Abstract
OBJECTIVE
To examine determinants of racial/ethnic differences in diabetes incidence among postmenopausal women participating in the Women’s Health Initiative.
RESEARCH DESIGN AND METHODS
Data on race/ethnicity, baseline diabetes prevalence, and incident diabetes were obtained from 158,833 women recruited from 1993–1998 and followed through August 2009. The relationship between race/ethnicity, other potential risk factors, and the risk of incident diabetes was estimated using Cox proportional hazards models from which hazard ratios (HRs) and 95% CIs were computed.
RESULTS
Participants were aged 63 years on average at baseline. The racial/ethnic distribution was 84.1% non-Hispanic white, 9.2% non-Hispanic black, 4.1% Hispanic, and 2.6% Asian. After an average of 10.4 years of follow-up, compared with whites and adjusting for potential confounders, the HRs for incident diabetes were 1.55 for blacks (95% CI 1.47–1.63), 1.67 for Hispanics (1.54–1.81), and 1.86 for Asians (1.68–2.06). Whites, blacks, and Hispanics with all factors (i.e., weight, physical activity, dietary quality, and smoking) in the low-risk category had 60, 69, and 63% lower risk for incident diabetes. Although contributions of different risk factors varied slightly by race/ethnicity, most findings were similar across groups, and women who had both a healthy weight and were in the highest tertile of physical activity had less than one-third the risk of diabetes compared with obese and inactive women.
CONCLUSIONS
Despite large racial/ethnic differences in diabetes incidence, most variability could be attributed to lifestyle factors. Our findings show that the majority of diabetes cases are preventable, and risk reduction strategies can be effectively applied to all racial/ethnic groups.
More than 25 million Americans have diabetes, and an estimated 300 million worldwide will be diagnosed with diabetes by the year 2025 (1,2). Diabetes is the seventh leading cause of death in the U.S. and is an underlying factor in cardiovascular and cancer mortality (1,3). Non-Hispanic blacks have been reported to be 1.4–2.2 times more likely to receive a diagnosis of diabetes than non-Hispanic whites in the U.S. population (4). U.S. women of Hispanic and Asian ancestry also have a higher prevalence of diabetes than non-Hispanic whites (5). Although racial/ethnic disparities in diabetes risk have been identified, determinants of these differences have not been well studied. Previous studies have considered dietary and lifestyle factors individually, but few studies have considered these factors in aggregate in order to estimate the proportion of diabetes that might be avoided by adopting a pattern of low-risk behaviors (6,7). Moreover, few studies have been large or diverse enough to allow for the assessment of these relationships in individual racial/ethnic groups, particularly among women.
The Women's Health Initiative (WHI) provides a unique opportunity to assess racial/ethnic disparities in both diabetes prevalence and incidence and factors contributing to disparities in diabetes incidence within a large and well-characterized group of postmenopausal women.
RESEARCH DESIGN AND METHODS
The WHI participants
The design and baseline characteristics of the WHI have been described in detail elsewhere (8). In brief, the WHI enrolled postmenopausal women 50–79 years of age who provided written informed consent and had an expected survival and local residency of ≥3 years. Exclusion criteria included current alcoholism, drug dependency, dementia, or other conditions that would limit full participation in the study. A total of 161,808 women (68,132 in clinical trials and 93,676 in an observational study) were enrolled in the WHI between 1993 and 1998. Data used in this report were obtained from women at baseline and then at periodic follow-up points over an average of 10.4 years. The protocol and consent forms were approved by the institutional review boards of all participating institutions.
Identification of diabetes
Prevalent diabetes was defined as a self-report at baseline of “ever having received a physician diagnosis of diabetes when not pregnant.” Incident diabetes was based on data collected at annual follow-up visits at which participants were asked, “Since the date given on the front of this form, has a doctor prescribed any of the following pills or treatments?” Choices included “pills for diabetes” and “insulin shots for diabetes.” Cases of incident treated diabetes reported as of 31 August 2009 were included in the analysis. A study of the accuracy of self-reported diabetes conducted in a subset of WHI participants indicated that self-reported disease status was a reasonably valid indicator of diagnosed diabetes when compared with medication and laboratory criteria (9). However, self-report fails to identify undiagnosed diabetes and tends to underestimate diabetes prevalence and incidence in our study group (9).
Race/ethnicity
At baseline, WHI participants self-reported their race/ethnicity, choosing from non-Hispanic white (referred to hereafter as white), non-Hispanic black (referred to hereafter as black), Hispanic, American Indian/Alaska Native, Asian (ancestry was Chinese, Indo-Chinese, Korean, Japanese, Pacific Islander, or Vietnamese), and other. We excluded 1,849 women who reported race/ethnicity as “other” and 413 women without race/ethnicity information. The sample size was limited among American Indians (n = 714); therefore, analyses were restricted to whites, blacks, Hispanics, and Asians.
