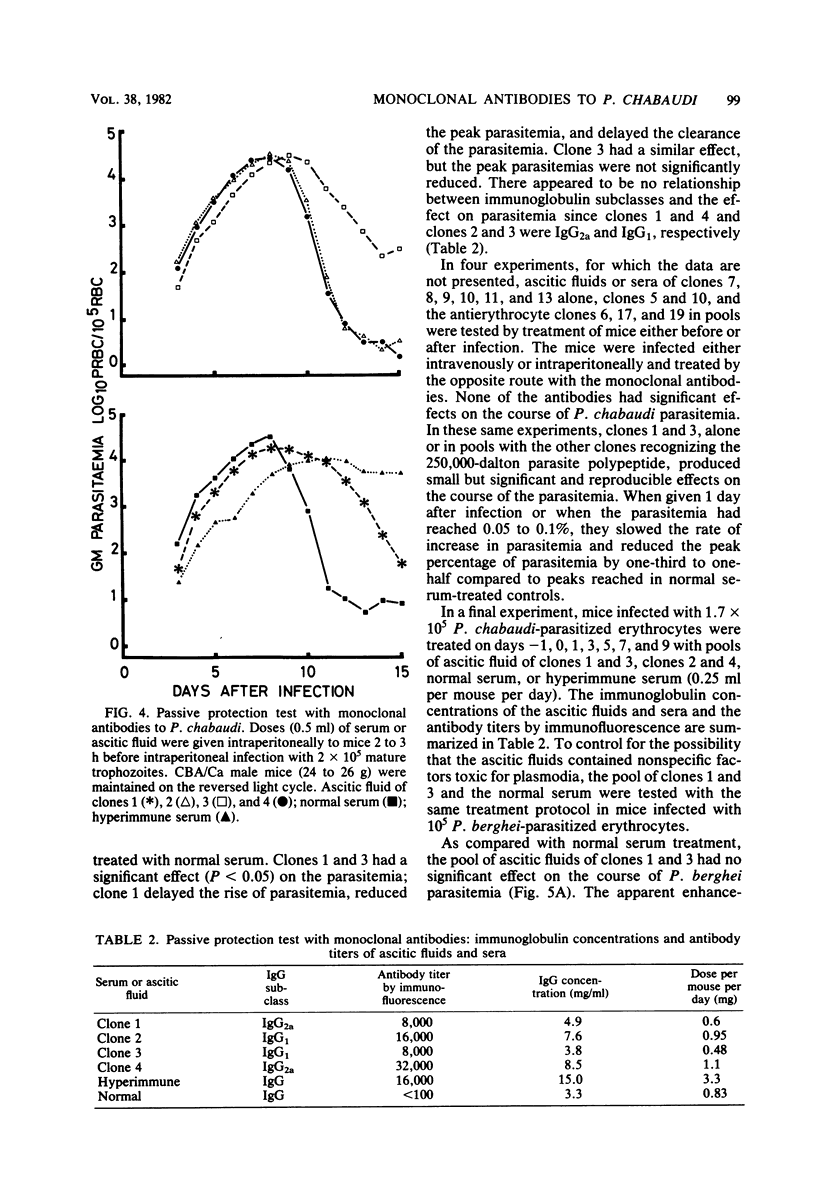
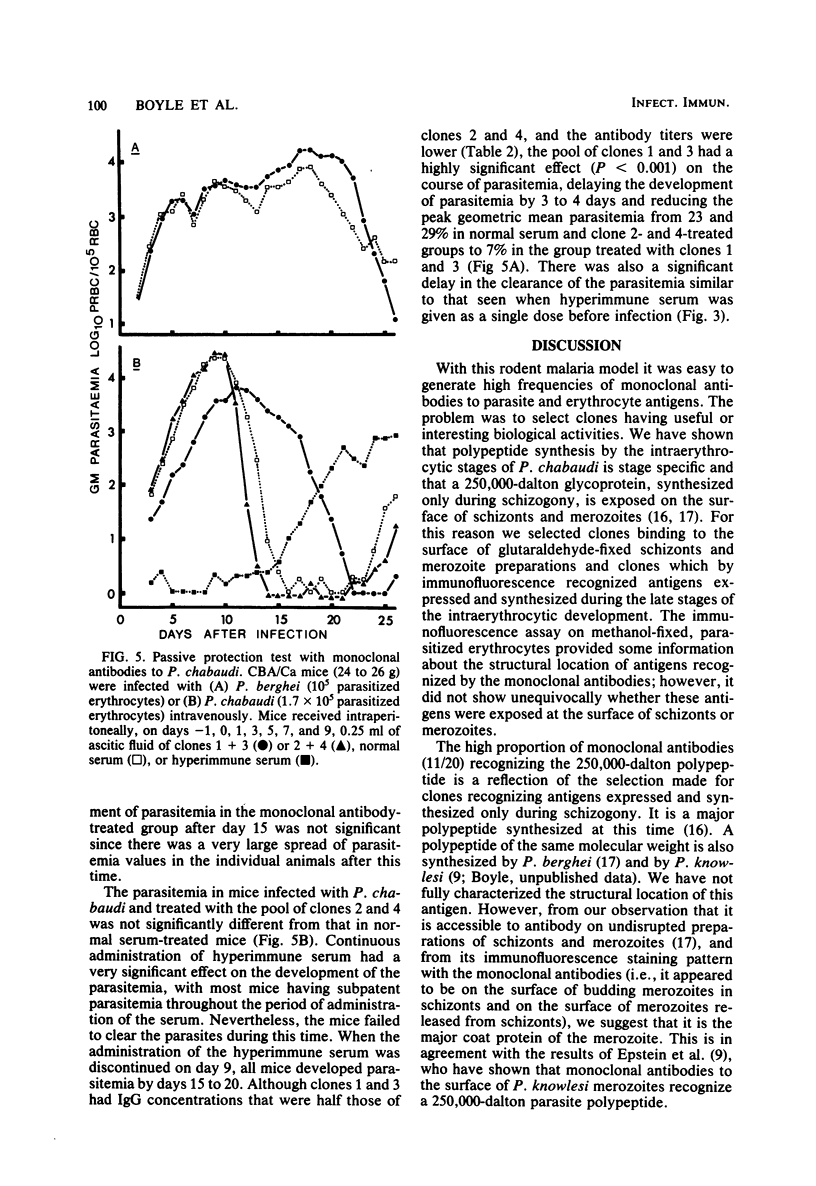
Abstract
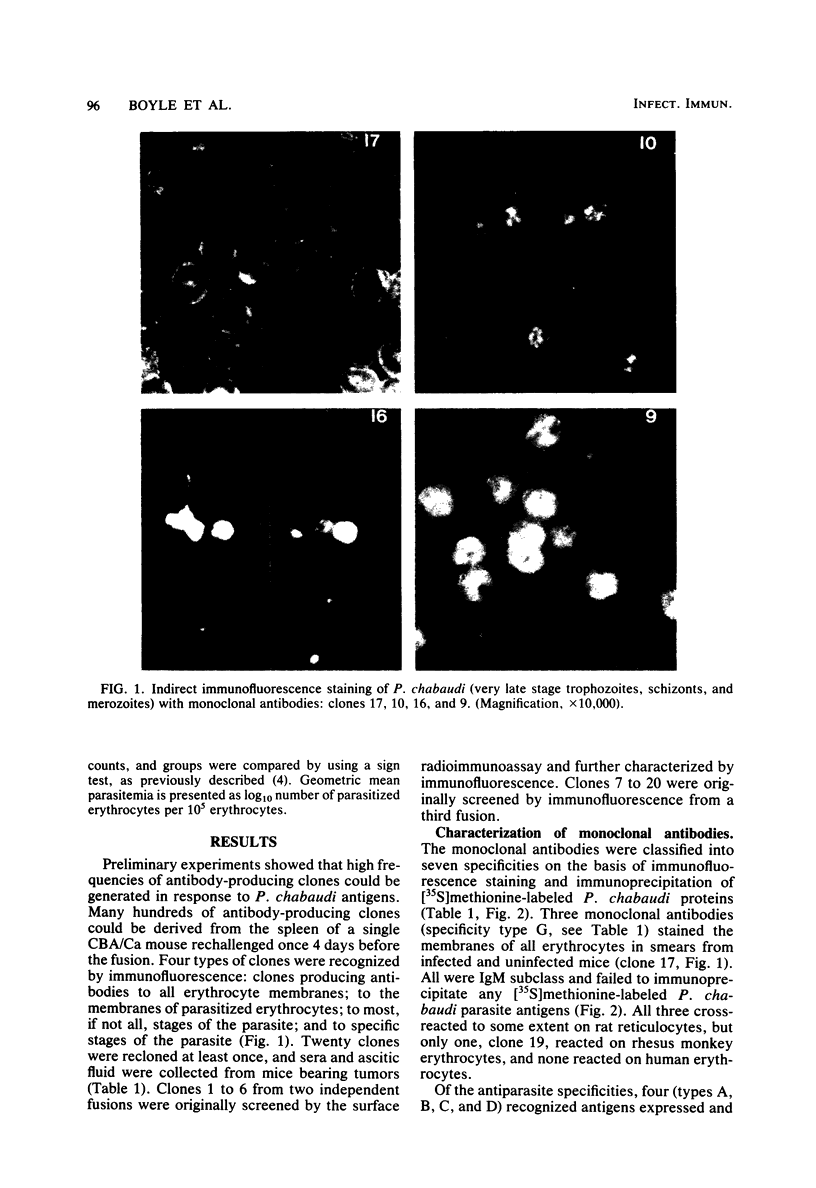
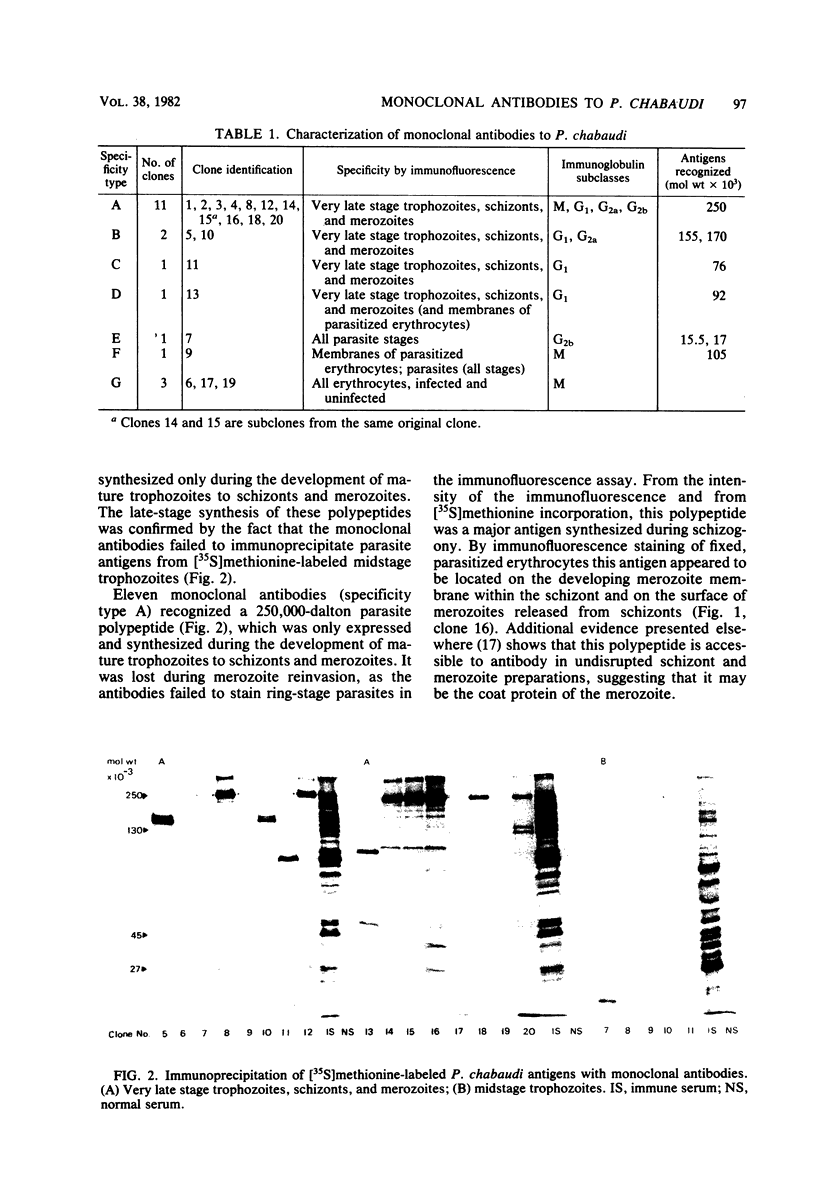
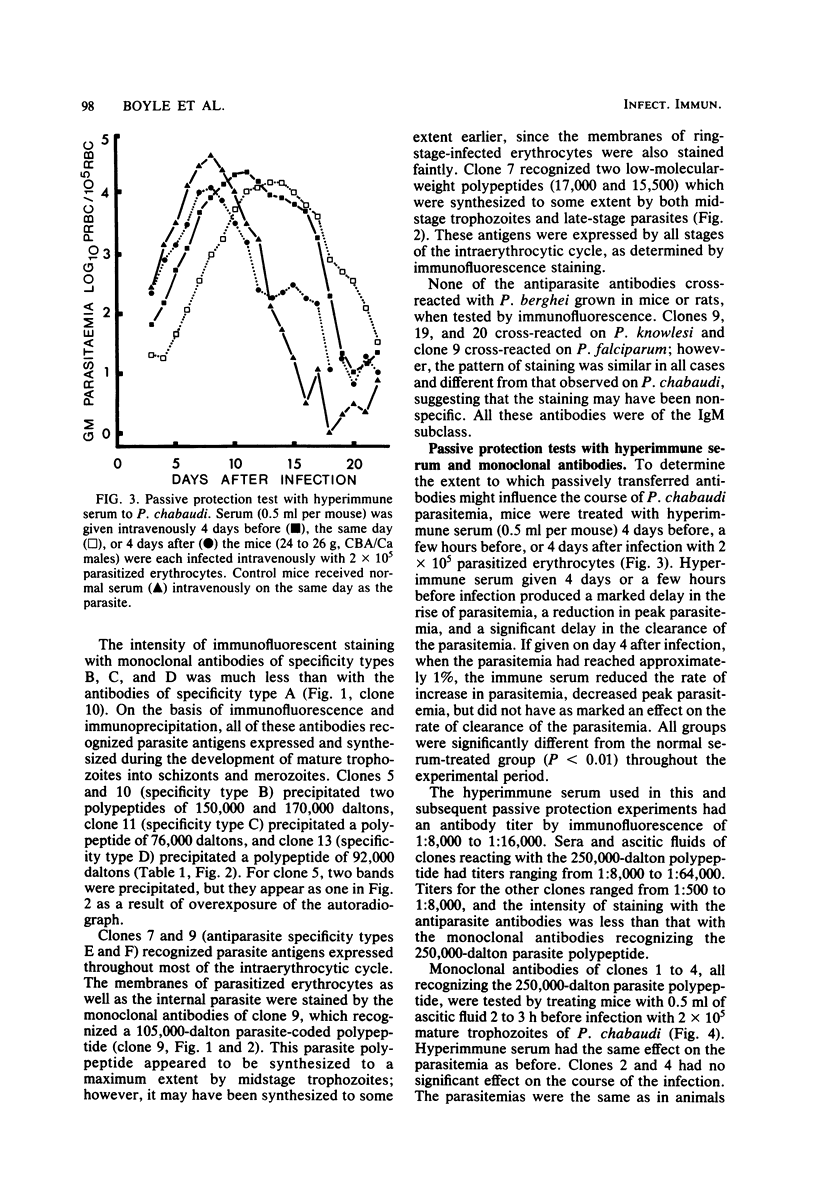
Twenty monoclonal antibodies have been prepared to the erythrocytes from CBA/Ca mice infected with the rodent malaria Plasmodium chabaudi. By immunofluorescence, 15 of these antibodies recognized parasite antigens expressed only during the development of mature trophozoites to schizonts and merozoites, 2 recognized parasite antigens that were expressed throughout most of the intraerythrocytic cycle, and 3 recognized the membranes of all infected and uninfected erythrocytes. By immunoprecipitation of [35S]methionine-labeled, parasitized erythrocytes, parasite antigens recognized by all of the antiparasite antibodies were characterized. Eleven precipitated a 250,000-dalton parasite polypeptide