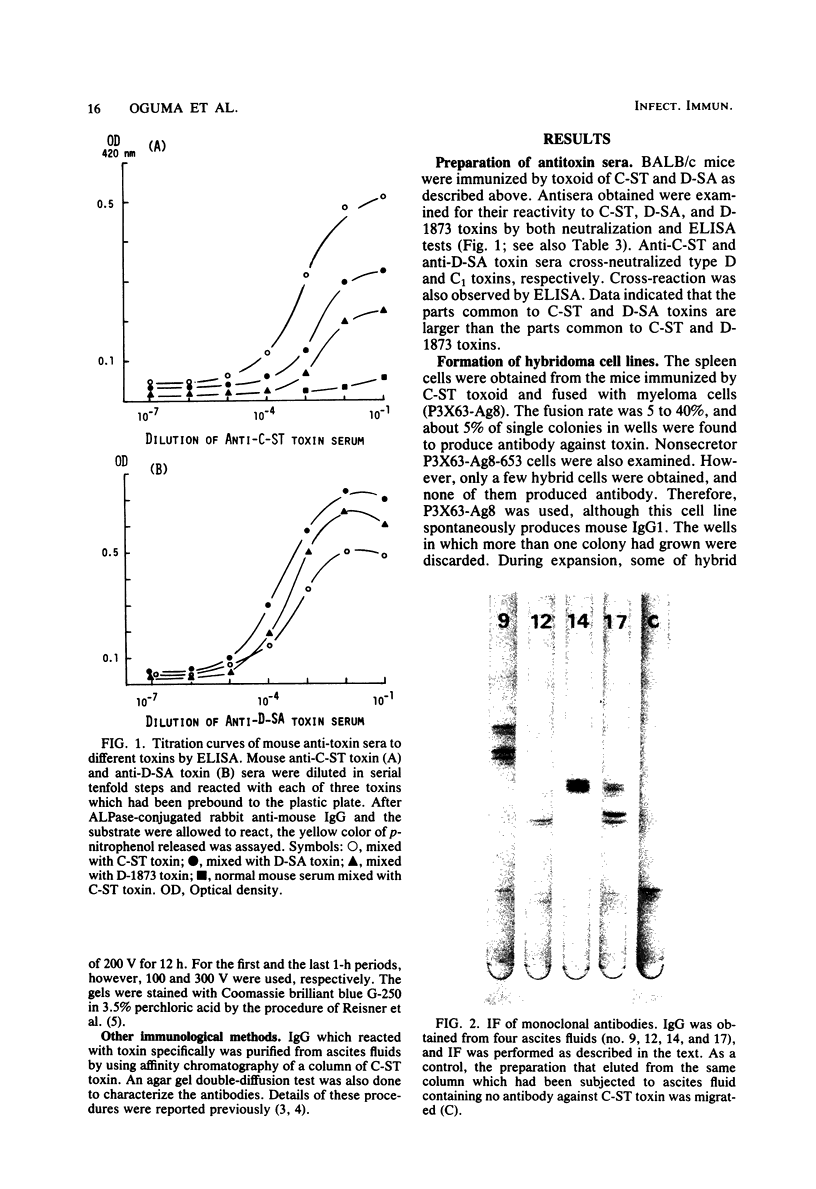

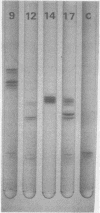
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Iida H., Shiozaki M., Inoue K. Antigenicity of converting phages obtained from Clostridium botulinum types C and D. Infect Immun. 1976 Mar;13(3):855–860. doi: 10.1128/iai.13.3.855-860.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Agui T., Iida H., Kubo S. Homogeneity and heterogeneity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1981 Nov;34(2):382–388. doi: 10.1128/iai.34.2.382-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]