Covariates
Body weight, height, and waist circumference were measured at baseline and year 3. BMI was computed as weight (kg) divided by height (m2). Demographic and health history data were self-reported at baseline and included age, years of education, cigarette smoking status, family history of diabetes, and hormone therapy use. The baseline physical activity questionnaire asked about usual frequency and duration of several types of recreational and household activities using a standardized classification of physical activity intensity (10). Total energy expenditure was summarized in metabolic equivalent (MET) hours per week (MET-h/week), computed as the summed product of frequency, duration, and intensity for reported activities. Participants also completed a standardized food frequency questionnaire (FFQ) developed for the WHI to estimate average daily nutrient intake over the 3-month period prior to baseline visit (11). Dietary quality, assessed by the Alternate Healthy Eating Index (AHEI) (12,13), was computed based on food items and nutrients derived from the FFQ, including 1) fruit, 2) vegetables, 3) nuts and legumes, 4) ratio of white to red meat, 5) total dietary fiber, 6) trans-fat, 7) ratio of polyunsaturated fat to saturated fat, 8) alcohol, and 9) multivitamin use. Higher AHEI scores are indicative of a better quality diet.
The reliability and validity of physical activity measurements were assessed among a random sample of 536 participants by a second measure of physical activity ∼10 weeks after the first measure. The test-retest reliability (weighted κ) for the physical activity variables ranged from 0.53 to 0.72, and the intraclass correlation for the total physical activity variable was 0.77 (8). For the FFQ, we assessed bias and precision of the FFQ by comparing the intake of 30 nutrients estimated from the FFQ with means from four 24-h dietary recalls and a 4-day food record from 113 women who participated in the WHI (11). For most nutrients, means estimated by the FFQ were within 10% of the records or recalls. Energy-adjusted correlation coefficients ranged from 0.2 (vitamin B12) to 0.7 (magnesium), with a mean of 0.5. The correlation for percentage energy from fat was 0.6. We concluded that the FFQ produced nutrient estimates, which were similar to those obtained in other studies comparing short-term dietary recall and recording methods. We acknowledge that the reliability and validity are not perfect; however, they provided reasonable measures in a large clinical trial, as published by several of our authors in high-impact articles (14–16).
Statistical analyses
Racial/ethnic differences in the prevalence of diabetes were assessed using logistic regression and are expressed as odds ratios (ORs) and associated 95% CIs, with whites used as the reference group. We present four logistic regression models: 1) unadjusted, including only race/ethnicity as a covariate; 2) age-adjusted; 3) adjusted for multiple potential confounding factors, including study arm, baseline age, BMI, waist circumference, physical activity, dietary quality, and smoking status; and 4) adjusted for the same covariates as in model 3 plus educational attainment.
Among women who were free of diabetes at baseline, incidence rate was calculated as the number of newly reported postbaseline diabetes cases divided by total follow-up time in person-years. The time to diabetes (i.e., time to event) was calculated as the interval between enrollment date and the earliest of the following: 1) date of annual medical history update when new diagnosis of diabetes and initiation of treatment for diabetes were ascertained (observed event); 2) date of last annual medical update during which participants were identified to be without diabetes (censored event); or 3) date of death from any cause (censored event). Cox proportional hazards models were used to estimate the hazard ratios (HRs) and associated 95% CI of incident diabetes, with whites used as the reference group. Four Cox regression models that parallel those described for the analysis of diabetes prevalence are presented.
To identify determinants of diabetes incidence that might vary by racial/ethnic group, the impact of several covariates was evaluated by assessing the extent to which the HR estimate for a specific race/ethnicity group changed when each covariate was added individually to the unadjusted model. The percentage change in HR between the model with and without the covariate was used to describe the contribution of each covariate, considered singly, to diabetes risk estimated by race/ethnic group. To identify the most parsimonious prediction model, a stepwise Cox proportional hazards regression analysis was conducted (P values for entry =0.25 and for retaining in the model =0.05). Subgroup analyses also were conducted by race/ethnicity. Weight change from baseline to 3 years was evaluated in relation to risk of incident disease by race/ethnicity among women who reported being free of diabetes at the 3-year measurement point. A similar analysis was conducted for waist circumference.
To assess how the combined role of healthy lifestyle habits in diabetes prevention may vary by race/ethnicity (6,7), categories of four modifiable lifestyle factors were defined as follows: physical activity (upper vs. lower two tertiles), dietary quality score (upper vs. lower two tertiles), smoking (nonsmokers vs. past and former smokers combined), and BMI (<25 vs. ≥25 kg/m2). In subgroup analyses by race/ethnicity, HRs and 95% CIs were calculated for diabetes incidence for each lifestyle risk factor individually and several lifestyle factors in aggregate, adjusting for age, family history of diabetes, hormone therapy use, study arm, and lifestyle risk factors not already included in the model.
