Abstract
Nicotinic acetylcholine receptors (nAChRs) are oligomeric transmembrane proteins in which five subunits coassemble to form a central ion channel pore. Conventional agonists, such as acetylcholine (ACh), bind to an orthosteric site, located at subunit interfaces in the extracellular domain. More recently, it has been demonstrated that nAChRs can also be activated by ligands binding to an allosteric transmembrane site. In the case of α7 nAChRs, ACh causes rapid activation and almost complete desensitization. In contrast, allosteric agonists such as 4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c] quin oline-8-sulfonamide (4BP-TQS) activate α7 nAChRs more slowly and cause only low levels of apparent desensitization. In the present study, single-channel patch-clamp recording has been used to investigate differences in the mechanism of activation of α7 nAChRs by ACh and 4BP-TQS. The most striking difference between activation by ACh and 4BP-TQS is in single-channel kinetics. In comparison with activation by ACh, single-channel open times and burst lengths are substantially longer (∼160–800-fold, respectively), and shut times are shorter (∼8-fold) when activated by 4BP-TQS. In addition, coapplication of ACh and 4BP-TQS results in a further increase in single-channel burst lengths. Mean burst lengths seen when the two agonists are coapplied (3099 ± 754 ms) are ∼2.5-fold longer than with 4BP-TQS alone and ∼370-fold longer than with ACh alone. Intriguingly, the main single-channel conductance of α7 nAChRs, was significantly larger when activated by 4BP-TQS (100.3 ± 2.4 pS) than when activated by ACh (90.0 ± 2.7 pS), providing evidence that activation by allosteric and orthosteric agonists results in different α7 nAChRs open-channel conformations.
Introduction
Nicotinic acetylcholine (ACh) receptors (nAChRs) are excitatory neurotransmitter-gated ion channels that belong to the Cys loop receptor superfamily (Lester et al., 2004). Seventeen nAChR subunit genes have been identified in vertebrates (α1–α10, β1–β4, γ, δ, and ε) and can assemble into a variety of heteropentameric and homopentameric receptors (Le Novère et al., 2002; Millar and Gotti, 2009). Particular attention has been focused recently on homomeric α7 nAChRs. In part, this is a consequence of evidence that they may play a role in neurological and psychiatric disorders such as Alzheimer's disease and schizophrenia (Taly et al., 2009). As a consequence, there has been great interest in the identification and characterization of α7-selective agonists and allosteric modulators (Arneric et al., 2007; Bertrand and Gopalakrishnan, 2007; D'hoedt and Bertrand, 2009; Williams et al., 2011b).
Nicotinic receptors are allosteric proteins (Changeux and Edelstein, 2005) that are activated by the binding of the endogenous agonist ACh to an extracellular binding site, located at the interface of two subunits (Taly et al., 2009). In addition to this well characterized orthosteric binding site, nAChRs can be modulated by a variety of ligands acting at distinct allosteric sites (Arneric et al., 2007; Bertrand and Gopalakrishnan, 2007; D'hoedt and Bertrand, 2009; Williams et al., 2011b). For example, evidence has suggested that some positive allosteric modulators (PAMs) of α7 nAChRs bind in an intrasubunit cavity located in the transmembrane domain (Young et al., 2008; Collins et al., 2011; Gill et al., 2011, 2012). TQS is a well characterized allosteric modulator of α7 nAChR that has no pharmacological effect when applied alone but causes dramatic potentiation of agonist responses when coapplied with ACh (Grønlien et al., 2007; Gill et al., 2011, 2012). In addition, whereas activation by ACh causes rapid desensitization of α7 nAChRs (Couturier et al., 1990), coapplication of ACh with TQS causes an almost complete loss of agonist-induced desensitization (Grønlien et al., 2007; Gill et al., 2011). In recent studies, it has been demonstrated that 4BP-TQS, a compound with close chemical similarity to TQS, is able to activate α7 nAChRs in the absence of ACh and appears to do so by acting as an allosteric agonist and interacting with a transmembrane binding site similar to that proposed for allosteric modulators such as TQS (Gill et al., 2011, 2012).
The aim of the present study was to investigate the contrasting effects of the classic orthosteric agonist ACh and the allosteric agonist 4BP-TQS. In previous studies (Gill et al., 2011, 2012), the properties of allosteric agonists such as 4BP-TQS have been examined only by whole-cell recordings. Here, the mechanism of action of 4BP-TQS on the human α7 nAChR has been examined by single-channel analysis. The results indicate that activation of α7 nAChRs by 4BP-TQS causes significantly increased open times, increased open probability, and increased burst lengths compared with those for activation by ACh. Further differences in single-channel kinetics are observed when these two agonists are coapplied. In addition, differences in single-channel conductance resulting from activation by orthosteric or allosteric ligands provide further evidence for differences in the mechanisms by which they cause receptor activation.
Materials and Methods
Materials.
4-(4-Bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS) and 4-(napthalen-1-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (TQS) were obtained from Chembridge Corporation (San Diego, Ca). 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid was obtained from Calbiochem (San Diego, CA), and all other chemicals were obtained from Sigma-Aldrich (Poole, UK).
In Vitro Synthesis of cRNA.
The human α7 nAChR subunit cDNA subcloned into the plasmid expression vector pSP64GL has been described previously (Broadbent et al., 2006). Plasmid DNA was linearized using the restriction enzyme BamHI and purified using a QIAquick PCR purification kit (QIAGEN, Dorking, Surrey, UK). In vitro reverse transcription was performed using an SP6 mMessage mMACHINE kit (Ambion, Warrington, UK).
Expression in Xenopus Oocytes.
