Abstract
BH3 mimetics such as ABT-737 and navitoclax bind to the BCL-2 family of proteins and induce apoptosis through the intrinsic apoptosis pathway. There is considerable variability in the sensitivity of different cells to these drugs. Understanding the molecular basis of this variability will help to determine which patients will benefit from these drugs. Furthermore, this understanding aids in the design of rational strategies to increase the sensitivity of cells which are otherwise resistant to BH3 mimetics. We discuss how the expression of BCL-2 family proteins regulates the sensitivity to ABT-737. One of these, MCL-1, has been widely described as contributing to resistance to ABT-737 which might suggest a poor response in patients with cancers that express levels of MCL-1. In some cases, resistance to ABT-737 conferred by MCL-1 is overcome by the expression of pro-apoptotic proteins that bind to apoptosis inhibitors such as MCL-1. However, the distribution of the pro-apoptotic proteins amongst the various apoptosis inhibitors also influences sensitivity to ABT-737. Furthermore, the expression of both pro- and anti-apoptotic proteins can change dynamically in response to exposure to ABT-737. Thus, there is significant complexity associated with predicting response to ABT-737. This provides a paradigm for the multiplicity of intricate factors that determine drug sensitivity which must be considered for the full implementation of personalized medicine.
Keywords: ABT-737, Navitoclax, ABT-263, BH3 mimetic, Apoptosis, Personalized medicine
Introduction
The concept of personalized medicine envisages that therapy is tailored to take into account in each patient the pharmacodynamic and pharmacokinetic factors which may affect drug safety and efficacy. In oncology, this differs from the traditional approach in which the therapy is often based on the tissue of origin of the tumor. In personalized medicine, a treatment is chosen to address the molecular defects associated with the patient’s personal disease. In principle, it appears relatively straightforward to identify genetic (or epigenetic) abnormalities in a patient’s cancerous cells and select therapy accordingly. However, simply knowing the genes affected may be inadequate without a detailed understanding of the regulation of the signalling pathways involved. An often quoted example is provided by cetuximab, a therapeutic antibody which targets the EGF receptor. Cetuximab is effective in colon cancer driven by activation of the EGF pathway but less efficacious in patients whose tumors also are activated downstream of the EGF receptor by mutation of the gene encoding RAS. Furthermore, understanding signalling pathways in detail allows the rational design of drug combinations. We anticipate that a full implementation of personalized medicine will require profound analysis of the signalling pathways involved in the drug response. We exemplify this argument by a discussion of ABT-737, together with its cogener Navitoclax (ABT-263). ABT-737 is a “tool” compound that has been widely used, whereas ABT-263 possesses superior oral bioavailability and is currently being evaluated in clinical trials. The efficacy of these drugs depends on their ability to activate the intrinsic apoptosis pathway which we review first.
The intrinsic apoptosis pathway
The increase in oncogenic signalling (oncogene stress) and the DNA damage that occurs in genetically unstable cancer cells generate pro-apoptotic signals which, if executed, would effectively curtail tumorigenesis. Cancer cells are obliged by selective pressure to evolve mechanisms which suppress these pro-apoptotic signals. This has led to the ability of tumor cells to evade cell death through apoptosis being recognised as one of the hallmarks of cancer [1]. The dependency of cancer cells on the pathways suppressing apoptosis provides a therapeutic strategy for the treatment of cancer, because agents that inactivate these pathways should cause cell death. There are two key apoptosis pathways. The extrinsic apoptosis pathway is activated by extracellular pro-apoptotic stimuli, whereas the intrinsic pathway responds to intracellular cues. The intrinsic apoptosis pathway is controlled by the BCL-2 family of proteins (Table 1). This family comprises several classes of proteins which are considered as “effectors” (BAX, BAK), “inhibitors” (BCL-2, BCL-XL, BCL-2 MCL-1, BFL/A1, BCL-B), “activators” (BIM, BID, PUMA [2-4], possibly BMF and NOXA [5]) and “sensitizers” (eg BAD, BMF, NOXA). The family share several conserved “BH” domains termed BH1, BH2, BH3 and BH4. Of these domains, the activators and the sensitizers possess only the BH3 domain, and hence are often referred to as “BH3-only” proteins. These proteins are induced by cellular stress, e.g. chemotherapy. The other family members possess BH1, 2, 3 and 4 domains. The inhibitors are consequently referred to as multi-domain apoptosis inhibitors. For clarity, we shall refer to these as “apoptosis inhibitors” throughout this review, but these should not be confused with other inhibitors of apoptosis that are not part of the BCL-2 family. These multi-domain proteins possess a hydrophobic groove that serves as a binding site for BH3 domains. BAX and BAK can form homo-oligomers that form a pore in the mitochondrial outer membrane. This allows the release of several mediators of apoptosis including cytochrome C, triggering the subsequent activation of caspases. BAK normally resides in the mitochondrial outer membrane, whereas BAX translocates from the cytosol to mitochondria following activation. Two competing models have been proposed for how BAX and BAK are activated, but recent data supports a “direct activation” model in which the activator BH3-only proteins bind directly but transiently to BAX or BAK to activate them [3]. The ability of the activator BH3-only proteins to trigger BAK and BAX is restrained by the apoptosis inhibitors which sequester the BH3-only activators by binding to their BH3 domain. The apoptosis inhibitors can also suppress apoptosis by binding to the BH3 domain of the effectors and have been shown in the case of BCL-XL to promote the translocation of BAX from mitochondria to the cytosol [6]. However, the apoptosis inhibitors may be prevented from suppressing apoptosis by the BH3-only sensitizers whose BH3 domain can also bind to the apoptosis inhibitors. The BH3-only sensitizers occupy the inhibitors, reducing the capacity of the apoptosis inhibitors to sequester the BH-3 only activators or the effectors (Figure 1). Thus, the BH3-only sensitizers reduce the reservoir of unliganded inhibitors available to suppress apoptosis, facilitating pro-apoptotic signalling to BAK and BAX by the BH3-only activators. The alternative “indirect” model envisages that the apoptosis inhibitors sequester the effectors. In this model, the effectors are not considered to require activation, but are continually repressed by the apoptosis inhibitors. The BH3-only proteins induce apoptosis indirectly by liberating the effectors from the apoptosis inhibitors. Variations on both these models have been proposed and it is possible that aspects of both are correct.
Table 1.
BCL-2 family proteins
| BH3 only pro-apoptotic proteins | Multi-domain pro-apoptotic | Anti-apoptotic proteins |
|---|---|---|
|
Activators |
|
|
| BIM |
BAK |
BCL-2 |
| BID |
BAX |
BCL-XL |
| PUMA |
BOK |
BCL-W |
| |
|
MCL-1 |
|
Sensitizers |
|
BFL/BCL-2A1 |
| NOXA |
|
BCL-B |
| BAD |
|
|
| BMF |
|
|
| HRK |
|
|
| BIK |
|
|
| BNIP3 |
Of the BH3 only proteins, BID, BIM and possibly PUMA are considered apoptosis activators and the others as apoptosis sensitizers. BOK is an apoptosis effector expressed primarily in the ovary.
Figure 1.
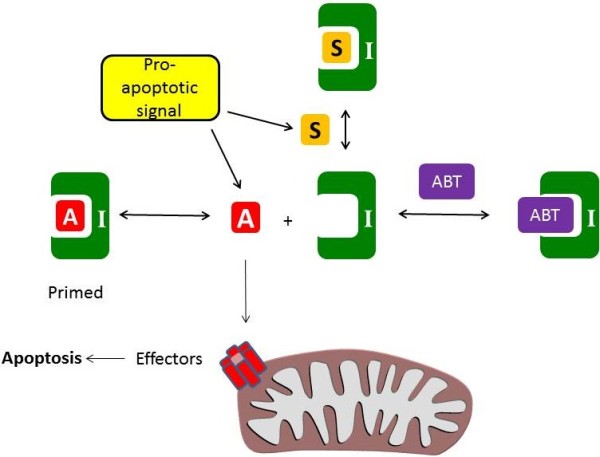
The intrinsic apoptosis pathway. Apoptosis is triggered by the formation of a pore in the mitochondrial outer membrane formed from the effectors. This is controlled by the activator molecules (BID, BIM, PUMA, labelled “A”) which directly stimulate the effectors (BAK and BAX). A pro-apoptotic signal (such as oncogene stress or chemotherapy) induces the expression of apoptosis activators and sensitizers. The activators may be sequestered by the inhibitors (labelled “I”, e.g. BCL-2, BCL-XL, MCL-1) and this prevents apoptosis. However, sensitizer molecules (labelled “S”, eg NOXA, BAD) or BH3 mimetics (eg ABT-737, labelled “ABT”) can occupy the inhibitors, preventing them from binding the activators. In cells in which the inhibitors are already primed with an activator, the effective displacement of the activator from the inhibitor by ABT-737 induces apoptosis.