RESULTS
Population characteristics
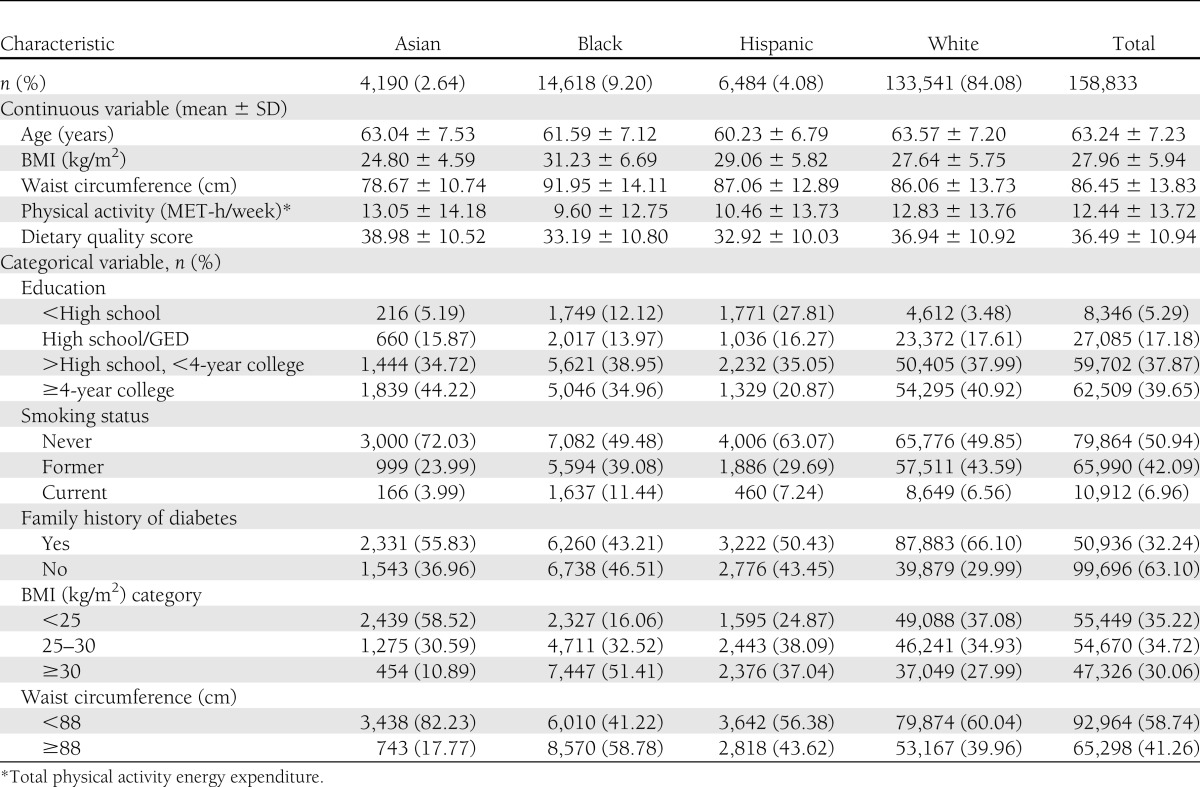
At baseline, the average age of the 158,833 women with evaluable data was 63 years. The racial/ethnic distribution was 84.1% white, 9.2% black, 4.1% Hispanic, and 2.6% Asian. Approximately one-third of participants had a family history of diabetes. Approximately two-thirds had completed at least some college education. The prevalence of current smoking was 7%. Compared with whites, blacks and Hispanics tended to have more risk factors, whereas Asians tended to have fewer (Table 1).
Table 1.
Baseline characteristics of participants in the WHI 1993–2009 (N = 158,833)
Racial/ethnic disparities in diabetes prevalence and incidence
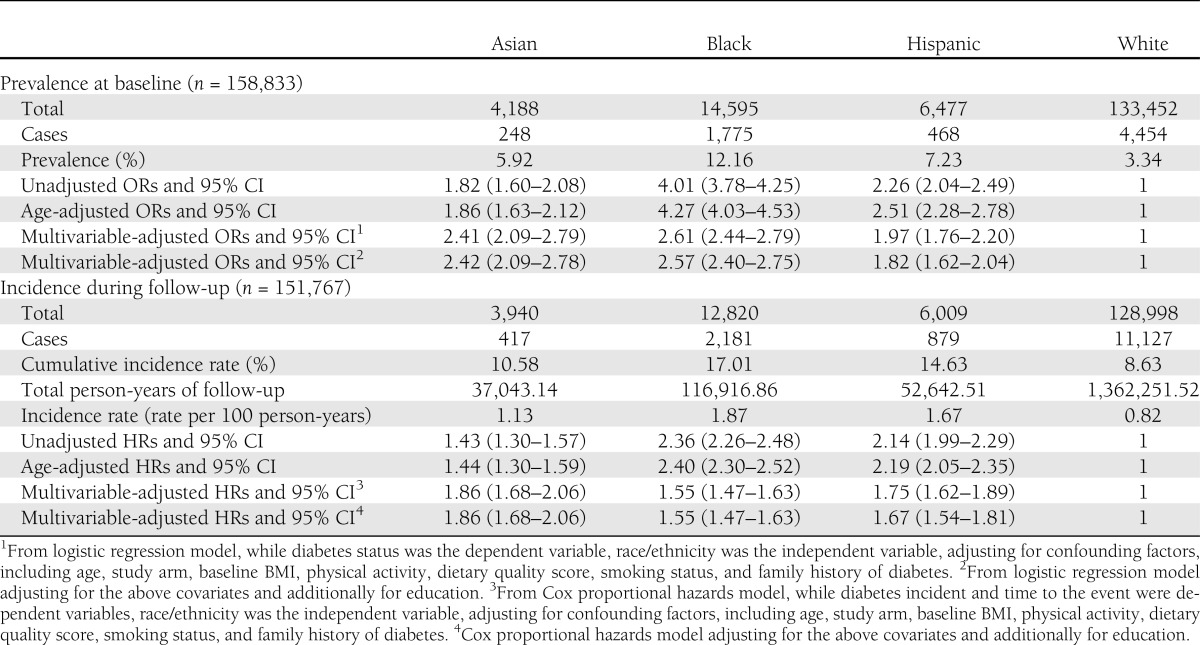
At enrollment, diabetes prevalence was highest among blacks (12.2%), followed by Hispanics (7.2%), Asians (5.9%), and whites (3.3%) (Table 2). Compared with the unadjusted ORs, which were significantly higher in all three race/ethnic groups when compared with whites, the age-adjusted and multivariable-adjusted ORs were attenuated for blacks and Hispanics but strengthened for Asians. Additional adjustment for educational attainment further attenuated the ORs for blacks and Hispanics; however, ORs for all three racial/ethnic minority groups remained significantly higher than that of whites.