Adult female Xenopus laevis frogs were obtained from the European Xenopus Resource Centre, Portsmouth University (Portsmouth, UK). Stage V to VI oocytes were isolated and defolliculated as described previously (Young et al., 2007). Human α7 cRNA was injected into the oocyte cytoplasm using a Nanoject II variable volume microinjector (Drummond Scientific, Broomall, PA). cRNA was injected in a total injection volume of 36.8 nl/oocyte. Injected oocytes were incubated at 18°C in a modified Barth's solution (Young et al., 2007) for 3 to 5 days.
Two-Electrode Voltage-Clamp Recording.
Two-electrode voltage-clamp recording was performed using an Axon GeneClamp 500B amplifier with a Axon Digidata 1200 and pClamp software (Molecular Devices, Sunnyvale, CA). A solution containing 3 mM ACh diluted in external saline was applied using a BPS-8 solution exchange system (ALA Scientific Instruments, Westbury, NY). Currents were recorded from oocytes impaled by microelectrodes filled with 3 M KCl. A holding potential of −60 mV was applied.
Single-Channel Recording.
Oocytes showing significant expression levels of the receptor (0.5–2 μA response to ACh) were selected for single-channel outside-out patch-clamp recordings. Oocytes were placed in hyperosmotic medium (containing 100 mM NaCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 222 mM gluconic acid lactone, and 222 mM N-methyl-d-glucamine) to aid removal of the vitelline membrane. Oocytes were placed in a recording chamber on the stage of a Zeiss upright microscope with Nomarski digital interface contrast optics, and the edge of the oocyte was examined at 400× magnification. Oocytes were continuously bathed in the recording solution (10 mM HEPES, 150 mM NaCl, and 1 mM CaCl, pH 7.4). Patch pipettes were made from thick-walled borosilicate glass (GC150F; Harvard Apparatus, Kent, UK) and had a region approximately 15 mm in length up to 2 mm from the tip covered with Sylgard (Dow Corning, Midland, MI). Pipettes were fire-polished to a final resistance of 6 to 8 MΩ and filled with saline solution (150 mM NaOH, 10 mM HEPES, 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 150 mM gluconic acid lactone, and 10 mM NaCl, pH 7.2). Outside-out patches were voltage-clamped at −60 mV (or from −80 to +50 mV for generation of current-voltage curves). A microperfusion system was used to deliver agonists and allosteric modulators. Drugs were applied to each patch for periods ranging between 15 s and 18 min, and the number of drug applications made to each patch ranged from 1 to 5. The data presented in this study were derived from a total of 39 patches that were used to determine both single-channel amplitude and kinetic data. In five patches, ACh, 4BP-TQS, and ACh + 4BP-TQS were investigated on the same patch, and in these patches ACh was applied first, followed by 4BP-TQS, and then the two agonists were coapplied. Patches were washed with drug-free recording solution between applications. All experiments were performed at room temperature. Single-channel currents were recorded using an Axopatch 200B patch-clamp amplifier (Axon Instruments) and filtered at 10 kHz by an eight-pole Bessel filter. Acquired data were further filtered at 2 kHz and digitized at 20 kHz using a Micro1401 interface (Cambridge Electronic Design, Cambridge, UK) and stored to computer using WinEDR (Dempster, 2001).
Analysis of Single-Channel Data.
Single-channel activity was analyzed using the time course fitting program SCAN (Colquhoun and Sigworth, 1995). Distributions of current amplitudes, open times, shut times, burst lengths, and total open time per burst were obtained using EKDIST software (Colquhoun and Sigworth, 1995). Amplitude distributions were created for openings longer than two filter rise times (322 μs); open and shut time distributions were made for intervals longer than 100 μs. Amplitude distributions were then fitted with the sum of three Gaussian components using the maximum likelihood method. Open and shut time distributions, burst length, and total open time per burst distributions were fitted with a mixture of exponential components using the maximum likelihood method. Mean open and shut time, mean burst length, and total open time per burst were calculated by integrating the exponential components. Bursts were defined as openings that were separated by shut times shorter than a critical time (tcrit). Critical times were calculated using the best-fit parameters of the two longest exponential components of the shut time distribution. Probability of opening (Popen) was calculated by dividing the mean open time by the sum of mean open time and shut time in the data record. Stability plots (Weiss and Magleby, 1990) for amplitudes, open times, shut times, and Popen were also generated to check whether channel activity was stable during recordings.
Statistical Analysis.
Multiple comparisons of single-channel data were performed by one-way ANOVA with Tukey's post hoc test. Pairwise comparisons were performed by Student's unpaired t test.
Results
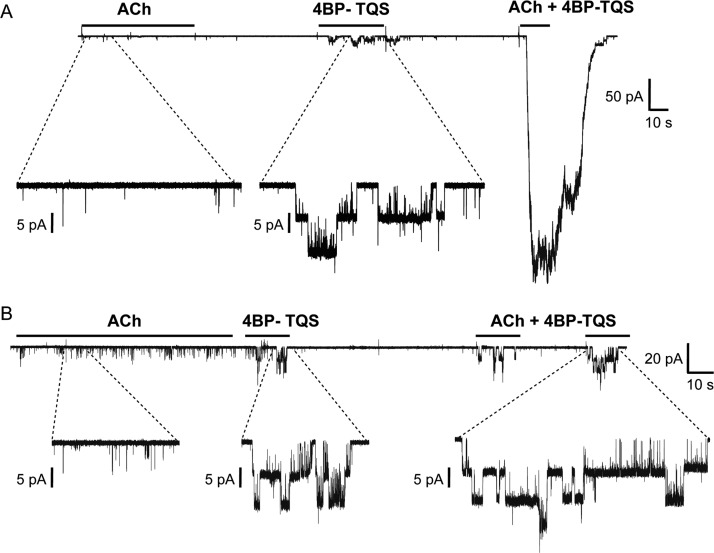
The single-channel properties of human α7 nAChRs, expressed in Xenopus oocytes, were recorded from a total of 39 outside-out patches treated with either the orthosteric agonist ACh or an allosteric agonist, 4BP-TQS. In several recordings, multiple superimposed channel openings were observed, suggesting that some patches contain several receptors, despite the observation that many isolated membrane patches gave no response to either agonist. Recordings from two multichannel patches are shown in Fig. 1 to illustrate the remarkable differences observed in response to activation by ACh alone, 4BP-TQS alone, and coapplication of the two agonists. Although these effects are qualitatively similar to the effects observed when these two agonists are examined by whole-cell recording (Gill et al., 2011, 2012), these examples also illustrate the wide variation in responses that can be observed in isolated patches. For analysis of channel kinetics, channel records were selected, assuming that only a single channel was active if no double openings were observed during application of 4BP-TQS.