A further layer of complexity is involved because of the different binding specificity of the apoptosis inhibitors for their ligands (Figure 2). Some BH3-only proteins can bind to all the apoptosis inhibitors, whereas other BH3-only proteins exhibit specificity for a subset of the apoptosis inhibitors. For example, BAD bind preferentially to BCL-2, BCL-XL and BCL-w [7,8], whereas NOXA binds preferentially to MCL-1 [7,8] and possibly BFL/A1 [7]. The BH3-only apoptosis activators BIM and PUMA are each able to bind to all the apoptosis inhibitors whereas BID has a more restricted binding profile [7,9]. Whether the induction of a BH3-only protein leads to apoptosis depends on whether the repertoire of apoptosis inhibitors expressed is adequate to dampen the pro-apoptotic signal arising from the activation of BH3-only proteins.
Figure 2.
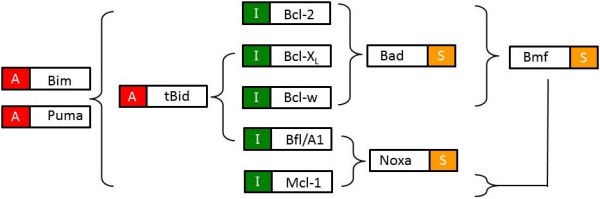
Binding specificity of BH3 only proteins. The activators (denoted with an “A”), BIM and PUMA bind to all the apoptosis inhibitors (denoted with an “I”). However, tBID and the sensitizers (denoted with a “S”) bind to a subset of the apoptosis inhibitors. There are some discrepancies in the literature (e.g., whether BMF binds MCL-1) and it is important to note that this diagram summarizes interactions (adapted from [7-9] in a binary fashion (interaction or no interaction) whereas in reality the interactions are better described by a range of binding affinities.
ABT-737 and other BH3 mimetics
The recognition that BCL-2 family proteins regulate apoptosis has led to the development of “BH3 mimetics”, drugs which compete with BH3 domains to bind the apoptosis inhibitors. These drugs function as sensitizer molecules – although unable to directly stimulate apoptosis, they prevent the apoptosis inhibitors from sequestering the pro-apoptotic proteins through their BH3 domains (reviewed in [10]). In cells where the apoptosis inhibitors are already bound to a BH3-only apoptosis activator, the cell are considered “primed for death” because this allows a BH3 mimetic to induce apoptosis through liberation of a BH3-only activator (Figure 1). BH3 mimetics have shown remarkable single agent activity in primed cells [8] which appear addicted to apoptosis inhibitors for survival. In cells where the apoptosis inhibitors are not already primed with a BH3-only activator, exposure to chemotherapeutic agents induces BH3-only proteins that can be liberated from the inhibitors by a BH3 mimetic. Consequently, BH3 mimetics have shown synergy with a wide range of chemotherapeutic agents.
Several BH3 mimetics have been described. The best characterized is probably ABT-737 [11] and its analogue ABT-263 [12]. These compounds bind with high affinity to BCL-2, BCL-XL and BCL-W but weakly to MCL-1 and BFL/A1. ABT-737 can induce apoptosis in cells in which the apoptosis inhibitors are primed with BH3-only activator proteins and ABT-737. Chemotherapeutic agents may induce priming, and consequently ABT-737 and ABT-263 are synergistic with several different chemotherapeutic agents [10,13]. Whilst the activity of ABT-737 is substantially reduced in cells lacking BAX or BAK, several BH3 mimetics other than ABT-737 retain cytotoxicity in BAX−/−/BAK−/− cells [14,15] and unexpectedly increase the level of NOXA [16]. This suggests that the BH3 mimetics other than ABT-737 and ABT-263 possess additional activities that have yet to be fully defined. As the goal of this review is to summarize what determines sensitivity to BH3 mimetics, this undefined pharmacological activity significantly complicates a rational discussion of the factors that determine sensitivity to these drugs. Thus, this review will focus on ABT-737 (and ABT-263).
Determinants of sensitivity to ABT-737
Knowledge of the tissue of origin of the malignant cells is not redundant, even in the era of personalized medicine, because some genetic aberrations are commonly associated with a particular cancer. For example, BCL-2 is commonly amplified in chronic lymphocytic leukemia (CLL). However, the fact that cancer is a disease involving significant genetic instability means that cancer cells sharing the same tissue origin may be genetically quite distinct. The genetic background of malignant cells can have a profound effect on drug response. Indeed, cancers of different tissue origins may share similar pharmacological sensitivities because they share common genetic anomalies. For example, chronic myelogenous leukemia and non-small cell lung cancer patients may share a polymorphism in the gene encoding BIM which leads to reduced expression of BIM isoforms possessing the BH3 domain that is necessary for its pro-apoptotic activity. Cells with this polymorphism are resistant to tyrosine kinase inhibitors, but this can be overcome with ABT-737 [17].
BCL-2, BCL-XL and BCL-W
The most obvious determinant of sensitivity to any drug is whether cells express the intended targets. In the case of ABT-737, these are BCL-2, BCL-XL and BCL-W. It is often assumed that increased expression of an oncogene indicates a tumor cell’s dependence on it. Elevated expression of these apoptosis inhibitors may be necessary to tolerate elevated levels of BH3-only proteins. However, the situation is significantly more complex, because the expression of BCL-2, BCL-XL or BCL-W is a necessary but not a sufficient condition to confer drug sensitivity [18,19]. Cells in which the apoptosis inhibitors are already primed with BH3-only proteins provide an example of oncogene addiction and are particularly sensitive to ABT-737. In contrast, cells in which BCL-2, BCL-XL or BCL-W are expressed but which are unoccupied by either BH3-only proteins or by BAX or BAK are not likely to be sensitive to ABT-737. Thus, measurement of expression levels of BCL-2, BCL-XL and BCL-W alone may be a poor predictor of sensitivity.
An added complication has recently arisen because at least two groups have noted a discrepancy between the activity of ABT-737 expected from in vitro experiments and that observed in live cells. The binding profile of ABT-737 suggests that it should inhibit the binding of BH3-only proteins to BCL-2, BCL-XL or BCL-W. However, in cells ABT-737 appears to more readily prevent BIM from binding to BCL-2 than to BCL-XL or BCL-W [20]. This might reflect the somewhat lower affinity of BIM for BCL-2. Another report suggests that the interaction of BIM with BCL-2 or BCL-XL is altered by the subcellular localization of the complex, and mitochondrial BIM complexes with BCL-2 or BCL-XL are relatively insensitive to ABT-737 [21]. These observations are particularly relevant to personalized medicine, because it underscores the difficulty in making predictions of drug sensitivity using measurement of protein expression and knowledge of binding interactions measured in vitro.
MCL-1 and BFL/A1
Several lines of evidence indicate that MCL-1 confers resistance to ABT-737 because of the poor affinity of ABT-737 for MCL-1. Silencing MCL-1 by RNA interference increases the sensitivity of several cancer cell lines to ABT-737 [22-32] while ectopic expression of MCL-1 can render cells resistant to ABT-737 [24,29,33]. Similarly, prolonged exposure to ABT-737 to render cells resistant to ABT-737 leads to the up-regulation of MCL-1 [34]. Short-term exposure to ABT-737 has also been reported to elevate expression of MCL-1 [16,35,36]. However, expression of MCL-1 is not always sufficient to cause resistance to ABT-737 because occupancy of MCL-1 by pro-apoptotic proteins can effectively inactivate MCL-1 [18,37] (and see below). In the context of personalized medicine, this argues that expression of MCL-1 on its own should not necessarily preclude the use of ABT-263.
It also noteworthy that some cells appear to be more dependent on MCL-1 for survival rather than on other BCL-2 family members [38], and these cells may be sensitive to a recently described MCL-1 inhibitor “Maritoclax” [39]. By causing the degradation of MCL-1, this compound has the potential to overcome some forms of resistance to ABT-737, and has shown synergy with ABT-737 [39].
Expression of BFL1/A1 may also contribute to the sensitivity of cells to ABT-737 because, like MCL-1, BFL/A1 does not bind to ABT-737 with high affinity. Knockdown of BFL/A1 increases sensitivity to ABT-737 [27] whereas BFL/A1 over-expression reduces sensitivity [40]. Resistance to ABT-737 may also lead to up-regulation of BFL1/A1 [34].
NOXA
The observation that MCL-1 and BFL/A1 can confer resistance to ABT-737 suggests that BH3-only proteins that occupy these apoptosis inhibitors should increase sensitivity to ABT-737.
NOXA is particularly significant because it binds to both MCL-1 and BFL/A1, the apoptosis inhibitors implicated in resistance to ABT-737. Ectopic expression of NOXA has been shown to increase sensitivity to ABT-737 [26,32,41], whereas inhibition of NOXA expression by RNA interference decreases sensitivity to ABT-737 [40,41]. By blocking MCL-1, NOXA prevents MCL-1 from sequestering BH3-only proteins that are released from other BCL-2 family members by ABT-737. For example, ectopic expression of NOXA prevents MCL-1 sequestering BIM [41]. ABT-737 itself also increases expression of NOXA in some cells [41] but whether this is a significant and frequent phenomenon is debateable because if it were it would prevent MCL-1 from conferring resistance to ABT-737.
The expression of NOXA is increased following exposure to several chemotherapeutic agents and consequently NOXA is implicated in the synergy between these drugs and ABT-737. The importance of NOXA expression is demonstrated by several observations in which synergy between ABT-737 and chemotherapeutic agents is reduced if the expression of NOXA is repressed using RNAi. Repression of NOXA reduces synergy between ABT-737 and temozolomide [42], etoposide [27,43], vinblastine [27], cisplatin [43], CPT-11 [44] and oxaliplatin [45]. NOXA is also necessary for synergy with the proteasome inhibitors bortezomib [44] and MG132 [28].
