Abstract
Green tea polyphenolic catechins exhibit biological activity in a wide variety of cell types. Although reports in the lay and scientific literature suggest therapeutic potential for improving cardiovascular health, the underlying molecular mechanisms of action remain unclear. Previous studies have implicated a wide range of molecular targets in cardiac muscle for the major green tea catechin, (−)-epigallocatechin-3-gallate (EGCG), but effects were observed only at micromolar concentrations of unclear clinical relevance. Here, we report that nanomolar concentrations of EGCG significantly enhance contractility of intact murine myocytes by increasing electrically evoked Ca2+ transients, sarcoplasmic reticulum (SR) Ca2+ content, and ryanodine receptor type 2 (RyR2) channel open probability. Voltage-clamp experiments demonstrate that 10 nM EGCG significantly inhibits the Na+-Ca2+ exchanger. Of importance, other Na+ and Ca2+ handling proteins such as Ca2+-ATPase, Na+-H+ exchanger, and Na+-K+-ATPase were not affected by EGCG ≤1 μM. Thus, nanomolar EGCG increases contractility in intact myocytes by coordinately modulating SR Ca2+ loading, RyR2-mediated Ca2+ release, and Na+-Ca2+ exchange. Inhibition of Na+-K+-ATPase activity probably contributes to the positive inotropic effects observed at EGCG concentrations >1 μM. These newly recognized actions of nanomolar and micromolar EGCG should be considered when the therapeutic and toxicological potential of green tea supplementation is evaluated and may provide a novel therapeutic strategy for improving contractile function in heart failure.
Introduction
A number of reports indicate that green tea consumption is beneficial to cardiovascular health and can reduce the risk of cardiovascular diseases (Wolfram, 2007; Babu and Liu, 2008). Polyphenol catechins constitute approximately 30% of the dry weight of green tea leaves and have been shown to possess a wide spectrum of biological activities (Feng, 2006; Wang and Ho, 2009). (−)-Epigallocatechin-3-gallate (EGCG) is among the most abundant green tea catechins and has been extensively studied (Wolfram, 2007). Oral consumption of EGCG results in rapid distribution to the blood and organs, including heart, skeletal muscles, and brain (Suganuma et al., 1998). A pharmacokinetic study of EGCG after supplementation of fasting individuals with an oral dose of 1200 mg revealed peak plasma EGCG of 7.4 ± 3.6 μM free EGCG (Chow et al., 2005).
Previous cellular and molecular studies of the biological actions of EGCG often use very high concentrations of EGCG (10–200 μM) to define its mechanisms of action (Stangl et al., 2007; Babu and Liu, 2008). In the last two decades, studies have demonstrated that EGCG and related catechins interact strongly with phospholipid membranes, and concentrations ≥30 μM can damage lipid membranes (Ikigai et al., 1993; Tamba et al., 2007). It is therefore likely that results from in vitro experiments at high concentrations could mask more specific mechanisms by which EGCG exerts its biological actions at pharmacologically relevant doses (<10 μM). Recent reports suggest that EGCG increases cardiac contractility at low micromolar concentrations (1–5 μM) (Lorenz et al., 2008) without altering ECG parameters and cardiac ion channels (Kang et al., 2010). The molecular mechanisms responsible for the positive inotropic effect of EGCG remain unclear. In the present study, we found that EGCG, at concentrations 100- to 500-fold lower than those previously reported, significantly enhances evoked Ca2+ transient amplitude and contractility in murine myocytes. At these pharmacological concentrations, the actions of EGCG are mediated by selective activation of ryanodine receptor type 2 (RyR2) and inhibition of plasmalemma Ca2+ fluxes via the Na+-Ca2+ exchanger (NCX), with negligible influence on Ca2+-ATPase (SERCA2), Na+-H+ exchanger, and Na+-K+-ATPase. Previous work has shown that nanomolar EGCG has also no effect on L-type Ca2+ current in ventricular myocytes (Kang et al., 2010). These coordinated actions of EGCG result in a net shift of Ca2+ transport during the cardiac cycle away from the plasma membrane to the energetically more favorable sarcoplasmic reticulum (SR) Ca2+ transport, which may represent a novel therapeutic strategy for increasing cardiac contractility in patients with heart failure.
Materials and Methods
Myocyte Isolation and Ca2+ Indicator Loading.
All experiments were approved by the institutional animal care and use committees at Vanderbilt University and performed in accordance with National Institutes of Health guidelines. Adult C57BL/6 mice (12–16 weeks old) were used for myocyte experiments. Single ventricular myocytes were isolated by a modified collagenase/protease method as described previously (Knollmann et al., 2006). All the experiments were conducted in Tyrode's solution containing the following: 2 mM CaCl2, 134 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH adjusted to 7.4 with NaOH. The final concentration of Ca2+ was 2 mM. After isolation of myocytes, myocytes were loaded with Fura-2 acetoxymethyl ester (Fura-2 AM) as described by us previously (Chopra et al., 2007). In brief, myocytes were incubated with 2 μM Fura-2 AM for 6 min at room temperature to load the indicator in the cytosol. Myocytes were washed twice for 10 min with Tyrode's solution containing 250 μM probenecid to retain the indicator in the cytosol. A minimum of 30 min was allowed for de-esterification before the cells were imaged.
Measurement of Intracellular [Ca2+]i and Cell Shortening.