Fig. 1.
Representative recordings (A and B) from two multichannel outside-out patchs isolated from Xenopus oocytes expressing human α7 receptors. Patches were exposed sequentially to 10 μM ACh alone, to 10 μM 4BP-TQS alone, and then to the coapplication of ACh + 4BP-TQS. With ACh, multiple openings were never observed, whereas 4BP-TQS resulted in the simultaneous activation of two or more channels. When ACh and 4BP-TQS were coapplied, the response was, in some cases, very markedly enhanced. In each panel (A and B), the upper trace shows a continuous low-gain trace of the recording, whereas the lower trace shows expanded segements of the trace.
Single-Channel Kinetics.
Application of ACh resulted in infrequent single-channel openings that were of very short duration (mean open time 1.49 ± 0.28 ms) (Table 1; Fig. 2). These findings were as expected, on the basis of the very rapid desensitization of α7 nAChR whole-cell responses (Couturier et al., 1990) and are in agreement with previous single-channel studies of α7 nAChRs (Mike et al., 2000; Fucile et al., 2002; Williams et al., 2011a; daCosta et al., 2011). In contrast, activation of α7 nAChRs by 4BP-TQS resulted in mean open times that were approximately 160 times longer and mean shut times that were approximately 10 times shorter (Table 1; Fig. 2). Although some patches gave initial multichannel responses, data segments were chosen for kinetic analysis assuming that only a single channel was active if no double openings were observed during application of 4BP-TQS. Because it is not possible to determine whether multiple receptors are active in the presence of ACh, this approach, if anything, will tend to underestimate the difference in Popen between ACh and 4BP-TQS. The calculated open probability with 4BP-TQS was approximately 800 times greater than that for ACh (Table 1). In addition, when receptors were activated by 4BP-TQS, openings had mean burst lengths approximately 370-fold longer and mean total open time within bursts approximately 600 times greater than those observed with ACh (Table 1).
TABLE 1.
Single-channel kinetics
Numbers of patches selected as being suitable for burst analysis were as follows: ACh, n = 3; ACh + TQS, n = 11; 4BP-TQS, n = 13; and ACh + 4BP-TQS, n = 7. Data are means ± S.E.M.
| Agonist | Open Time | Shut Time | Open Probability | Burst Length | Open Time within Burst |
|---|---|---|---|---|---|
| ms | |||||
| ACh (n = 9) | 1.49 ± 0.28§ | 4113 ± 1419§§§ | 0.0004 ± 0.0015§ | 3.28 ± 2.24§ | 2.00 ± 1.26 |
| ACh + TQS (n = 14) | 24.7 ± 5.8††† | 379 ± 103*** | 0.13 ± 0.04††† | 165 ± 55 | 141 ± 42 |
| 4BP-TQS (n = 20) | 245 ± 68* | 532 ± 184*** | 0.32 ± 0.09* | 1228 ± 364* | 1207 ± 360 |
| ACh + 4BP-TQS (n = 8) | 330 ± 56** | 590 ± 135*** | 0.36 ± 0.09††† | 3099 ± 754**,‡ | 2845 ± 745**,§ |
Significant differences (determined by ANOVA) to ACh (* P < 0.05; ** P < 0.01; *** P < 0.001) or to 4BP-TQS (§ P < 0.05; §§§ P < 0.001) are indicated. Otherwise, significant differences determined by pairwise comparisons to ACh (††† P < 0.001) or to 4BP-TQS (‡ P < 0.05) are indicated.
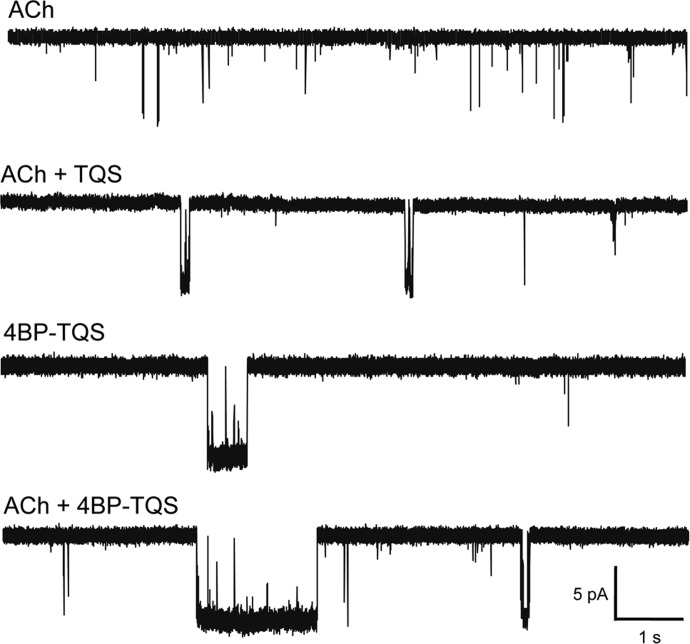
Fig. 2.
Representative single-channel traces from an outside-out patch isolated from Xenopus oocytes expressing human α7 receptors. Illustrated from top to bottom are traces obtained in response to application of ACh (10 μM), 4BP-TQS (10 μM), coapplication of ACh + 4BP-TQS (both 10 μM), and coapplication of ACh + TQS (both 10 μM).