Table 2.
Prevalence and incidence of diabetes in the WHI 1993–2009
During an average of 10.4 years (SD = 3.2) of follow-up, 14,604 new cases of diabetes were reported (11,127 whites, 2,181 blacks, 879 Hispanics, and 417 Asians), with incidence being highest in blacks and lowest in whites. Compared with whites, unadjusted analyses showed a significantly higher diabetes incidence of 136% in blacks, 114% in Hispanics, and 43% in Asians. After adjusting for age, study arm, BMI, physical activity, smoking status, and educational attainment, HRs increased for Asians and decreased for blacks.
Determinants of racial/ethnic disparities in diabetes incidence
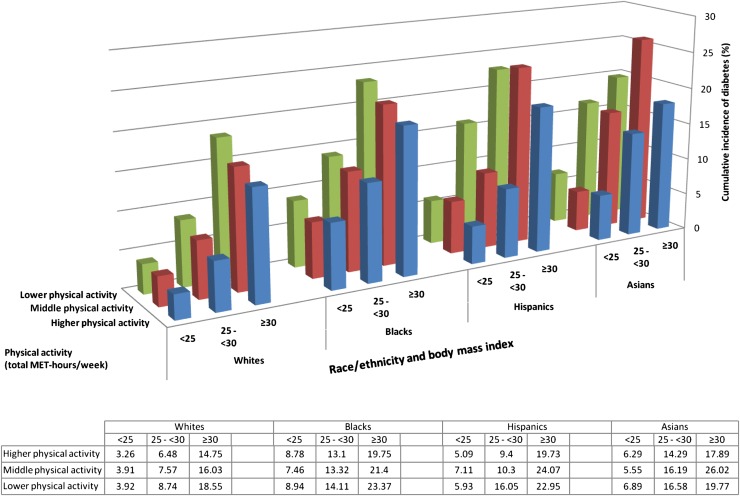
In each racial/ethnic group (Fig. 1), the highest cumulative incidence of diabetes was seen among women who had both high BMI and low levels of physical activity. The cumulative incidence of diabetes was 23.4% among black women who were both obese and in the lowest tertile of physical activity, but decreased to 8.8% in those who had a healthy weight and exercised. White women who were overweight (BMI = 25–29.9 kg/m2) and in the lowest tertile of physical activity had a cumulative incidence of 8.7%, whereas white women who were obese (BMI ≥30 kg/m2) and in the lowest tertile of physical activity had a cumulative incidence of 18.6%. Across all racial/ethnic groups, women who were normal weight (BMI <25 kg/m2) and in the highest tertile of physical activity had less than one-third to one-sixth the incidence of diabetes compared with women with BMI ≥30 kg/m2 and in the lowest tertile of physical activity.
Figure 1.
Cumulative incidence of diabetes by race/ethnicity, physical activity level, and BMI category.
Analyses conducted to estimate the influence of individual factors that may account for the observed racial/ethnic variation in diabetes incidence by comparing HRs obtained from unadjusted versus fully adjusted models (results not shown) revealed that if Asian women had the same waist circumference, BMI, and dietary quality intake as whites, their HR for diabetes would be increased by 44, 29, and 3.5%, respectively. Among blacks, if they had the same BMI, waist circumference, family history of diabetes, dietary quality intake, and physical activity levels as whites, their HR for diabetes would be decreased by 25, 19, 11, 7, and 6%, respectively. Among Hispanics, if they had the same educational attainment, BMI, family history of diabetes, dietary quality intake, and physical activity levels as whites, their HR for diabetes would be decreased by 14, 10, 9, 7, and 6%, respectively.
Variables retained in the final model predicting diabetes incidence
Results from the stepwise Cox proportional hazards regression analysis conducted to identify the most parsimonious predictive model for diabetes incidence, fitting candidate predictors plus race/ethnicity, showed that race/ethnicity, age, BMI, waist circumference, education, smoking status, physical activity, family history of diabetes, and dietary quality score entered the model.