which was synthesized and expressed late in the intraerythrocytic cell cycle and which appeared to be the major coat protein of the merozoites. In passive protection experiments, transfer of hyperimmune serum before infection with the parasite resulted in a delay in the rise of parasitemia, reduction in peak parasitemias, and a delay in the clearance of the parasitemia. Two monoclonal antibodies to the 250,000-dalton polypeptide had a similar but not as marked effect on parasitemia when given as a single dose before infection. When mixed and administered throughout the course of infection, their effects were greater. They had no influence on the course of Plasmodium berghei KSP11 parasitemia. Monoclonal antibodies to other parasite antigens and normal erythrocyte antigens failed to have a significant and reproducible effect on P. chabaudi parasitemia. The results suggest that this 250,000-dalton malaria parasite antigen may be important in the induction and expression of antibody-mediated immunity to malaria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown I. N., Brown K. N., Hills L. A. Immunity to malaria: the antibody response to antigenic variation by Plasmodium knowlesi. Immunology. 1968 Jan;14(1):127–138. [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., Hills L. A. The possible role of isoantigens in protective immunity to malaria. Bull World Health Organ. 1979;57 (Suppl 1):135–138. [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Hills L. A. T cells and protective immunity to Plasmodium berghei in rats. Infect Immun. 1976 Oct;14(4):858–871. doi: 10.1128/iai.14.4.858-871.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979 Jan 15;203(1153):323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Parish C. R. Plasmodium yoelii: antibody and the maintenance of immunity in BALB/c mice. Exp Parasitol. 