Previous whole-cell electrophysiological studies with α7 nAChRs have shown that the macroscopic effects of agonist activation by 4BP-TQS are broadly similar to the effects of activation with ACh in the presence of a PAM such as TQS (Gill et al., 2011, 2012). Here we examined single-channel kinetics on coapplication of ACh with TQS. In comparison with single-channel kinetic properties observed with ACh alone, the channel kinetics were qualitatively similar when either ACh was coapplied with TQS or when 4BP-TQS was applied alone. These effects included increased open times, decreased shut times, and increased burst lengths (Table 1; Fig. 2).
Previous studies have demonstrated that, in addition to acting as an allosteric agonist, 4BP-TQS is also an extremely effective potentiator of responses to ACh (Gill et al., 2011, 2012). For example, it has been found that coapplication of ACh and 4BP-TQS generates much larger whole-cell responses than are seen with either agonist alone (Gill et al., 2011). The effect of coapplication of ACh and 4BP-TQS on single-channel kinetics was therefore examined. As is illustrated in Fig. 2 and summarized in Table 1, coapplication of ACh and 4BP-TQS resulted in a significantly longer mean burst length and longer total open time per burst than were observed with 4BP-TQS alone (2.5- and 2.4-fold, respectively).
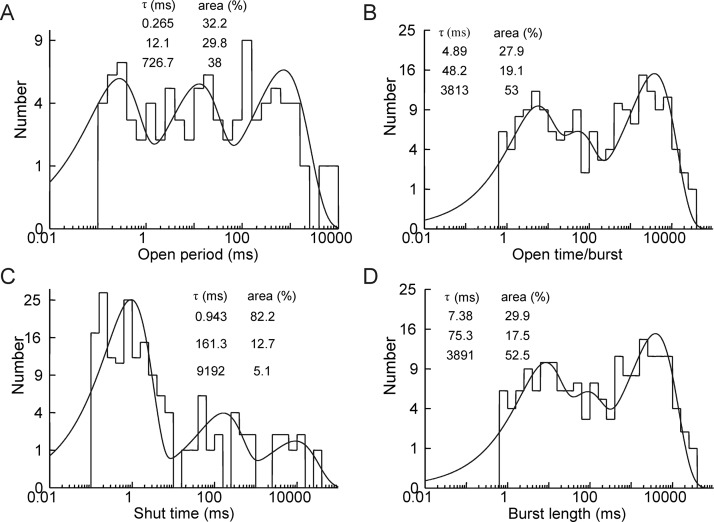
Activation by ACh resulted in channel open time distributions that were dominated by a single fast exponential component of time constant (1.02 ± 0.24 ms and relative area of 92 ± 4.7%). In contrast, when receptors were activated by 4BP-TQS, three exponential components were apparent in distributions of channel open times (Fig. 3) with the most prominent component (59.0 ± 3.8% of the distribution area) having the longest time constant (626 ± 386 ms). The significance of these extra exponential components, visible in the presence of the allosteric agonist and also with ACh in the presence of either 4BP-TQS or TQS, is considered further under Discussion. Channel closed times during activation by ACh were dominated by long closed periods (4742 ± 3436 ms; n = 5), reflecting the very low steady-state open probability observed with the endogenous neurotransmitter. In contrast, when receptors were activated by 4BP-TQS, the channel closed time distribution (Fig. 3C) could be described with three exponential components (as was the case with ACh); however, the proportion of long closed times (4332 ± 2422 ms; n = 10) observed in the data record was much reduced (24.4 ± 6.7%; n = 10), consistent with the idea that desensitized states of the receptor occur much less frequently in the presence of the allosteric agonist (Fig. 3C). From these studies, is clear that allosteric modulators (both allosteric agonists such as 4BP-TQS and PAMs such as TQS) exert a dramatic effect on the single-channel kinetics of α7 nAChRs.
Fig. 3.
Kinetic properties of α7 receptors activated by 4BP-TQS. Distributions of channel open times (A), open time per burst (B), channel closed times (C), and burst length (D) are shown fitted with multiple exponential components. Values for the component time constants and associated areas are inset. Bisection of the shut time distribution second and third exponential components gave a tcrit value (see Materials and Methods) used in identifying bursts of channel openings. Data are from a single patch recording.
Single-Channel Amplitudes.