BIM
Early work in the field highlighted the importance of the association of BIM with BCL-2 in determining sensitivity to ABT-737 [46]. Cancer cells from a number of lymphoid malignancies show significant (IC50 < 100 nM) sensitivity to ABT-737 as a single agent. In these cells, BCL-2 is already primed with BIM [8,47,48]. However, if BIM is associated with MCL-1, the cells are resistant to ABT-737 unless NOXA is also expressed and occupies MCL-1 [18]. Thus, the apoptosis inhibitor to which BIM is bound as well as which other BH3-only proteins are expressed helps determine sensitivity to ABT-737. Resistance to ABT-737 may also result from reduced expression of BIM. Small cell lung cancer (SCLC) cells grown as xenografts that have developed resistance to ABT-737 show a reduction in BIM:BCL-2 complexes compared to sensitive cells [49]. These observations underline the multi-parametric nature of sensitivity to ABT-737, and emphasize the complexity of its accurate estimation in personalized medicine.
Several chemotherapeutic agents induce the expression of BIM and probably priming of the apoptosis inhibitors with BIM. ABT-737 disrupts complexes of BIM and BCL2 (in some cells, docetaxel appears to induce priming of BCL-2 with BIM), and ABT-737 and docetaxel in combination show improved activity against the same cells grown as xenografts, compared to the activity of either agent alone [50]. Similarly, the displacement of BIM from BCL-2 by ABT-737 contributes to synergy observed between ABT-737 and TRAIL [51]. Thus, the use of ABT-263 in personalized medicine in combination with other cancer therapeutics offers yet more challenges in accurately predicting patient response because of the response to drug exposure.
PUMA
Recent work has suggested that PUMA functions as a direct activator of BAX and BAK [2-4]. In the presence of ABT-737 to occupy apoptosis inhibitors, the expression of PUMA may be sufficient to induce apoptosis [4]. PUMA co-operates with other BH3-only activators and ABT-737 to induce apoptosis [52] while loss of PUMA reduces the apoptotic activity of ABT-737 [2,4].
BID
BID is a BH3-only activator whose caspase-mediated cleavage promotes its pro-apoptotic activity. ABT-737 has been shown to trigger the cleavage of BID in leukaemia [53] and neuroblastoma cells [54]. Cleavage of BID is observed following activation of the extrinsic apoptosis pathway, so how is this enhanced by ABT-737? This could reflect cross-talk between the intrinsic and extrinsic apoptosis pathways, or we speculate it may be linked to the up-regulation of the TRAIL receptor DR5 by ABT-737 [55]. From a personalized medicine perspective, this raises the question whether it is important to consider both the extrinsic as well as the intrinsic apoptosis pathway when predicting patient response to ABT-263.
BAD
BAD is classified as a BH3-only sensitizer, and so its liberation by ABT-737 indirectly induces apoptosis by reducing the reservoir of apoptosis inhibitors. BAD and ABT-737 bind to the same apoptosis inhibitors suggesting that expression of BAD would be anticipated to reduce the concentration of ABT-737 required to occupy BCL-2/BCL-XL/BCL-W. However, there is additional complexity because by preventing the association of BAD with BCL-XL, ABT-737 slows the turnover of BAD, leading to a striking increase in BAD protein [56]. The relative contributions of BAD prior to, and after, exposure to ABT-737 remain unclear.
Summary of factors affecting sensitivity
From the foregoing discussion, it is clear that there is no single determinant of sensitivity to ABT-737. In several cases, various BH3-only proteins have been identified as being “necessary” for ABT-737 to exhibit pro-apoptotic activity. Discrepancies between different studies probably reflect differences in the experimental setting: whether cells are primed; the genetic background of the cells being studied; the BH3-only proteins and the apoptosis inhibitors expressed and the effect of ABT-737 on their expression. The over-arching factor that determines whether ABT-737 induces apoptosis appears to be whether sufficient BH3-only proteins can be released to exceed the buffering capacity of the apoptotic inhibitors when cells are exposed to ABT-737. This is determined by the repertoire of apoptosis inhibitors, BH3-only activators and sensitizers expressed in a particular cell. As we will discuss below, this creates a significant dilemma for predicting drug sensitivity in the clinic. Analysis of protein expression alone may not be sufficient to understand the drug sensitivity of an individual tumor, rather measurement of the functional status of the intrinsic apoptosis pathway may be a superior predictor.
Strategies for overcoming resistance to ABT-737
One aspect of personalized medicine envisages that patients may not receive a drug where assessment of the molecular abnormalities in their tumor predicts that the drug is unlikely to be effective. But through an understanding of the pathways regulated by ABT-737 and ABT-263, can we rationally design drug combinations which increase the sensitivity of cells to these drugs? This may benefit patients predicted to be unresponsive to ABT-263. Two general strategies have been advanced, categorized as either decreasing the expression of MCL-1, or increasing the expression of BH3-only proteins, or in some cases both of these factors are involved.
Decreasing the expression of MCL-1
The observation that MCL-1 can confer resistance to ABT-737 suggests that strategies to decrease the expression of MCL-1 might increase sensitivity to ABT-737. Several drugs have been proven effective in preclinical studies (Figure 3). This approach is also particularly important because exposure to ABT-737 can itself increase expression of MCL-1 [16,35,36] thus providing a mechanism by which resistance to ABT-737 can be caused by the drug itself.
Figure 3.
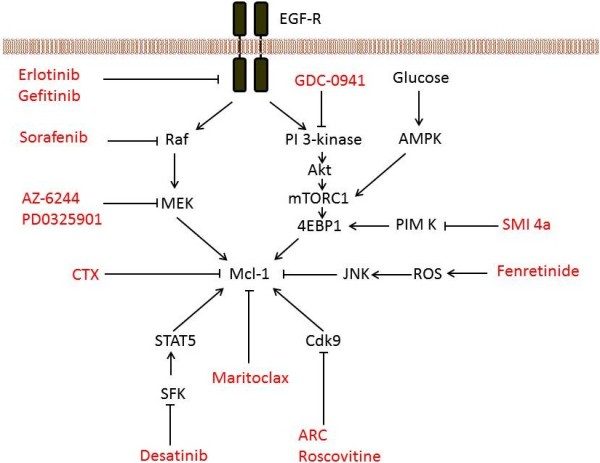
Strategies to decrease the expression of MCL-1. The sensitivity of cells to ABT-737 can be increased using drugs which decrease the expression of MCL-1. The ERK (including RAF and MEK) and PI 3-kinase pathways are key regulators of MCL-1 expression so drugs inhibiting this pathway show synergy with ABT-737. Drugs inhibiting other regulators of MCL-1 are also shown, and these drugs also increase sensitivity to ABT-737. ARC, 4-amino-6-hydrazino-7-beta-D-ribofuranosyl-7 H-pyrrolo(2,3-d)-pyrimidine-5-carboxamide; CTX, chemotherapy; EGF-R, EGF receptor; ROS, reactive oxygen species; SFK, SRC family kinase. For references, the reader is referred to the main text.
Activation of the MAP-kinase (ERK) signalling cascade increases levels of MCL-1 through increased transcription [57] and increased protein stability [58]. Consequently inhibition of components of this cascade increase sensitivity to ABT-737. This appears to be a particularly important locus for intervention, because exposure to ABT-737 activates the MAP-kinase pathway [59], and increases expression of MCL-1 [31,34,53,60,61]. Thus, repression of the ERK pathway through inhibition of RAF with sorafenib or inhibition of MEK reduces levels of MCL-1 and is synergistic with ABT-737 in vitro [59,62,63]. ABT-737 combined with a MEK or RAF inhibitor is more effective in xenograft studies than the single agents [31,59,63].
Dasatinib is an inhibitor of BCRABL and SRC-family kinases. In haematological malignancies, dasatinib has been shown to inhibit SRC-family kinase-mediated activation of the transcription factor STAT5, and correspondingly decrease the expression of MCL-1 [40,64-66]. Dasatinib also reduces the expression of MCL-1 by inhibiting the SRC-family kinase LYN which suppresses the expression of miR-181. This microRNA recognizes the MCL-1 3’ UTR [67], decreasing expression of MCL-1 and contributes to the synergy between dasatinib and ABT-737 in CLL cells [40]. As LYN is widely expressed, it will be of interest to evaluate this strategy in other cancers. Synergy between ABT-737 and two other BCRABL inhibitors, imatinib and nilotinib, that are used in the treatment of CML has also been reported [68-71]. In part, this synergy may also reflect reduction of MCL-1 by imatinib [72]. This is of particular interest because resistance to imatinib can lead to treatment failure. Potential mechanisms of resistance to imatinib include increased expression of the apoptosis inhibitors [68,71] or diminished expression of BH3-only proteins [71]. This offers the prospect of combining ABT-263 with BCRABL inhibitors to treat CML.