Subgroup analyses by race/ethnicity (results not shown) conducted to assess the effect of interval (i.e., baseline to 3 years) changes in factors found to be significant in the Cox proportional hazards models revealed a significant effect of ∼5% increased risk of diabetes for each 5-cm increase in waist circumference (HR 1.05 [95% CI 1.04–1.07]). The observed effect was consistent across all race/ethnicity groups. An alternative model fit to assess if weight gain could explain some of the observed racial/ethnic variation in diabetes incidence revealed an ∼3% increase in subsequent risk for each 1-kg/m2 increment in BMI (1.03 [1.02–1.04]).
HR of incident diabetes by single and specific combinations of lifestyle risk factors and race/ethnicity
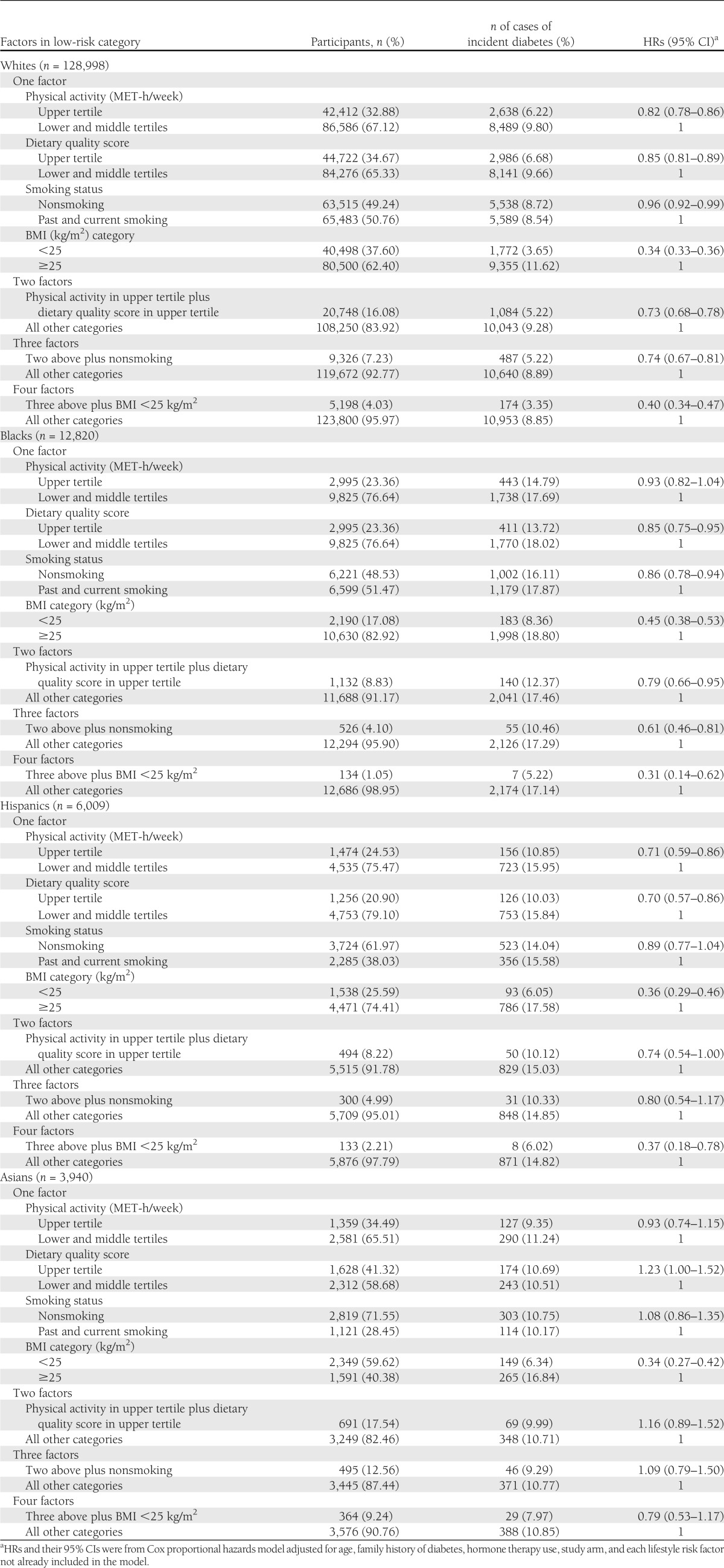
Higher levels of physical activity, better diet, and having a healthy weight tended to be associated with a significantly lower risk of diabetes in each racial/ethnic group, with some exceptions. Whites, blacks, and Hispanics with all factors in the low-risk category (4.0, 1.1, and 2.2%) had 60, 69, and 63% lower risk for incident diabetes (Table 3). Healthy weight (BMI <25 kg/m2) demonstrated the greatest role in reducing risk of diabetes in each racial/ethnic group: 66% in whites, 55% in blacks, 64% in Hispanics, and 66% in Asians.
Table 3.