1981 Aug;52(1):18–24. doi: 10.1016/0014-4894(81)90056-4. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Trejdosiewicz A. J., Cross G. A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980 Mar 27;284(5754):366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Newbold C. I., Boyle D. B., Smith C. C., Brown K. N. Identification of a schizont- and species-specific surface glycoprotein on erythrocytes infected with rodent malarias. Mol Biochem Parasitol. 1982 Jan;5(1):45–54. doi: 10.1016/0166-6851(82)90005-6. [DOI] [PubMed] [Google Scholar]
- Newbold C. I., Boyle D. B., Smith C. C., Brown K. N. Stage specific protein and nucleic acid synthesis during the asexual cycle of the rodent malaria Plasmodium chabaudi. Mol Biochem Parasitol. 1982 Jan;5(1):33–44. doi: 10.1016/0166-6851(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Jones V. E. Immunity to Plasmodium berghei in rats: maximum levels of protective antibody activity are associated with eradication of the infection. Parasitology. 1972 Feb;64(1):117–127. doi: 10.1017/s0031182000044693. [DOI] [PubMed] [Google Scholar]
- Scott-Finnigan T. J., Wilson R. J., Williamson J. A compact semi-automated continuous cultivation system for Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1981;75(2):292–298. doi: 10.1016/0035-9203(81)90338-2. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Kim K. J., Munoz P. A., Evans C. B., Asofsky R. Monoclonal antibodies to stage-specific, species-specific, and cross-reactive antigens of the rodent malarial parasite, Plasmodium yoelii. Infect Immun. 1981 May;32(2):563–570. doi: 10.1128/iai.32.2.563-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. II. A reproducible method. Immunology. 1970 Jun;18(6):875–881. [PMC free article] [PubMed] [Google Scholar]