The PI3-KINASE pathway is frequently activated in cancer, leading to activation of AKT and several downstream effectors. Amongst these is mTORC1 which regulates 5’ cap-dependent mRNA translation through phosphorylation of 4EBP1. This is particularly important because the short half-life of MCL-1 suggests that interfering with MCL-1 protein synthesis should have a dramatic effect on expression levels. Examples of inhibition at several points on the PI 3-KINASE/AKT/mTORC1 pathway and its impact on MCL-1 are discussed below, although it is important to note that factors other than regulation of MCL-1 synthesis may contribute to the impact on protein level.
GDC-0941 is a PI 3-KINASE inhibitor which reduces the expression of MCL-1 [35]. In part, this reflects the induction of BH3-only proteins which may promote turnover of MCL-1. GDC-941 and ABT-737 synergistically induce cell death in vitro and in combination inhibit xenograft growth [35]. Importantly, ectopic expression of MCL-1 reduces this effect of the drug combination. Inhibition of other members of the PI 3-KINASE signalling pathway also provides potential routes for increasing sensitivity because inhibition of AKT or mTORC1 is also synergistic with ABT-737 [73,74].
MCL-1 expression is maintained in the presence of extracellular glucose, but it rapidly diminished in its absence. Inhibition of glycolysis with 2-deoxyglucose (2-DG) can also inhibit the expression of MCL-1 [75,76]. This may in part be mediated through regulation of protein synthesis by the PI 3-KINASE/AKT/mTORC1 pathway. Inhibition of glucose metabolism and consequently mTORC1 reduces phosphorylation of 4EBP1 and decreases expression of MCL-1 [75,76]. However, activation of this pathway by constitutively active AKT is not sufficient to maintain the expression of MCL-1 in the absence of extracellular glucose [75], suggesting that glucose regulates MCL-1 expression through more than one pathway. Nonetheless, resistance to ABT-737 could be overcome by inhibition of glycolysis with 2-DG or mTORC1 with PP242 [75]. In other cells, 2-DG does not substantially reduce expression of MCL-1, but it does block its ability to form a complex with BAK [37]. Furthermore, the combination of 2-DG with ABT-737 or ABT-263 improved the survival of animals with an experimental model of lymphoma [77] or prostate cancer [37] beyond that seen in animals treated with either agent alone.
PIM kinases are also involved in the regulation of transcription, translation, cell survival and energy metabolism [78], including regulation of phosphorylation of 4EBP1. Of particular interest, inhibition of PIM kinases decreases translation of MCL-1 mRNA, as well as increasing degradation of MCL-1 protein [33]. The reduced level of MCL-1 (as well as up-regulation of NOXA, see below) is thought to underlie the more than additive activity seen with a PIM inhibitor and ABT-737 both in vitro and in xenograft studies [33].
CDK9 is part of the transcription elongation factor b complex that regulates elongation of mRNA [79]. ARC is a nucleoside analogue which inhibits CDK9 and reduces the expression of MCL-1 [80]. In combination with ABT-737, ARC induces apoptosis and causes a synergistic inhibition of cell survival. The transcription of MCL-1 can also be reduced by exposure to roscovitine which inhibits several cyclin dependent kinases including CDK9 and this also increases sensitivity to ABT737 [15,24,40]. Synergy with flavopiridol, another pan-CDK inhibitor that down-regulates MCL-1, has also been observed [30].
Fenretinide is a synthetic retinoid whose cytotoxic activity depends in part on the generation of reactive oxygen species (ROS). Subsequent activation of JNK by ROS leads to phosphorylation and decreased levels of MCL-1 [53]. Synergy has been observed between fenretinide and ABT-737 in acute lymphoid leukemia (ALL) and neuroblastoma cells in vitro [53,54] and the drug combination improves the survival of mice bearing neuroblastoma xenografts beyond that seen with either agent alone [54].
Several chemotherapeutic agents have shown synergy with ABT-737 (reviewed in [10]) and in some cases this reflects the decreased MCL-1 caused by the chemotherapy, for example by etoposide or cisplatin [43,81]. In other cases, the induction of the pro-apoptotic protein NOXA may lead to the ubiquitination and proteasomal degradation of MCL-1 [82]. However, down-regulation of MCL-1 has also been observed without an accompanying increase in NOXA [43][83], suggesting that the chemotherapeutic agents decreased the expression of MCL-1 through several mechanisms. The transcriptional inhibitor actinomycin has been shown to reduce MCL-1 [29,83]. The activity of this chemotherapeutic agent is reminiscent of the effect seen when targeted agents are used to inhibit transcription and translation through inhibition of cyclin-dependant kinases, mTORC or PIM kinases.
Other agents which have shown synergy with ABT-737 by virtue of down-regulating MCL-1 include methylseleninic acid [84] and L-asparaginase [85].
Induction of BH3 proteins
An alternative to decreasing the expression of MCL-1 is to induce BH3-only proteins to occupy MCL-1 and hence increase sensitivity to ABT-737. Both chemotherapeutic and molecularly-targeted agents have been used successfully.
Induction of NOXA appears to be one key strategy to increase sensitivity to ABT-737, because NOXA binds to the BCL-2 family proteins MCL-1 and BFL/A1 that are insensitive to antagonism by ABT-737. Consequently, NOXA can co-operate with ABT-737 to occupy the full repertoire of anti-apoptotic BCL-2 family inhibitors. NOXA has also been reported to induce the degradation of MCL-1 [82,86,87] but not in all cells [41]. NOXA is induced by exposure to several chemotherapeutic agents including fludarabine [40], CPT11 [88], imiquimod [25], dacarbazine [25] and actinomycin [83] and in each of these cases, synergy with ABT-737 has been observed. Although temozolomide as a single agent does not appreciably affect NOXA, its combination with ABT-737 induces NOXA, leading to a more than additive induction of apoptosis and improved activity in a xenograft model [42]. In several cases, the induction of NOXA appears crucial, as inhibition of the expression of NOXA using RNA interference reduces the effect of the drug combination [25,42,44]. Similarly, ectopic expression of NOXA enhances the effect of the combination of actinomycin and ABT-737 [83].
Expression of NOXA is also increased by exposure to targeted agents including several proposed BH3 mimetics [16], bortezomib [30,40], a PIM kinase inhibitor [33], and inhibition of signalling through the NOTCH pathway using a γ-SECRETASE inhibitor [89]. NOXA appears to contribute to the synergy observed between ABT-737 and these agents because knockdown of NOXA reduces the effect of the drug combination [40,89].
BIM binds to all the known apoptosis inhibitors, including MCL-1. Inhibition of the epidermal growth factor receptor (EGFR) with erlotinib or gefitinib leads to increased expression of BIM [46,90,91], probably through transcriptional and translational regulation [90], and apoptosis. In these experiments, ABT-737 exhibited minimal activity when tested on its own, but when combined with erlotinib or gefitinib, a more than additive increase in cell death was observed. Combining ABT-737 with an EGFR inhibitor may avoid early resistance to EGFR inhibitors that arises from increased BCL-2/ BCL-XL expression [92].
These data suggest that EGFR suppresses the expression of BIM. One of the downstream pathways mediating this is likely to be the ERK (MAP) kinase pathway because inhibition of RAF or MEK increased the expression of BIM. Sorafenib (which inhibits several kinases including RAF) induces BIM and apoptosis and is synergistic with ABT-737 in AML cells [93]. In AML cells that are relatively insensitive to ABT-737 alone, a MEK inhibitor was synergistic with ABT-737 [59]. This synergy was dependent on BIM because suppression of BIM by RNAi reduced apoptosis that was induced by the drug combination. A MEK inhibitor also improved the efficacy of ABT-737 in an AML xenograft model [59]. In NSCLC cells, a MEK inhibitor caused predominantly G1 arrest and ABT-737 on its own minimally induced apoptosis [63]. However, when the drugs were combined, a more than additive induction of apoptosis was observed and the drug combination showed substantially improved activity in a xenograft models.
An inhibitor of JAK2 induced BIM and also showed synergy with ABT-737 in cells harbouring a JAK2 activating mutation [94].
Histone deacetylase (HDAC) inhibitors have also been shown to induce expression of BIM and the preferential priming by BIM of BCL-2 and BCL-XL than of MCL-1. Consequently, the HDAC inhibitor suberoyl bis-hydroxamic acid was found to induce apoptosis synergistically with ABT-737 [95]. ABT-737 released BIM from complexes with BCL-2 or BCL-XL, and knockdown of BIM substantially blunted the effect of the drug combinations. Vorinostat (also known as SAHA, another HDAC inhibitor) induces histone acetylation, the expression of the genes encoding BMF, BIM and NOXA and consequent priming of BCL-2 [96]. As a result, vorinostat showed synergy with ABT-737 which was reduced by inhibition of BMF expression by shRNA.
Although BAD does not bind to MCL-1, it is possible that it could still improve sensitivity to ABT-737 by reducing the total capacity of free apoptosis inhibitors. Both sorafenib [61,93] and ABT-737 [56] can increase BAD protein levels. Sorafenib increased the transcription of BAD [61] whereas ABT-737 decreased the turnover of BAD [56]. Sorafenib also regulates BAD through inhibition of BAD phosphorylation, promoting its pro-apoptotic activity. Sorafenib and ABT-737 have been shown to display synergistic activity in a number of cell types [61,93]. However, it is difficult to ascribe this solely to the effect of sorafenib on BAD, because of the impact of sorafenib on MCL-1.