HRs of incident diabetes corresponding to single and specific combinations of lifestyle risk factors in race/ethnicity subgroups in the WHI 1993–2009
CONCLUSIONS
Previous reports, mainly focused on younger men and women, have indicated significant racial/ethnic disparities in diabetes in the U.S. (1,4,5). The 2007–2009 National Health Interview Survey found that 7.1% of whites, 8.4% of Asians, 11.8% of Hispanics, and 12.6% of blacks reported having a diagnosis of diabetes (1). The WHI allows a unique opportunity to deepen understanding of the patterns and determinants of diabetes in older women, including racial/ethnic minorities that represent growing segments of the U.S. population.
We found that the prevalence and incidence of diabetes ranged from approximately two to three times higher in blacks and approximately two times higher in Hispanics and in Asians, compared with whites. Observed racial/ethnic differences in diabetes incidence were explained, in large part, by modifiable lifestyle factors that included diet quality, physical activity, and smoking status or factors resulting from lifestyle behaviors including BMI and waist circumference. Adjustment for differences in these variables indicates that Asians have the highest inherent risk, though women of all four racial/ethnic groups would experience a large reduction in diabetes risk by maintaining a healthy body weight, healthy diet, and a physically active lifestyle. Maintaining a BMI <25 kg/m2 appears to be particularly important, and interval changes in both BMI and waist circumference predicted newly incident disease.
In this study, both BMI and waist circumference were found to be related to prevalent diabetes, and interval changes were associated with risk of incident disease. Because waist circumference reflects centralized obesity, and the propensity toward large waist circumference varies according to race/ethnicity, its effect may differ from that of BMI (17,18). Although BMI and waist circumference among Asian, postmenopausal women in the WHI were relatively lower than whites, Asians were at higher risk of diabetes at lower levels of BMI and with smaller waist circumferences (i.e., by 44 and 29%, respectively) compared with whites, a result consistent with that seen in the Multi-Ethnic Study of Atherosclerosis (19). Our results on Asians suggest that additional risk factors, which might be biological, social, or a combination, drive the higher prevalence and incidence of diabetes in this population. BMI and waist circumference also contributed to disparities in diabetes for blacks and Hispanics. Diabetes risk in blacks with the same BMI and waist circumference as whites was lower by 25 and 17%, respectively. Similarly, diabetes risk in Hispanics with the same BMI and waist circumference as whites was lower by 10 and 2%, respectively. We noted that within each group, BMI was the most important determinant for diabetes incidence.
Physical inactivity also was found to be associated with increased risk of diabetes in all groups. In a previous publication using WHI data, we reported the association between physical inactivity and development of diabetes (20). Our present study showed that, compared with whites, given the same level of physical activity, the risk for diabetes for blacks and Hispanics is 6% lower.
Nutritional factors may play a role in the development of diabetes (21–25). Several studies have found differences in dietary intake by race/ethnicity (26,27). Our analysis suggested that Asians appear to be particularly sensitive to poor dietary intake. Although their diet quality was better than that of whites, we found, for example, if their overall dietary quality were to decrease to that of whites, their risk of diabetes would increase by 4% relative to whites. Poorer dietary intake among blacks and Hispanics put them at increased risk of diabetes. In fact, if blacks and Hispanics improved their diet to a quality similar to that of whites, their risk of diabetes would be 7% lower.
Although previous studies demonstrated that diabetes is preventable by relatively simple lifestyle modifications (6,7), our data suggest that some minority groups might obtain greater benefit from improving lifestyle factors (i.e., blacks and Hispanics) than others (i.e., Asians) due to differences in the amount of change possible or the vulnerability to these factors. The divergent findings in Asian women are of interest, suggesting that additional approaches to prevention deserve attention. For example, Asians may need to achieve even greater weight loss to have the same low risk of diabetes as nonoverweight whites, and the World Health Organization already uses a lower BMI cut point for Asians. The fact that Asians in the WHI had much healthier body weights at baseline set the stage for much lower overall diabetes risk. However, further research with this population is warranted because our statistical models, with additional covariates, may be unstable and less reliable due to small numbers.
We found that blacks and Hispanics are more sensitive to lifestyle modifications and weight loss than whites, and this is corroborated by our previous lifestyle intervention study results from a Hispanic population (28). Hispanic sensitivity to the development of an insulin-resistant state or diabetes with lower weight gain is well described (29). Less is understood about corresponding sensitivity to weight loss in lifestyle interventions. If confirmed in weight-loss studies, the sensitivity to modest weight loss that we observed in high-risk groups in the WHI will have important clinical and public health implications. It will also be important to explore possible social and genetic underpinnings for such population sensitivity.
Two recent studies indicate that social economic status (SES) may account for some of the observed race/ethnicity disparities in diabetes prevalence (30,31). Similarly, in our study, adjustment for educational attainment resulted in the largest decrease in diabetes incidence in Hispanics; if Hispanics had achieved the same education levels as whites, their risk of diabetes would be 14% lower. However, education is only one component of SES, and thus consideration of an adjustment for other SES parameters (e.g., income and occupation) would likely account for a greater proportion of the disparities observed in black and Hispanic women.