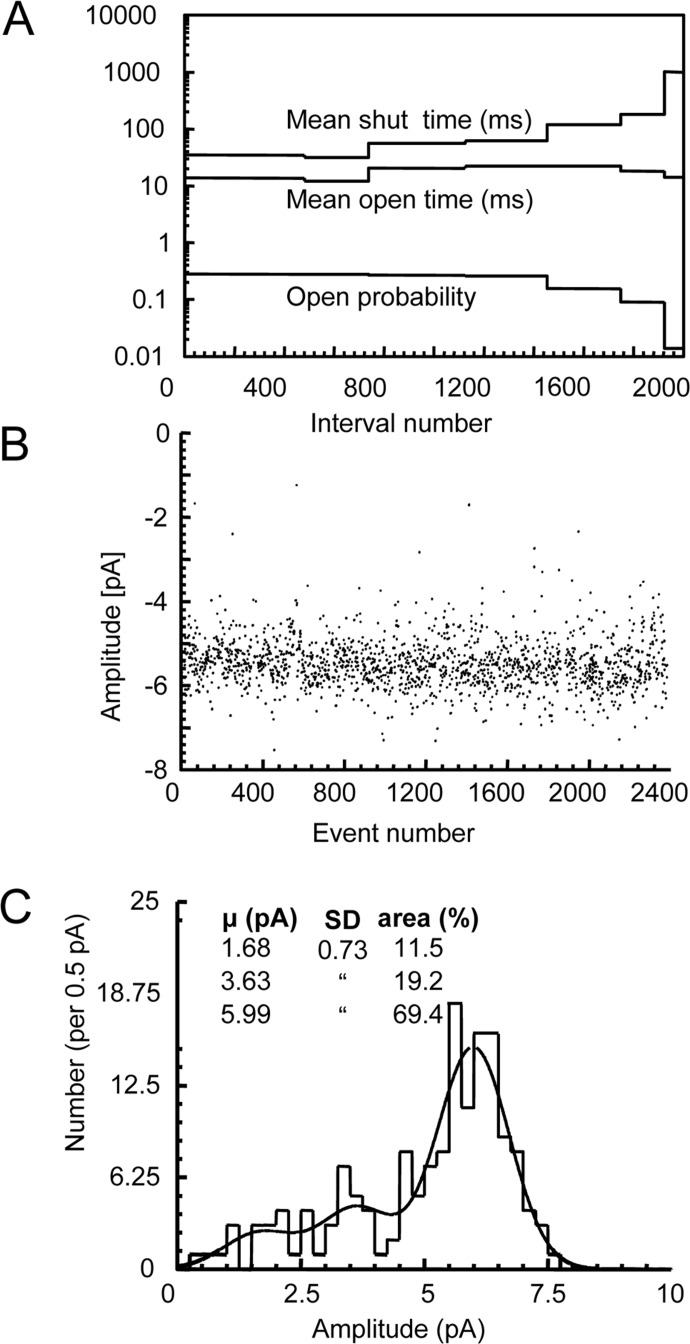
In addition to their effects on single-channel kinetics, allosteric modulators were found to exert more subtle, but significant, effects on single-channel amplitudes. For both ACh and 4BP-TQS, three Gaussian components were required to adequately describe the amplitude distribution of single-channel currents (Fig. 4; Table 2). Although ACh and 4BP-TQS generated single-channel openings of broadly similar amplitudes, the single-channel conductance of the largest component (which was also the most frequent component: relative area, 79.7 ± 3%) was significantly larger with 4BP-TQS than with ACh (cord conductances of 100 ± 2.4 and 90 ± 2.7 pS, respectively; P < 0.05).
Fig. 4.
Kinetic and amplitude stability of 4BP-TQS-activated channel currents. A, kinetic stability plot analysis illustrated that the running mean of channel open and closed times was stable in this recording for approximately 1400 events before the channel Popen began to decrease associated with increasing duration of channel closed intervals. B, stability plot analysis of channel amplitudes from the same patch as illustrated in A, demonstrating that channel amplitudes were stable throughout the recording. C, distribution of channel amplitudes in α7 receptors activated by 4BP-TQS. Each entry in the distribution corresponds to the amplitude of a single channel opening. The distribution is shown fitted with the sum of three Gaussian components with the S.D. constrained to be the same for all three components. Data are from a single patch recording of duration 80.8 s.
TABLE 2.
Single-channel conductance
Data are means ± S.E.M. The frequency of the three conductance classes was calculated as a weighted mean ± S.E.M., based on the number of openings in each patch.
| Agonist | Conductance 1 [Frequency] | Conductance 2 [Frequency] | Conductance 3 [Frequency] |
|---|---|---|---|
| pS [%] | |||
| ACh (n = 9) | 32.7 ± 3.3 [2.8 ± 2.9] | 63.6 ± 2.7 [5.2 ± 2.9] | 90.0 ± 2.7 [92.0 ± 12.9]‡ |
| ACh + TQS (n = 14) | 47.0 ± 4.6 [14.0 ± 2.0]* | 81.1 ± 5.1 [33.4 ± 4.4] | 109.4 ± 5.5 [52.4 ± 7.0]** |
| 4BP-TQS (n = 20) | 40.0 ± 2.4 [8.5 ± 2.6] | 67.6 ± 3.7 [12.7 ± 2.9] | 100.3 ± 2.4 [78.7 ± 5.2]† |
| ACh + 4BP-TQS (n = 8) | 40.6 ± 2.6 [6.3 ± 2.2] | 63.9 ± 6.6 [18.9 ± 1.9] | 107.3 ± 2.8 [74.8 ± 2.3]* |
Significant differences in conductance (determined by ANOVA) to ACh (* P < 0.05; ** P < 0.01) are indicated. Otherwise, significant differences determined by pairwise comparisons to ACh († P < 0.05) or to 4BP-TQS (‡ P < 0.05) are indicated.