BAD is also likely to play a role in the change in sensitivity to ABT-737 when cells are deprived of glucose or exposed to 2-DG (discussed above). In its unphosphorylated state, BAD promotes apoptosis, but when phosphorylated it regulates glucose metabolism [97]. Dephosphorylation of BAD is promoted by glucose deprivation [98], suggesting that 2-DG may do the same, and that this may contribute to the synergy observed between ABT-737 and 2-DG. However, 2-DG also induces the expression of BIM, BMF and NOXA [99,100] and glucose deprivation induces PUMA, BID and BIM [75,101], again making it difficult to assess the contribution of BAD.
Factors that complicate measurement of sensitivity to BH3 mimetics
The underlying goal of the work discussed in the previous section was to develop methods that can be translated to the clinic to improve patients’ response to ABT-737/ABT-263. This presupposes that experimental measurements of the sensitivity to ABT-737 made in the laboratory are predictive of clinical outcome. How good are our preclinical models? While xenograft studies are considered by many to remain the best predictors of clinical activity, they are costly and relatively slow. This makes it crucially important that cellular assays are as realistic as possible so that appropriate hypotheses are tested in animal and clinical studies. Perhaps the most common setting in which sensitivity to drugs is measured uses cancer cell lines growing in nutrient and growth-factor rich media as a monolayer attached to cell culture plasticware in 21 % O2 (i.e. atmospheric oxygen). The past decade has seen a growing appreciation that this is perhaps not the most realistic model, although it is experimentally convenient. In patients, tumor cells may be located in hypoxic, nutrient-poor environments comprising 3-dimensional tumors. Each of these factors has been reported to affect cellular sensitivity to ABT-737.
Metabolism
Tumor cells are thought to depend more on glycolysis than oxidative phosphorylation to provide ATP and as we have noted, the extracellular glucose concentration affects sensitivity to ABT-737. Metabolic factors that influence sensitivity are not limited to carbohydrate metabolism. Starvation of amino acids and growth factors leads to the up-regulation of PUMA [102], and its subsequent priming of BCL-XL renders cells sensitive to ABT-737.
ABT-737 is further linked to metabolism through its ability to stimulate autophagy. The nucleation phase of autophagy involves the activation of the lipid kinase VPS34 by the BH3-only protein BECLIN. BCL-2 can sequester BECLIN and the release of BECLIN by ABT-737 triggers autophagy [103,104]. However, ABT-737 also activates other regulators of autophagy including AMPK and IKK, both of which appear to be necessary for ABT-737 to stimulate autophagy [104]. How these factors conspire with the pro-apoptotic activity of ABT-737 to determine the final cellular response to ABT-737 is unclear, as autophagy can promote both cell survival and cell death. Nevertheless it is clear that metabolism and the activity of ABT-737 are intimately linked.
Hypoxia
Several reports indicate that hypoxia increases the sensitivity of tumor cells to ABT-737 [105-107]. This may reflect down-regulation of MCL-1 [105], or up-regulation of BH3-only proteins including PUMA, NOXA, BNIP3 or NOXA [107]. It is noteworthy that cells resistant to anoxia show increased expression of apoptosis inhibitors [107]. In xenograft studies, ABT-737 induces apoptosis preferentially in hypoxic regions of the tumor [105]. Finally, the extent of synergy seen between chemotherapeutic agents and ABT-737 is also dependent on oxygen concentration [105]. Thus, it may be advisable to evaluate ABT-737 activity in laboratory experiments under a more physiological oxygen tension.
3-dimensional culture
An alternative to testing the sensitivity of cells in a monolayer is to use 3-dimensional cultures such as spheroids. These are aggregates of growing tumor cells prevented from adhering to a solid surface and which mimic a small tumor. The repertoire of BCL-2 family proteins appears to change in cells grown as spheroids, compared to the same cell line grown as a monolayer. Increases in the expression of BCL-2 and BCL-XL have been observed in spheroids [108-111], and decreases in the expression of MCL-1 has also been reported [108,109]. This implies a switch towards an ABT-737-sensitive phenotype in cells grown as spheroids. Thus, even though increased resistance to either cisplatin or the combination of bortezomib and TRAIL was observed when cells were grown as spheroids, this could be overcome by exposure to ABT-737 [108,109]. The expression of pro-apoptotic family members may also be changed, and the growth of melanoma cells in spheroids leads to reduced expression of NOXA [26] but increased expression of BIM confers sensitivity to ABT-737 [112].
Although these observations highlight the usefulness of spheroids as tumor models, some caution is appropriate A decrease in drug sensitivity in spheroid culture may also be due to poor diffusion of the drug into the spheroid inner mass [26], or a reduced rate of proliferation. The changes in the expression of both pro- and anti-apoptotic BCL-2 family members observed in spheroids may also be linked to the oxygen gradient between the inside and outside of the spheroid [105].
These observations suggest that a more complete understanding of tumor cell sensitivity to ABT-737 requires that we adapt our experimental condition. It is interesting to note that in some cases ABT-737 inhibits the growth of xenografts in animals even though this is not predicted by experiments performed under standard cell culture conditions [81,113]. We suggest that although it is reasonable to evaluate the gross factors that determine sensitivity to ABT-737 using standard cell culture conditions, a refined understanding will be provided by using more complex models that recapitulate (patho)physiological conditions more closely. The clinical evaluation of the conclusions from laboratory experiments is then more likely to have a successful outcome.
Translational impact
Which patient groups might benefit from ABT-263? In addition to activity as monotherapy in animal models of lung cancer [12,49,114][11] and several lymphoid malignancies [11,12,15,62,85,115], ABT-737 or ABT-263 have also shown activity in combination with other drugs in animal models of numerous different cancer types. This includes the most prevalent cancers, breast [35,50], prostate [37], lung [92,116-118] and colorectal cancers [45] cancers. In principle, BH3 mimetics may improve the response to any chemotherapeutic agent that induces apoptosis through the intrinsic pathway. Thus, the use of such drugs may not be limited to cancers originating from particular tissues. Consequently, a key goal of clinical studies with ABT-263 will be to identify which patients should receive the drug, either alone or in combination with another drug. Bearing in mind the number of factors discussed above that influence sensitivity to ABT-737, how can this be achieved? There remain a number of complications which must be addressed.
Firstly, several groups have failed to demonstrate a correlation between drug sensitivity and expression of an individual apoptosis inhibitor [40,41,119,120]. The discovery that MCL-1 confers resistance to ABT-737 (and presumably ABT-263) suggests that measurement of MCL-1 could be used to exclude patients from treatment with ABT-263 and instead direct the use of ABT-263 in combination with a drug to repress MCL-1. The predictive value of MCL-1 measurement has been improved by measuring ratios of multiple ABT-737- sensitive and insensitive apoptosis inhibitors [30,120,121]. This suggests that the expression of all the BCL-2 family apoptosis inhibitors should be considered.
Secondly, the BH3-only proteins also influence sensitivity to ABT-737. Thus, correlations between NOXA/MCL-1 [40,41] and BIM/MCL-1 [73] ratios have been reported. This suggests that it would be helpful to include measurement of these in any biomarker panel.
Thirdly, even measuring the expression of both apoptosis inhibitors and BH3-only proteins may not be adequate, because some tumor cells exhibit dependency on more than one apoptosis inhibitor for survival. For example, cells containing BIM in complex with MCL-1 and BCL-2 may still be sensitive to ABT-737 if sufficient BIM can be liberated from BCL-2 to induce apoptosis [18]. Priming of the apoptosis inhibitors provides a good correlation with clinical response to chemotherapy [122] and the response to ABT-737 in primary cultures [47]. This suggests that determining the distribution of pro-apoptotic proteins among the various apoptosis inhibitors is likely to be a better predictor of sensitivity to ABT-737 than simply measuring expression [18,123]. Moreover, there are discrepancies between the sensitivities of these complexes in cells to ABT-737 and those anticipated from in vitro studies [20,21]. This suggests that extrapolating from knowledge of in vitro binding interactions of BH3-only proteins and apoptosis inhibitors to what happens in the clinical setting may be challenging. An alternative is to use a functional measure of the activity of ABT-263 in which the pharmacodynamic activity of the drug is directly measured, for example “BH3 profiling”. This provides a functional measurement of the sensitivity of cells to ABT-737 through assessment of the drug’s effect (or that of a peptide corresponding to a BH3 domain) on mitochondria isolated from cancer cells; this can also identify the apoptosis inhibitors that cells are dependent on for survival. Importantly, this assay has the potential to be used clinically [124], overcoming some of the difficulties discussed above. By directly measuring the effect of the drug, it is neither necessary to have knowledge of the expression of BCL-2 family members, nor to analyse their interactions.
Fourthly, short-term exposure to ABT-737 has also been reported to elevate expression of MCL-1 [16,35,36], BFL/A1 [34] as well as BAD [56,61] and NOXA [41]. Thus, measurement of BCL-2 family members prior to drug treatment may provide an inaccurate indicator of drug response. It may be preferable to briefly expose patients to ABT-263 prior to collecting malignant tissue (e.g. by biopsy or surgery). This will allow for any potential effects of the drug on the expression of BCL-2 family members to be assessed, prior to predicting the sensitivity of the tumor tissue to ABT-263.