There are several interesting nuances to our findings that are worth noting. First, observed prevalence of diabetes in the WHI was lower than expected, as relatively healthy postmenopausal women were enrolled in the study. Second, reported education level among black women in the WHI was considerably higher than education level among black women in the U.S. The education differential for blacks and Hispanics is much more extreme than that observed in whites, and this may underlie some of the other observations in the data (32,33). However, observed patterns are consistent with the population-based literature in terms of race/ethnic patterns of diabetes prevalence and incidence of diabetes. Third, Asian women appear to have the greatest “inherent” risk in that their risk factor profile was in fact better than whites, and large increases in diabetes risk would occur if the risk factors deteriorate. This is consistent with the report from Lutsey et al. (19), which showed that Asians had a higher diabetes risk per unit increase in BMI and waist circumference. Recognizing that each of the racial/ethnic groups is heterogeneous due to differences by national origin and other relevant parameters, future studies should examine disparities in incident diabetes by national origin. Fourth, it is encouraging to note the effect of interval changes in weight amounting to a 3% reduction in risk of diabetes for each unit decrease in BMI. Individual efforts to reduce weight and increase physical activity are difficult to sustain; however, current efforts are underway to tackle weight regulation through a variety of approaches that go beyond the individual. Lessons from the tobacco literature indicate that behavior change is influenced by our social and political structure; thus, multilevel approaches are likely needed.
In the U.S., the race/ethnicity categories used most often in medical and public health research are from self-report, the same as the U.S. Census categories. Genetic race/ethnicity information known as admixture data (i.e., ancestry-informative markers) from another study indicates great diversity within all four of the groups that we examined (34). Yet, few studies obtain such data due to issues concerning cost, feasibility, practicality, and comparability. Thus, the differences observed and reported here reflect any inherent biological differences across the groups studied as well as differences in life experiences (e.g., exposure to specific environments and stressors), which may also contribute to acquired physiological differences in reactivity, and in turn diabetes risk (35). The challenges for interpreting the results associated with studying self-reported racial/ethnic groups will likely increase in future studies, as more people identify themselves as belonging to multiple racial/ethnic groups.
This study has several limitations that are worth noting. First, the WHI participants are not a population-based random sample. Although geographically diverse, racial/ethnic groups vary in their representation of the general population. Data for whites show that many characteristics of the WHI participants are similar to white women participating in the National Health and Nutrition Examination Survey (11); however, ethnic groups are underrepresented in the WHI. Participants from each ethnic group were generally of higher SES than national averages. Women from parts of the country where we see large disparities in certain minorities (e.g., rural southern blacks) were not represented. Thus, both prevalence and racial differentials are smaller in the WHI than what we might expect to see in the U.S. as a whole. Second, only self-reported prevalence of diabetes and treated incident diabetes were ascertained; thus, prevalence and incidence of diabetes may be underestimated. We acknowledge that this is a limitation, and we did not account for nontreated diabetes. However, self-reported diabetes in the WHI was found to be reliable and sufficiently accurate to allow its use in epidemiologic studies (9). Third, there could be other factors for which we did not control that further contribute to racial/ethnic disparities, such as health care access (36). However, >90% of the WHI participants had insurance coverage, and diabetes prevalence and incidence were assessed at regular study visits. Fourth, although incident diabetes in older women is likely to be type 2 diabetes, the WHI question did not specify type of diabetes. Other limitations include missing data; however, the rates of retention in the WHI were >95% during an average of 7 years of follow-up (37).
Balancing the limitations, there are several major strengths. First, this study represents a racially diverse sample of well-characterized women. Secondly, the prospective design enables an examination of diabetes incidence. In addition, the WHI collected detailed information on a comprehensive range of diabetes risk factors relevant to this investigation, with a 10-year follow-up for diabetes outcome.
In conclusion, significant disparities exist between the major ethnic groups in diabetes prevalence and incidence in postmenopausal women; these differences withstand adjustment for a very comprehensive group of physiological and behavioral risk factors. Determinants of the disparities observed varied by race/ethnicity. Although these results highlight the potential benefits of tailored diabetes prevention strategies directed at those specific factors that are most likely to increase the risk of diabetes among each racial/ethnic group, it is prudent to recommend avoidance of weight gain, weight loss, a healthy diet, and adequate levels of physical activity to all postmenopausal women for the purpose of diabetes risk reduction.
Acknowledgments
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant 1R21-DK-083700-01A1 and by National Heart, Lung, and Blood Institute (NHLBI) Grant 1R01-HL-094575-01A1 to Y.M. It was also supported in part by Center Grant 5P30-DK-32520 from NIDDK. Y.M., M.C.R., and I.S.O. are members of the University of Massachusetts Diabetes and Endocrinology Research Center (DK32520). The WHI program is funded by the NHLBI, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01-WH-22110, -24152, -32100–32102, -32105, -32106, -32108, -32109, -32111–32113, -32115, -32118, -32119, -32122, -42107–42126, -42129–42132, and -44221.
No potential conflicts of interest relevant to this article were reported.