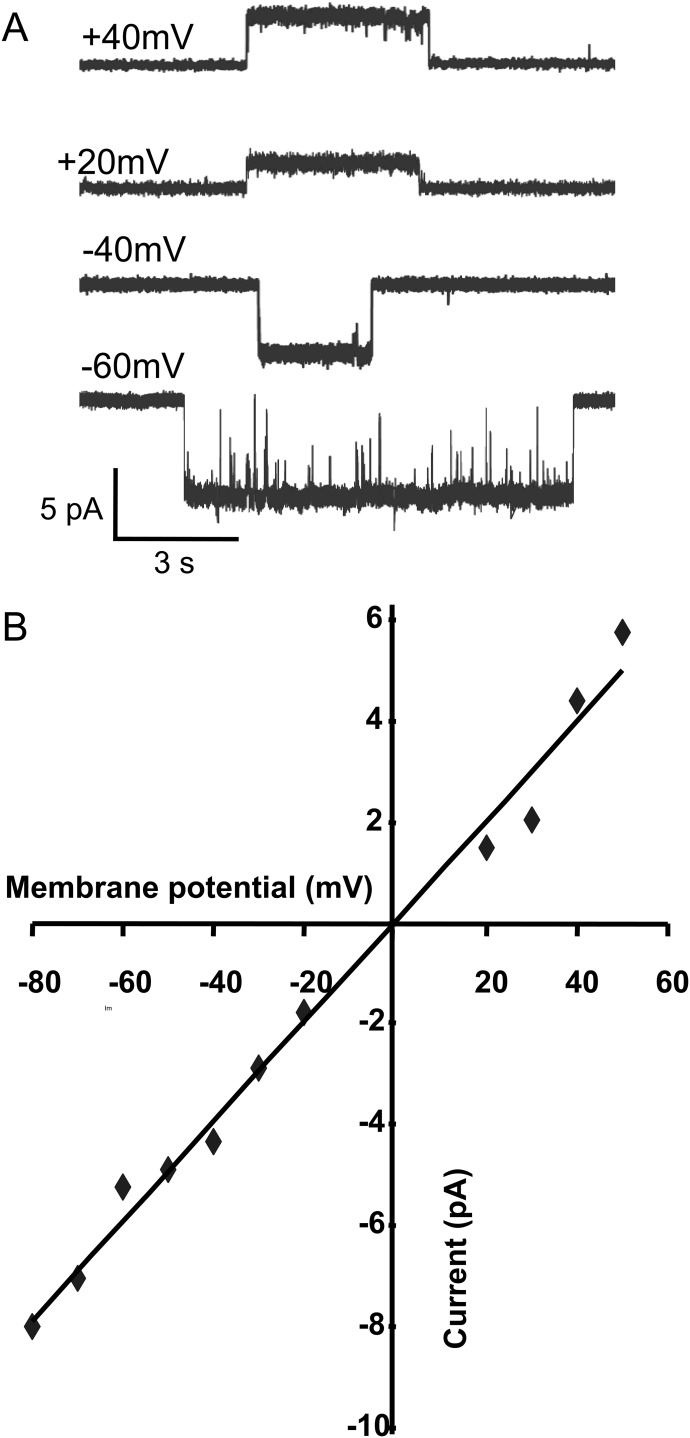
In addition, because of the greater ease of obtaining single-channel currents with 4BP-TQS than with ACh, it was possible to estimate the slope conductance for α7 nAChRs activated by 4BP-TQS from recordings performed at different membrane potentials (Fig. 5). An approximately linear current-voltage relationship was observed (Fig. 5), giving a slope conductance of 99 pS.
Fig. 5.
Slope conductance of α7 nAChRs activated by 4BP-TQS. Single-channel openings recorded at different membrane potentials (A) enabled a slope conductance to be determined (99 pS) from the plot of current versus membrane potential (B).
As was observed when α7 nAChRs were activated with ACh or 4BP-TQS alone, when ACh was coapplied with either TQS or with 4BP-TQS, the distribution of single-channel amplitudes could be described by the sum of three Gaussian components (Table 2). In addition, as was observed with 4BP-TQS alone, the amplitude of the largest component detected when ACh was coapplied with either TQS or with 4BP-TQS was significantly larger. It appears, therefore, that the binding of an allosteric ligand (TQS or 4BP-TQS) to its proposed transmembrane site results in larger single-channel currents than are seen when α7 nAChRs are activated only by ACh binding to its orthosteric extracellular site. This observation provides evidence that activation of α7 nAChRs by orthosteric and allosteric agonists results in different open channel conformations.
Discussion
There have been relatively few studies examining the single-channel properties of α7 nAChRs. To a large extent, this is probably a consequence of the difficulties that are associated with obtaining single-channel data from receptors that undergo such rapid agonist-induced desensitization. When single-channel data have been reported for α7 nAChRs in response to activation by ACh, openings have been infrequent and of very short duration. For example, studies conducted with human recombinant α7 nAChRs and also with native α7 nAChRs from rat and chick have reported single-channel mean open times of less than 1 ms (Mike et al., 2000; Fucile et al., 2002; Nai et al., 2003), similar to the results obtained with ACh in the present study.
Previous studies have reported much longer mean open times for α7 nAChRs when activated by ACh in the presence of the positive allosteric modulator 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea (PNU-120596) (Hurst et al., 2005; Williams et al., 2011a; daCosta et al., 2011). Our data, obtained from ACh coapplied with TQS, are consistent with these previous studies. An important novel aspect of the present study is the single-channel data obtained from receptors activated by an allosteric agonist (4BP-TQS) in the absence of ACh. The dramatically longer mean open time, longer mean burst length, and greater open probability observed with 4BP-TQS give new insight into previous studies of allosteric agonists conducted by whole-cell electrophysiological approaches and help to define the mechanism of action of allosteric potentiators at α7 nAChRs (Gill et al., 2011, 2012). Likewise, the increased mean burst length observed when ACh and 4BP-TQS are coapplied is consistent with evidence that, in addition to causing allosteric receptor activation in the absence of ACh, 4BP-TQS is also a potent allosteric potentiator of ACh-evoked responses (Gill et al., 2011, 2012).
The main focus of previous studies comparing activation of α7 nAChRs by ACh in the presence or absence of the positive allosteric modulator PNU-120596 appears to have been its effect on channel kinetics rather than on single-channel amplitudes (Hurst et al., 2005; Williams et al., 2011a; daCosta et al., 2011). This may be a consequence of difficulties in estimating the amplitude of the brief and infrequent openings in response to activation by ACh. For example, the authors of one study state that “it is difficult to draw a conclusion regarding the effect of PNU-120596 on the unitary conductance” (Hurst et al., 2005), whereas another study concluded that “the conductance measured in the presence of PNU-120596 is likely to be a reasonable estimate of single-channel α7 conductance in the absence of PNU-120596” (Williams et al., 2011a). Interestingly, we observed significantly larger single-channel amplitudes when α7 nAChRs are activated by an allosteric agonist or in the presence of a positive allosteric modulator. This result provides support for the conclusion that the binding of a ligand (4BP-TQS or TQS) to the α7 allosteric binding site may modify the open-channel conformation generated by the binding of ACh (alone) to its orthosteric site. An altered open channel conformation is also consistent with differences in rates of desensitization that have been observed with whole-cell responses in these two situations (Gill et al., 2011, 2012). These findings are also consistent with a report suggesting that allosteric modulators can increase the single-channel conductance of GABAA receptors (Gaul et al., 2007).