Finally, we have highlighted how experimental conditions can affect the sensitivity to ABT-737. Clearly any proposed biomarkers to predict patient response requires validation in patients, but in the present case it appears to be particularly important to consider the strength of the preclinical evidence prior to advancing potential biomarkers to clinical evaluation.
These considerations raise the issue whether accurately selecting subsets of patients to receive BH3 mimetic therapy is feasible. What does the clinical experience with ABT-263 so far tell us? Preliminary evidence for efficacy has been observed in several lymphoid malignancies [125]. In a recent phase 1 trial in CLL, 34 % of patients showed a partial response to ABT-263 [126]. However, there was no correlation between BCL-2 expression and patient response, although a high BIM:MCL-1 ratio (implying occupancy of MCL-1 overcoming resistance to ABT-263) was associated with a response. Somewhat less encouraging results were obtained in patients with relapsed SCLC [127] where only 2.6 % of patients showed a partial response (although drug-induced apoptosis was more pronounced in patients thought to express BCL-2 more highly). Importantly, in both CLL and SCLC, BCL-2 expression is elevated in the majority of patients yet the outcomes of the trials are clearly distinct. Thus, these clinical studies reinforce the notion that measuring the expression of the targets of ABT-263 is likely to be an inadequate predictor of response.
Considering the difficulties in predicting response that we have discussed above, it might even be argued that there is merit in considering using ABT-263 without attempting to identify which patients groups are most likely to respond. However, the lifespan of platelets is regulated by BCL-XL which consequently is shortened by ABT-737 [128,129]. Correspondingly, thrombocytopenia is a major adverse effect of ABT-263 [125-127,130] which may preclude its “non-selective” use. Patients with already reduced platelets (e.g. some leukemias) may be particularly at risk if treated with ABT-263. This also suggests the possibility that drug interactions may occur in patients co-treated with other drugs such as carboplatin that also cause thrombocytopenia. This may limit the use of ABT-263 in combination with carboplatin, which is unfortunate because synergy between ABT-737 and carboplatin has been observed in preclinical studies [113]. It is interesting to note that Abbot have recently developed ABT-199, an analog of ABT-263 that avoids antagonising BCL-XL that may overcome some of these difficulties. However, this drug is unlikely to be effective in tumors dependent on BCL-XL rather than BCL-2 (eg ovarian cancer, [113]) and we speculate that BCL-XL will emerge as a mechanism of resistance to ABT-199.
Taking into account the complexities of measuring drug sensitivity that we have discussed, our opinion is that it would be preferable not to select patients for entry into clinical trials of BH3 mimetics using measurement of the expression of BCL-2 family members alone (even if this includes both pro- and anti-apoptotic members). Although there have been notable successes in using biomarkers to identify patients who respond to a particular drug, we doubt whether measuring expression levels alone will identify all patients likely to respond to ABT-263. However, some method of patient selection is clearly desirable. We consider that assays that measure functional outcome as the response to a drug are preferable because they are independent of our assumptions regarding factors that control drug sensitivity. One such assay is BH3 profiling. Alternatively, we note that apoptosis can be measured in patients within 6 hours of SCLC patients receiving ABT-263 [130]. We believe that this is likely to provide a more robust prediction of drug therapeutic activity because it measures the drug’s anticipated pharmacological effect in patients. Such tests evaluating the pharmacodynamic activity of ABT-263 could be used as the basis of selecting patients to receive ABT-263. In principle, this approach could be applied more widely in personalized medicine in oncology, where drug-induced apoptosis is frequently a desired outcome.
Conclusion
The trend from cytotoxic chemotherapy towards targeted therapeutics offers the promise of improved efficacy accompanied by a reduction in the adverse effects seen with chemotherapy. Currently, cancer therapies take patient characteristics into consideration but with regards to the tumor itself, other than conventional factors such as stage and histological type, traditional therapy has been based on a ‘one size fits all’ approach. While this may have been acceptable with chemotherapy, targeted therapeutics are designed to be more selective so the possibility that tumors can evade the effects of the drug is substantially increased. The more we learn, the more we appreciate the complex heterogeneity that exists between and within tumor types. Thus, patients are likely to be more varied in their response to targeted therapies than to chemotherapy. The concept of personalised medicine is an appealing solution to this problem, but demands a depth of understanding of disease biology that is only just beginning to be translated from the laboratory to the clinic.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All the authors contribute to reviewing the literature in this review, helped to draft the manuscript and read and approved the final manuscript.
Authors’ information
VS is a final year Ph.D student and a trainee gynaecologist with a particular interest in gynaecological oncology.
CWR is a consultant gynecological oncology surgeon with a particular interest in ovarian cancer.
AR is a senior lecturer in pharmacology who has conducted drug discovery research in the pharmaceutical industry as well as translational research in academic institutions. He has a particular interest in ovarian cancer.
Contributor Information
Vasileios A Stamelos, Email: vasileios.stamelos@doctors.org.uk.
Charles W Redman, Email: Charles.Redman@uhns.nhs.uk.
Alan Richardson, Email: a.richardson1@keele.ac.uk.
Acknowledgements
Work in the authors’ laboratory on BH3 mimetics is supported by grants from The North Staffordshire Medical Institute, Keele University and the University Hospital of North Staffordshire. Work in the authors’ laboratory would not have been possible without the invaluable contribution of Dr. Geoffrey Richardson.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, Juin P. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SP, Phillips DC, Jeffers JR, Chipuk JE, Parsons MJ, Rehg JE, Opferman JT, Green DR, Zambetti GP. Genetically defining the mechanism of Puma- and Bim-induced apoptosis. Cell Death Differ. 2012;19:642–649. doi: 10.1038/cdd.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 Domains other than Bim and Bid Can Directly Activate Bax/Bak. J Biol Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang CX, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) Retrotranslocates Bax from the Mitochondria into the Cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Richardson A, Kaye SB. Pharmacological inhibition of the Bcl-2 family of apoptosis regulators as cancer therapy. Curr Mol Pharmacol. 2008;1:244–254. doi: 10.2174/1874467210801030244. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Chen J, Jin S, Abraham V, Huang XL, Liu B, Mitten MJ, Nimmer P, Lin XY, Smith M, Shen Y. et al. The Bcl-2/Bcl-X(L)/Bcl-w Inhibitor, Navitoclax, Enhances the Activity of Chemotherapeutic Agents In Vitro and In Vivo. Mol Cancer Ther. 2011;10:2340–2349. doi: 10.1158/1535-7163.MCT-11-0415. [DOI] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, Cohen GM. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albershardt TC, Salerni BL, Soderquist RS, Bates DJ, Pletnev AA, Kisselev AF, Eastman A. Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA. J Biol Chem. 2011;286:24882–24895. doi: 10.1074/jbc.M111.255828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, Ariyaratne PN, Takahashi N, Sawada K, Fei Y. et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, Kaufman JL, Lee KP, Lonial S, Boise LH. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith KC, Lestini BJ, Gross M, Ip L, Bhumbla A, Zhang X, Zhao H, Liu X, Hogarty MD. BH3 response profiles from neuroblastoma mitochondria predict activity of small molecule Bcl-2 family antagonists. Cell Death Differ. 2010;17:872–882. doi: 10.1038/cdd.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, Yue P, Robati M, Phipson B, Fairlie WD. et al. Bcl-2, Bcl-xL, and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranovich A, Liu Q, Collins T, Geng F, Dixit S, Leber B, Andrews DW. Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Mol Cell. 2012;45:754–763. doi: 10.1016/j.molcel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS One. 2009;4:e6651. doi: 10.1371/journal.pone.0006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestini BJ, Goldsmith KC, Fluchel MN, Liu X, Chen NL, Goyal B, Pawel BR, Hogarty MD. Mcl1 downregulation sensitizes neuroblastoma to cytotoxic chemotherapy and small molecule Bcl2-family antagonists. Cancer Biol Ther. 2009;8:1587–1595. doi: 10.4161/cbt.8.16.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- Weber A, Kirejczyk Z, Potthoff S, Ploner C, Hacker G. Endogenous Noxa Determines the Strong Proapoptotic Synergism of the BH3-Mimetic ABT-737 with Chemotherapeutic Agents in Human Melanoma Cells. Translational Oncol. 2009;2:73–83. doi: 10.1593/tlo.08223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, Weninger W, Haass NK, Allen JD. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- Zall H, Weber A, Besch R, Zantl N, Hacker G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol Cancer. 2010;9:164. doi: 10.1186/1476-4598-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Goldstein NB, Johannes WU, Walton CH, Fujita M, Norris DA, Shellman YG. BH3 mimetic ABT-737 and a proteasome inhibitor synergistically kill melanomas through Noxa-dependent apoptosis. J Invest Dermatol. 2009;129:964–971. doi: 10.1038/jid.2008.327. [DOI] [PubMed] [Google Scholar]