Y.M. wrote the manuscript and researched data. J.R.H., J.E.M., S.L., M.J.L., C.E.B., J.K.O., B.O., K.L.S., M.C.R., D.M.S., J.W.-W., M.L.S., L.S.P., I.S.O., R.C.K., G.E.S., L.G., and B.V.H. contributed to the discussion and reviewed and edited the manuscript. R.B. and Y.Q. performed data analyses and reviewed and edited the manuscript. Y.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the principal investigators of all WHI clinical centers and the data coordinating center for their contribution to the study. The authors are also indebted to the dedicated and committed participants of the WHI.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0412/-/DC1.
A complete list of Women’s Health Initiative investigators can be found in the Supplementary Data online.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NIDDK or NHLBI.
References
- 1.CDC National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431 [DOI] [PubMed] [Google Scholar]
- 3.Matthews CE, Sui X, LaMonte MJ, Adams SA, Hébert JR, Blair SN. Metabolic syndrome and risk of death from cancers of the digestive system. Metabolism 2010;59:1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 5.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 2004;27:66–69 [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 7.Reis JP, Loria CM, Sorlie PD, Park Y, Hollenbeck A, Schatzkin A. Lifestyle factors and risk for new-onset diabetes: a population-based cohort study. Ann Intern Med 2011;155:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13(Suppl.):S107–S121 [DOI] [PubMed] [Google Scholar]
- 9.Margolis KL, Lihong Qi, Brzyski R, et al. Women Health Initiative Investigators Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(Suppl.):S498–S504 [DOI] [PubMed] [Google Scholar]
- 11.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–187 [DOI] [PubMed] [Google Scholar]
- 12.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–1271 [DOI] [PubMed] [Google Scholar]
- 13.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 2006;9(1A):152–157 [DOI] [PubMed] [Google Scholar]
- 14.McTiernan A, Kooperberg C, White E, et al. Women’s Health Initiative Cohort Study Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA 2003;290:1331–1336 [DOI] [PubMed] [Google Scholar]
- 15.Tinker LF, Bonds DE, Margolis KL, et al. Women’s Health Initiative Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled dietary modification trial. Arch Intern Med 2008;168:1500–1511 [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Hébert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008;24:941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr 1999;69:381–387 [DOI] [PubMed] [Google Scholar]
- 18.Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960-2000. Prev Med 2004;39:197–206 [DOI] [PubMed] [Google Scholar]
- 19.Lutsey PL, Pereira MA, Bertoni AG, Kandula NR, Jacobs DR., Jr Interactions between race/ethnicity and anthropometry in risk of incident diabetes: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010;172:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia J, Wu L, Allen C, et al. Women’s Health Initiative Research Group Physical activity and diabetes risk in postmenopausal women. Am J Prev Med 2005;28:19–25 [DOI] [PubMed] [Google Scholar]
- 21.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Choi HK, Ford E, et al. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006;29:1579–1584 [DOI] [PubMed] [Google Scholar]
- 23.Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–1026 [DOI] [PubMed] [Google Scholar]
- 24.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–930 [DOI] [PubMed] [Google Scholar]
- 25.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care 2007;30:1753–1757 [DOI] [PubMed] [Google Scholar]
- 26.Dubowitz T, Heron M, Bird CE, et al. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. Am J Clin Nutr 2008;87:1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forshee RA, Storey ML. Demographics, not beverage consumption, is associated with diet quality. Int J Food Sci Nutr 2006;57:494–511 [DOI] [PubMed] [Google Scholar]
- 28.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health 2012;102:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006;29:1585–1590 [DOI] [PubMed] [Google Scholar]
- 30.Link CL, McKinlay JB. Disparities in the prevalence of diabetes: is it race/ethnicity or socioeconomic status? Results from the Boston Area Community Health (BACH) survey. Ethn Dis 2009;19:288–292 [PMC free article] [PubMed] [Google Scholar]
- 31.LaVeist TA, Thorpe RJ, Jr, Galarraga JE, Bower KM, Gary-Webb TL. Environmental and socio-economic factors as contributors to racial disparities in diabetes prevalence. J Gen Intern Med 2009;24:1144–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marmot M, Friel S, Bell R, Houweling TA, Taylor S, Commission on Social Determinants of Health Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008;372:1661–1669 [DOI] [PubMed] [Google Scholar]
- 33.Marmot MG. Status syndrome: a challenge to medicine. JAMA 2006;295:1304–1307 [DOI] [PubMed] [Google Scholar]
- 34.Qi L, Nassir R, Kosoy R, et al. Relationship between diabetes risk and admixture in postmenopausal African-American and Hispanic-American women. Diabetologia 2012;55:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird CE, Rieker PP. Gender and Health: The Effects of Constrained Choices and Social Policies. New York, NY, Cambridge University Press, 2008 [Google Scholar]
- 36.Waidmann TA, Rajan S. Race and ethnic disparities in health care access and utilization: an examination of state variation. Med Care Res Rev 2000;57(Suppl. 1):55–84 [DOI] [PubMed] [Google Scholar]
- 37.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA 2006;295:39–49 [DOI] [PubMed] [Google Scholar]