We observed multiple conductance levels for α7 nAChRs, a finding that is consistent with studies conducted previously with other nAChR subtypes (McGehee et al., 1995). Three distinct conductance levels were observed with all agonists examined in the present study. Although there have been relatively few detailed studies of the single-channel properties of the wild-type α7 nAChRs, a number of studies have been conducted with α7 nAChRs containing mutations in the M2 transmembrane domain that confer reduced levels of desensitization. In several of these studies with mutated α7 nAChRs, three broadly similar conductance states have been identified (Fucile et al., 2002; Tonini et al., 2003, 2004). Studies of native rat hippocampal α7 nAChRs reported a mean single-channel conductance of 91.5 pS, and although a single Gaussian function did not provide a good fit of the amplitude distribution, it was not possible to identify unambiguously multiple conductance levels (Mike et al., 2000). In addition, studies of human recombinant α7 nAChRs expressed in SHEP1 cells have identified “two to three different levels of channel amplitudes” (Vallés et al., 2009). Likewise, studies of native chick α7 nAChRs identified multiple conductance levels (Nai et al., 2003).
In comparison with most other members of nAChR family, α7 receptors are somewhat atypical. They exhibit very fast rates of desensitization and, being homomeric nAChRs, contain five potential ligand-binding sites (Couturier et al., 1990; Palma et al., 1996). Opening of heteromeric receptors is believed to occur most efficiently in response to the binding of ACh to two or three binding sites (Rayes et al., 2009), and binding of ACh to its extracellular orthosteric site stabilizes an open conformation that is associated with a rotation of the M2 transmembrane helices (Unwin, 1995). It has been reported previously that 4BP-TQS and PAMs such as TQS bind to α7 nAChR in an intrasubunit transmembrane cavity (Young et al., 2008; Collins et al., 2011; Gill et al., 2011), and it is reasonable to assume that there are five potential binding sites in a homomeric nAChR. It is possible that multiple conductance states observed after activation of α7 nAChRs, with either orthosteric or allosteric agonists, correspond to different levels of occupancy of agonist binding sites. Indeed, this explanation for multiple conductance states has been proposed previously for glutamate receptors (Rosenmund et al., 1998). However, preliminary experiments with a 100-fold higher concentration of ACh (1 mM) did not support this possibility.
The single-channel data presented here provide direct evidence that, in the presence of an allosteric modulator, long-lived open states can be observed. A model for the action of PAMs on α7 nAChR has been suggested recently, whereby α7 nAChRs exist in two open and two desensitized states (Williams et al., 2011b), and allosteric modulators were suggested to cause either destabilization of a fast desensitized state or its conversion into a long-lived open state. Below, we consider these two possible explanations in more detail in light of the new single-channel data we have obtained in this study and, in particular, using for the first time, data from an allosteric potentiator that can activate the receptor in the absence of ACh (Gill et al., 2011).
Conceptually, a desensitized state is simply a long-lived closed state of the channel that has a high affinity for the agonist. Were the allosteric potentiator to impede channel closure when the receptor undergoes the desensitizing conformational change, the result would be a new, long-lived open state. This interpretation is similar to that used previously to explain the effects of the L247T mutation in the chick α7 nAChR (Revah et al., 1991). In the presence of ACh alone, the majority of patches (five of eight) exhibit open time distributions that can be described by a single fast exponential (τ = 1.02 ± 0.24 ms) and, on average, this exponential component accounted for 92 ± 4.7% of ACh-evoked openings. This finding suggests that the receptor has predominantly a single open state when activated by ACh (Colquhoun and Hawkes, 1982; Shelley and Magleby, 2008). A mean open time of approximately 1 ms at room temperature translates to approximately 290 μs at 37°C (assuming a Q10 of approximately 2.5 for channel gating). If the effect of an allosteric modulator were to prolong the channel open times by stabilizing the open state (slowing the channel closing rate), we would expect to see the same number of exponential components in the channel open time distributions in the presence of TQS or 4BP-TQS as with ACh alone. However, in the presence of TQS or 4BP-TQS, channel open time distributions displayed predominantly three exponential components, as did the burst length and total open time per burst distributions. Because the number of exponential components observable in the open time distribution or total open time per burst distribution is a guide to the number of open states of the channel (Colquhoun and Hawkes, 1982; Shelley and Magleby, 2008), these data showing an increase in the number of kinetically distinguishable open states in the presence of TQS and 4BP-TQS are consistent with the idea that when the allosteric potentiator is bound, receptors undergoing the desensitizing conformational change continue to allow ions to flow through the channel: in effect, the desensitized state has been converted to a new open state.
If the effect of the allosteric modulator were to destabilize a fast desensitized state, we should expect to see this reflected particularly in a change in the channel closed times (Shelley and Magleby, 2008) without necessarily any change in the channel open times (although the effects of limited time resolution in single-channel recordings make predicting the effect of a dramatic shortening of the life time of a closed state difficult). The channel closed time distributions show a clear tendency for briefer closings with overall an approximately 8-fold decrease in channel closed times. However, remarkably long closed times were regularly observed in the presence of either 4BP-TQS or with ACh in the presence of either allosteric modulator. These results therefore suggest that the channel can still enter long-lived shut states (“desensitized” states), although less frequently in the presence of the allosteric modulators. Therefore, the channel shut time distributions are consistent with the idea that the potentiators destabilize a fast desensitized state, as has been suggested previously (Williams et al., 2011a,b). However, the dramatic prolongation of the channel open times is also consistent with a slowing of the rate of entry into desensitized states. Overall, the single-channel data support a combination of these two mechanisms in generating the remarkable allosteric modulation of α7 nAChR receptor responses observed in the presence of TQS and 4BP-TQS.