- Olberding KE, Wang X, Zhu Y, Pan J, Rai SN, Li C. Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression. Cancer Biol Ther. 2010;10:918–929. doi: 10.4161/cbt.10.9.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzeau C, Dousset C, Bodet L, Gomez-Bougie P, Bonnaud S, Moreau A, Moreau P, Pellat-Deceunynk C, Amiot M, Le Gouill S. ABT-737 induces apoptosis in mantle cell lymphoma cells with a Bcl-2high/Mcl-1low profile and synergizes with other antineoplastic agents. Clin Cancer Res. 2011;17:5973–5981. doi: 10.1158/1078-0432.CCR-11-0955. [DOI] [PubMed] [Google Scholar]
- Hikita H, Takehara T, Shimizu S, Kodama T, Shigekawa M, Iwase K, Hosui A, Miyagi T, Tatsumi T, Ishida H. et al. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology. 2010;52:1310–1321. doi: 10.1002/hep.23836. [DOI] [PubMed] [Google Scholar]
- Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW. et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- Song JH, Kraft AS. Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737. Cancer Res. 2012;72:294–303. doi: 10.1158/0008-5472.CAN-11-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Yang W, Zhang C, Ding WJ, Zhu H, Lin NM, Wu HH, He QJ, Yang B. GDC-0941 sensitizes breast cancer to ABT-737 in vitro and in vivo through promoting the degradation of Mcl-1. Cancer Lett. 2011;309:27–36. doi: 10.1016/j.canlet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Bhat UG, Pandit B, Gartel AL. ARC synergizes with ABT-737 to induce apoptosis in human cancer cells. Mol Cancer Ther. 2010;9:1688–1696. doi: 10.1158/1535-7163.MCT-09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Janssen E, Perkins G, Ellisman M, Kitada S, Reed JC. Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy. PLoS One. 2011;6:e24102. doi: 10.1371/journal.pone.0024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ. et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, Krishnegowda G, Awwad A, Dewey A, Liu X. et al. Discovery of Marinopyrrole A (Maritoclax) as a Selective Mcl-1 Antagonist that Overcomes ABT-737 Resistance by Binding to and Targeting Mcl-1 for Proteasomal Degradation. J Biol Chem. 2012;287:10224–10235. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp JM, Geest CR, Breij EC, Elias JA, van Laar J, Luijks DM, Kater AP, Beaumont T, van Oers MH, Eldering E. Tipping the Noxa/Mcl-1 balance overcomes ABT-737 resistance in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18:487–498. doi: 10.1158/1078-0432.CCR-11-1440. [DOI] [PubMed] [Google Scholar]
- Hauck P, Chao BH, Litz J, Krystal GW. Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Mol Cancer Ther. 2009;8:883–892. doi: 10.1158/1535-7163.MCT-08-1118. [DOI] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, Cooper DA, Fujita M, Norris DA, Shellman YG. The combination of BH3-mimetic ABT-737 with the alkylating agent temozolomide induces strong synergistic killing of melanoma cells independent of p53. PLoS One. 2011;6:e24294. doi: 10.1371/journal.pone.0024294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zang Y, Li C, Patel NS, Grandis JR, Johnson DE. ABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathway. Mol Pharmacol. 2009;75:1231–1239. doi: 10.1124/mol.108.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Huang S, Sinicrope FA. Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin Cancer Res. 2008;14:8132–8142. doi: 10.1158/1078-0432.CCR-08-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raats DA, de Bruijn MT, Steller EJ, Emmink BL, Borel-Rinkes IH, Kranenburg O. Synergistic killing of colorectal cancer cells by oxaliplatin and ABT-737. Cell Oncol (Dordr) 2011;34:307–313. doi: 10.1007/s13402-011-0026-8. [DOI] [PubMed] [Google Scholar]
- Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, Wong KK, Letai A. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–11875. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, Lin X, Hierman JS, Wilburn DL, Watkins DN, Rudin CM. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, Deb S, Ritchie ME, Takano E, Ward T. et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012;109:2766–2771. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Wan Z, Kang YH, Sposto R, Reynolds CP. Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst. 2008;100:580–595. doi: 10.1093/jnci/djn076. [DOI] [PubMed] [Google Scholar]
- Fang H, Harned TM, Kalous O, Maldonado V, DeClerck YA, Reynolds CP. Synergistic activity of fenretinide and the Bcl-2 family protein inhibitor ABT-737 against human neuroblastoma. Clin Cancer Res. 2011;17:7093–7104. doi: 10.1158/1078-0432.CCR-11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Kandasamy K, Kraft AS. ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem. 2008;283:25003–25013. doi: 10.1074/jbc.M802511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzoukhry Z, Louandre C, Francois C, Saidak Z, Godin C, Maziere JC, Galmiche A. The Bcl-2 homology domain 3 (BH3) mimetic ABT-737 reveals the dynamic regulation of bad, a proapoptotic protein of the Bcl-2 family, by Bcl-xL. Mol Pharmacol. 2011;79:997–1004. doi: 10.1124/mol.110.070565. [DOI] [PubMed] [Google Scholar]
- Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, Craig RW. Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem. 1999;274:1801–1813. doi: 10.1074/jbc.274.3.1801. [DOI] [PubMed] [Google Scholar]
- Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo P. et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2011;26:778-787. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–2786. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- Galmiche A, Ezzoukhry Z, Francois C, Louandre C, Sabbagh C, Nguyen-Khac E, Descamps V, Trouillet N, Godin C, Regimbeau JM. et al. BAD, a proapoptotic member of the BCL2 family, is a potential therapeutic target in hepatocellular carcinoma. Mol Cancer Res. 2010;8:1116–1125. doi: 10.1158/1541-7786.MCR-10-0029. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T. et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651–3659. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Williams A, Vultur A, List A, Bhalla K, Smith D, Lee FY, Jove R. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol Cancer Ther. 2007;6:1400–1405. doi: 10.1158/1535-7163.MCT-06-0446. [DOI] [PubMed] [Google Scholar]
- Veldurthy A, Patz M, Hagist S, Pallasch CP, Wendtner CM, Hallek M, Krause G. The kinase inhibitor dasatinib induces apoptosis in chronic lymphocytic leukemia cells in vitro with preference for a subgroup of patients with unmutated IgVH genes. Blood. 2008;112:1443–1452. doi: 10.1182/blood-2007-11-123984. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Williams AH, Estes ML, Matsushita N, Boschelli F, Jove R, List AF. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT) Leuk Res. 2008;32:893–903. doi: 10.1016/j.leukres.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Zimmerman EI, Dollins CM, Crawford M, Grant S, Nana-Sinkam SP, Richards KL, Hammond SM, Graves LM. Lyn kinase-dependent regulation of miR181 and myeloid cell leukemia-1 expression: implications for drug resistance in myelogenous leukemia. Mol Pharmacol. 2010;78:811–817. doi: 10.1124/mol.110.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A. et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl + leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Kimura S, Andreeff M, Ashihara E, Kamitsuji Y, Yokota A, Kawata E, Takeuchi M, Tanaka R, Murotani Y. et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br J Haematol. 2008;140:181–190. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- Shimada K, Tomita A, Minami Y, Abe A, Hind CK, Kiyoi H, Cragg MS, Naoe T. CML cells expressing the TEL/MDS1/EVI1 fusion are resistant to imatinib-induced apoptosis through inhibition of BAD, but are resensitized with ABT-737. Exp Hematol. 2012;40:724–737. doi: 10.1016/j.exphem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Airiau K, Mahon FX, Josselin M, Jeanneteau M, Turcq B, Belloc F. ABT-737 increases tyrosine kinase inhibitor-induced apoptosis in chronic myeloid leukemia cells through XIAP downregulation and sensitizes CD34(+) CD38(−) population to imatinib. Exp Hematol. 2012;40:367–378. doi: 10.1016/j.exphem.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Mak DH, Wang RY, Schober WD, Konopleva M, Cortes J, Kantarjian H, Andreeff M, Carter BZ. Activation of apoptosis signaling eliminates CD34+ progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia. 2012;26:788–794. doi: 10.1038/leu.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spender LC, Inman GJ. Phosphoinositide 3-kinase/AKT/mTORC1/2 Signaling Determines Sensitivity of Burkitt's Lymphoma Cells to BH3 mimetics. Mol Cancer Res. 2012;10:347–359. doi: 10.1158/1541-7786.MCR-11-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Zou Y, Rahman JS, Lu B, Massion PP. Synergy between phosphatidylinositol 3-kinase/Akt pathway and Bcl-xL in the control of apoptosis in adenocarcinoma cells of the lung. Mol Cancer Ther. 2009;8:101–109. doi: 10.1158/1535-7163.MCT-08-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloff JL, Macintyre AN, Nichols AG, Liu T, Gallo CA, Plas DR, Rathmell JC. Akt-dependent glucose metabolism promotes Mcl-1 synthesis to maintain cell survival and resistance to Bcl-2 inhibition. Cancer Res. 2011;71:5204–5213. doi: 10.1158/0008-5472.CAN-10-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, Auberger P, Pende M, Ricci JE. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. 2010;29:1641–1652. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- Meynet O, Beneteau M, Jacquin MA, Pradelli LA, Cornille A, Carles M, Ricci JE. Glycolysis inhibition targets Mcl-1 to restore sensitivity of lymphoma cells to ABT-737-induced apoptosis. Leukemia. 2011;26:1145–47. doi: 10.1038/leu.2011.327. [DOI] [PubMed] [Google Scholar]
- Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- Cho S, Schroeder S, Ott M. CYCLINg through transcription: posttranslational modifications of P-TEFb regulate transcription elongation. Cell Cycle. 2010;9:1697–1705. doi: 10.4161/cc.9.9.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat UG, Gartel AL. Nucleoside analog ARC targets Mcl-1 to induce apoptosis in leukemia cells. Leukemia. 2010;24:851–855. doi: 10.1038/leu.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K, Chen HY, Karp CM, May M, Ganesan S, Karantza-Wadsworth V, DiPaola RS, White E. Bcl-2 modulation to activate apoptosis in prostate cancer. Mol Cancer Res. 2009;7:1487–1496. doi: 10.1158/1541-7786.MCR-09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Bougie P, Menoret E, Juin P, Dousset C, Pellat-Deceunynck C, Amiot M. Noxa controls Mule-dependent Mcl-1 ubiquitination through the regulation of the Mcl-1/USP9X interaction. Biochem Biophys Res Commun. 2011;413:460–464. doi: 10.1016/j.bbrc.2011.08.118. [DOI] [PubMed] [Google Scholar]
- Xu H, Krystal GW. Actinomycin D decreases Mcl-1 expression and acts synergistically with ABT-737 against small cell lung cancer cell lines. Clin Cancer Res. 2010;16:4392–4400. doi: 10.1158/1078-0432.CCR-10-0640. [DOI] [PubMed] [Google Scholar]
- Yin S, Dong Y, Li J, Fan L, Wang L, Lu J, Vang O, Hu H. Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by targeting Mcl-1 and Bad. Apoptosis. 2012;17:388–399. doi: 10.1007/s10495-011-0687-9. [DOI] [PubMed] [Google Scholar]
- High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O'Brien R, Khaw SL, Vikstrom IB, Roberts AW, Lock RB. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77:483–494. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM. Mutation to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–7131. doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima W, Tanaka N. Noxa induces apoptosis in oncogene-expressing cells through catch-and-release mechanism operating between Puma and Mcl-1. Biochem Biophys Res Commun. 2011;413:643–648. doi: 10.1016/j.bbrc.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Zhang B, Pan SH, Zhao HM, Zhang Y, Feng WH, Li YY, Cao XC. Bcl-2 inhibitor ABT-737 enhances the cisplatin-induced apoptosis in breast cancer T47D cells. Zhonghua Zhong Liu Za Zhi. 2011;33:891–895. [PubMed] [Google Scholar]
- Li M, Chen F, Clifton N, Sullivan DM, Dalton WS, Gabrilovich DI, Nefedova Y. Combined inhibition of Notch signaling and Bcl-2/Bcl-xL results in synergistic antimyeloma effect. Mol Cancer Ther. 2010;9:3200–3209. doi: 10.1158/1535-7163.MCT-10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1689. doi: 10.1371/journal.pmed.0040316. discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Tang Z, Yin L, Morrison B, Hafez-Khayyata S, Fu P, Huang H, Bagai R, Jiang S, Kresak A. et al. MET-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. Cancer Res. 2011;71:4494–4505. doi: 10.1158/0008-5472.CAN-10-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, McCubrey J, Cortes J, Andreeff M. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- Will B, Siddiqi T, Jorda MA, Shimamura T, Luptakova K, Staber PB, Costa DB, Steidl U, Tenen DG, Kobayashi S. Apoptosis induced by JAK2 inhibition is mediated by Bim and enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid cells. Blood. 2010;115:2901–2909. doi: 10.1182/blood-2009-03-209544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmans AP, Alsop AE, Bots M, Cluse LA, Williams SP, Banks KM, Ralli R, Scott CL, Frenzel A, Villunger A, Johnstone RW. Deciphering the molecular events necessary for synergistic tumor cell apoptosis mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT-737. Cancer Res. 2011;71:3603–3615. doi: 10.1158/0008-5472.CAN-10-3289. [DOI] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD. et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB. et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Zagorodna O, Martin SM, Rutkowski DT, Kuwana T, Spitz DR, Knudson CM. 2-Deoxyglucose-induced toxicity is regulated by Bcl-2 family members and is enhanced by antagonizing Bcl-2 in lymphoma cell lines. Oncogene. 2011;31:2738–2749. doi: 10.1038/onc.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Peinado S, Alcazar-Limones F, Lagares-Tena L. El Mjiyad N, Caro-Maldonado A, Tirado OM, Munoz-Pinedo C: 2-deoxyglucose induces Noxa-dependent apoptosis in alveolar rhabdomyosarcoma. Cancer Res. 2011;71:6796–6806. doi: 10.1158/0008-5472.CAN-11-0759. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283:36344–36353. doi: 10.1074/jbc.M803580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun F, Bertin-Ciftci J, Gallouet AS, Millour J, Juin P. Serum-nutrient starvation induces cell death mediated by Bax and Puma that is counteracted by p21 and unmasked by Bcl-x(L) inhibition. PLoS One. 2011;6:e23577. doi: 10.1371/journal.pone.0023577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- Malik SA, Orhon I, Morselli E, Criollo A, Shen S, Marino G, BenYounes A, Benit P, Rustin P, Maiuri MC, Kroemer G. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene. 2011;30:3918–3929. doi: 10.1038/onc.2011.104. [DOI] [PubMed] [Google Scholar]
- Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C, Roberts D. et al. Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest. 2011;121:1075–1087. doi: 10.1172/JCI43505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymenko T, Brandenburg M, Morrow C, Dive C, Makin G. The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol Cancer Ther. 2011;10:2373–2383. doi: 10.1158/1535-7163.MCT-11-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetschko H, Voss V, Senft C, Seifert V, Prehn JH, Kogel D. BH3 mimetics reactivate autophagic cell death in anoxia-resistant malignant glioma cells. Neoplasia. 2008;10:873–885. doi: 10.1593/neo.07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber J, Ellerkamp V, Wenz J, Kirchner B, Warmann SW, Fuchs J, Armeanu-Ebinger S. Apoptosis sensitizers enhance cytotoxicity in hepatoblastoma cells. Pediatr Surg Int. 2012;28:149–159. doi: 10.1007/s00383-011-2988-z. [DOI] [PubMed] [Google Scholar]
- Yang TM, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaitan D, Chandna S, Arya MB, Dwarakanath BS. Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J Transl Med. 2006;4:12. doi: 10.1186/1479-5876-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Ho WJ, Wu BM. The role of the 3D environment in hypoxia-induced drug and apoptosis resistance. Anticancer Res. 2011;31:3237–3245. [PubMed] [Google Scholar]
- Barbone D, Ryan JA, Kolhatkar N, Chacko AD, Jablons DM, Sugarbaker DJ, Bueno R, Letai AG, Coussens LM, Fennell DA, Broaddus VC. The Bcl-2 repertoire of mesothelioma spheroids underlies acquired apoptotic multicellular resistance. Cell Death Dis. 2011;2:e174. doi: 10.1038/cddis.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, Richardson A. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13:7191–7198. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- Park CM, Bruncko M, Adickes J, Bauch J, Ding H, Kunzer A, Marsh KC, Nimmer P, Shoemaker AR, Song X. et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. J Med Chem. 2008;51:6902–6915. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- Mason KD, Vandenberg CJ, Scott CL, Wei AH, Cory S, Huang DC, Roberts AW. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci U S A. 2008;105:17961–17966. doi: 10.1073/pnas.0809957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, Hu YZ, Lin NM, He QJ, Yang B. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther. 2011;10:1264–1275. doi: 10.1158/1535-7163.MCT-10-1091. [DOI] [PubMed] [Google Scholar]
- Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–6105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan N, Malek M, Zha JP, Yue P, Kassees R, Berry L, Fairbrother WJ, Sampath D, Belmont LD. Navitoclax Enhances the Efficacy of Taxanes in Non-Small Cell Lung Cancer Models. Clin Cancer Res. 2011;17:1394–1404. doi: 10.1158/1078-0432.CCR-10-2353. [DOI] [PubMed] [Google Scholar]
- Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie WD, Grigg AP, Seymour JF, Szer J, Huang DC, Roberts AW. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–2041. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- Al-Harbi S, Hill BT, Mazumder S, Singh K, Devecchio J, Choudhary G, Rybicki LA, Kalaycio M, Maciejewski JP, Houghton JA, Almasan A. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood. 2011;118:3579–3590. doi: 10.1182/blood-2011-03-340364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maiga S, Avet-Loiseau H, Bataille R, Moreau P, Le Gouill S. et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118:3901–3910. doi: 10.1182/blood-2010-11-317438. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Letai A. BH3 profiling - Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2012. in press. [DOI] [PMC free article] [PubMed]
- Wilson H, O'Connor A, Czuczman S, LaCasce S, Gerecitano F, Leonard P, Tulpule A, Xiong H, Chiu YL, Busman T. et al. Phase 1/2a Study of Navitoclax (Abt-263) in Relapsed or Refractory Lymphoid Malignancies. Haematol-Hematol J. 2010;95:116–117. [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS. et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW. et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, Iciek LA, Morgan SJ, Nasarre MC, Nelson R. et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, de Oliveira MR, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM. et al. Phase I Study of Navitoclax (ABT-263), a Novel Bcl-2 Family Inhibitor, in Patients With Small-Cell Lung Cancer and Other Solid Tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]