In summary, we have examined the single-channel properties of α7 nAChR when activated by either an orthosteric agonist (ACh) or an allosteric agonist (4BP-TQS). The differences that we have observed in single-channel kinetics and conductance help to explain the very different effects these two types of agonists exert on whole-cell responses and also provide evidence that they act through distinct open-channel conformations.
This work was supported by the Wellcome Trust [Grant 085141]. M.J. was supported by a Traveling Fellowship from The Company of Biologists.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- ACh
- acetylcholine
- nAChR
- nicotinic acetylcholine receptor
- PAM
- positive allosteric modulator
- TQS
- 4-(napthalen-1-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide
- 4BP-TQS
- 4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide
- ANOVA
- analysis of variance
- PNU-120596
- 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea.
Authorship Contributions
Participated in research design: Pałczyńska, Jindrichova, Gibb, and Millar.
Conducted experiments: Pałczyńska, Gibb, and Jindrichova.
Performed data analysis: Pałczyńska, Jindrichova, Gibb, and Millar.
Wrote or contributed to the writing of the manuscript: Pałczyńska, Jindrichova, Gibb, and Millar.
References
- Arneric SP, Holladay M, Williams M. (2007) Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol 74:1092–1101 [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 74:1155–1163 [DOI] [PubMed] [Google Scholar]
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, Sivilotti LG. (2006) Incorporation of the β3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol Pharmacol 70:1350–1357 [DOI] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ. (2005) Allosteric mechanisms of signal transduction. Science 308:1424–1428 [DOI] [PubMed] [Google Scholar]
- Collins T, Young GT, Millar NS. (2011) Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology 61:1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. (1982) On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci 300:1–59 [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. (1995) Fitting and statistical analysis of single-channel records, in Single-Channel Recording (Sakmann B, Neher E. eds) pp 483–587, Plenum Press, New York [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. (1990) A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5:847–856 [DOI] [PubMed] [Google Scholar]
- D'hoedt D, Bertrand D. (2009) Nicotinic acetylcholine receptors: an overview on drug discovery. Expert Opin Ther Targets 13:395–411 [DOI] [PubMed] [Google Scholar]
- daCosta CJ, Free CR, Corradi J, Bouzat C, Sine SM. (2011) Single-channel and structural foundations of neuronal α7 acetylcholine receptor potentiation. J Neurosci 31:13870–13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster J. (2001) The Laboratory Computer: a Practical Guide for Physiologists and Neuroscientists, Academic Press, London [Google Scholar]
- Fucile S, Palma E, Martinez-Torres A, Miledi R, Eusebi F. (2002) The single-channel properties of human acetylcholine α7 receptors are altered by fusing α7 to the green fluorescent protein. Proc Natl Acad Sci USA 99:3956–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul S, Ozsarac N, Liu L, Fink RH, Gage PW. (2007) The neuroactive steroids alphaxalone and pregnanolone increase the conductance of single GABAA channels in newborn rat hippocampal neurons. J Steroid Biochem Mol Biol 104:35–44 [DOI] [PubMed] [Google Scholar]
- Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS. (2012) A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol Pharmacol 81:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. (2011) Agonist activation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 108:5867–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. (2007) Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724 [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Corringer PJ, Changeux JP. (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53:447–456 [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. (2004) Cys-loop receptors: new twists and turns. Trends Neurosci 27:329–336 [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. (1995) Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269:1692–1696 [DOI] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX. (2000) Choline and acetylcholine have similar kinetic properties of activation and desensitization on the α7 nicotinic receptors in rat hippocampal neurons. Brain Res 882:155–168 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Nai Q, McIntosh JM, Margiotta JF. (2003) Relating neuronal nicotinic acetylcholine receptor subtypes defined by subunit composition and channel function. Mol Pharmacol 63:311–324 [DOI] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D. (1996) Neuronal nicotinic α7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol 491:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes D, De Rosa MJ, Sine SM, Bouzat C. (2009) Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci 29:6022–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiéry A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux JP. (1991) Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353:846–849 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. (1998) The tetrameric structure of a glutamate receptor channel. Science 280:1596–1599 [DOI] [PubMed] [Google Scholar]
- Shelley C, Magleby KL. (2008) Linking exponential components to kinetic states in Markov models for single-channel gating. J Gen Physiol 132:295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8:733–750 [DOI] [PubMed] [Google Scholar]
- Tonini R, Palma E, Miledi R, Eusebi F. (2003) Properties of neuronal α7 mutant nicotinic acetylcholine receptors gated by bicuculline. Neuropharmacology 44:765–771 [DOI] [PubMed] [Google Scholar]
- Tonini R, Renzi M, Eusebi F. (2004) Unliganded human mutant α7 nicotinic receptors are modulated by Ca2+ and trace levels of Zn2+. Neuropharmacology 46:727–733 [DOI] [PubMed] [Google Scholar]
- Unwin N. (1995) Acetylcholine receptor channel imaged in the open state. Nature 373:37–43 [DOI] [PubMed] [Google Scholar]
- Vallés AS, Roccamo AM, Barrantes FJ. (2009) Ric-3 chaperone-mediated stable cell-surface expression of the neuronal α7 nicotinic acetylcholine receptor in mammalian cells. Acta Pharmacol Sin 30:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, Magleby KL. (1990) Voltage dependence and stability of the gating kinetics of the fast chloride channel from rat skeletal muscle. J Physiol 426:145–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011a) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011b) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Broad LM, Zwart R, Astles PC, Bodkin M, Sher E, Millar NS. (2007) Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular β4 amino acids that are implicated in the coupling of binding to channel gating. Mol Pharmacol 71:389–397 [DOI] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. (2008) Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA 105:14686–14691 [DOI] [PMC free article] [PubMed] [Google Scholar]