For experiments with field stimulation, myocytes were loaded with membrane-permeable Fura-2 AM or Fluo-4 AM. After a 5-min exposure to either EGCG or vehicle, myocytes were field-stimulated at 1 Hz, and Ca2+ transients and cell shortening were recorded. At the end of a 20-s recording, myocytes were exposed to 10 mM caffeine for 5 s using a rapid concentration clamp system. Amplitudes of caffeine-induced Ca2+ transients were used as estimates of SR Ca2+ content. [Ca2+]i measurements were reported as fluorescence ratios (Fratio). Ca2+ transients and ventricular myocyte shortenings were analyzed using commercially available data analysis software (IonWizard; IonOptix, Milton, MA). Data were collected from three to four independent myocyte preparations.
Measurement of NCX.
NCX current was measured as the Ni2+-sensitive current recorded with a 1-s slow ramp-pulsing protocol applied from +60 to −100 mV at a holding potential of −40 mV, as described elsewhere (Woo and Morad, 2001; Reppel et al., 2007a). In brief, mouse ventricular myocytes were whole cell-patched in K+-free solution containing 10 mM CsCl, 135 mM NaCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4. The pipette solution contained 136 mM CsCl, 10 mM NaCl, 20 mM tetraethylammonium-Cl, 3 mM MgCl2, 100 mM CaCl2, 5 mM Mg-ATP, 10 mM HEPES, and 10 mM glucose, pH 7.2. To measure NCX currents, myocytes were held at −40 mV to inactivate both sodium and T-type calcium currents. Other unrelated overlapping currents were eliminated with drugs: 10 μM nifedipine to block the L-type calcium channel (ICa-L), 500 μM 4-aminopyride to suppress the transient outward K+ current (ITo), 200 μM BaCl2 to remove background K+ current (IK1), and 10 μM ouabain to inhibit Na+-K+-ATPase. The experiments were performed at room temperature (22–23°C).
Preparations of Cardiac Muscle Membranes Enriched in RyR2.
For measurements of RyR2 and SERCA2 activities, SR enriched in RyR2 was isolated from rabbit cardiac left ventricles (New Zealand White; Charles River Laboratories, Wilmington, MA) as described previously (Pessah et al., 1985, 1990). In brief, the left ventricle, prepared at 4°C, was carefully washed and then homogenized in iced 300 mM sucrose containing 40 mM Tris-histidine, pH 7.0, three times at 20,000 rpm for 30 s using PowerGen 700D (Thermo Fisher Scientific, Waltham, MA). The homogenate was centrifuged at 4°C for 20 min at 1000g; the supernatant was poured through four layers of cheesecloth and then centrifuged for 20 min at 8000g. The resulting supernatant was centrifuged for 30 min at 45,000g; the pellet was then resuspended in 10 ml of 600 mM KCl and 40 mM Tris-histidine, pH 7.0, and centrifuged for 30 min at 45,000g. The final pellet was resuspended in 300 mM sucrose containing 10 mM imidazole, pH 7.0, and quickly frozen with liquid nitrogen and stored at −80°C.
Crude cardiac membranes were prepared using a method described previously (Wang et al., 2001) and used for measuring the effects of EGCG on Na+-K+-ATPase. Homogenates were centrifuged at 6000g for 15 min. Supernatants were subsequently centrifuged at 100,000g for 60 min, and pellets were resuspended at 10 to 15 mg/ml protein, flash-frozen, and stored at −80°C until thawed to perform assays.
Measurements of [3H]Ryanodine Binding.
Measurements of equilibrium, high-affinity [3H]ryanodine ([3H]Ry) binding specifically to cardiac muscle membrane preparations (50–100 μg of protein/ml) were performed as described previously by us (Pessah et al., 1985; Pessah and Zimanyi, 1991). Incubations were performed in the presence or absence of freshly prepared EGCG introduced into assay buffer consisting of 10 mM HEPES, pH 7.4, 250 mM KCl, 15 mM NaCl, 1 to 10,000 μM CaCl2, and 1 to 5 nM [3H]Ry for 15 h at 25°C. The reactions were quenched by filtration through GF/B glass fiber filters (Brandel Inc., Gaithersburg, MD) and washed twice with ice-cold harvest buffer: 20 mM HEPES, 250 mM KCl, 15 mM NaCl, and 0.05 mM CaCl2, pH 7.1. Nonspecific binding was assessed by addition of a 1000-fold excess of unlabeled ryanodine to the assay medium in the presence or absence of EGCG.
Analysis of RyR2 Single Channel Incorporated in Planner Lipid Bilayer.
Single-channel recording and analysis were performed as described previously (Feng et al., 2008). In brief, RyR2 single channels were incorporated by inducing fusion of cardiac SR vesicles with a planar bilayer membrane composed of phosphatidylethanolamine-phosphatidylserine-phosphatidylcholine (5:3:2 w/w, 30 mg/ml in decane). Both cis (cytoplasmic) and trans (luminal) solutions were buffered by 20 mM HEPES at pH 7.4, with 500 mM Cs+ in cis and 50 mM in trans. To prevent additional fusion of SR vesicles after incorporation of a single channel, the cis chamber was immediately perfused with >20 volumes of identical solution without SR protein. Once a channel was reconstituted the free Ca2+ concentration was adjusted cis and trans as indicated in the figure legends, and baseline channel activity was measured for at least 2 min. EGCG was subsequently added to cis solution. Single-channel recordings were made for >1 min at −40 mV applied to the trans side with cis held as a virtual ground. Data were filtered at 1 kHz (Low-Pass Bessel Filter 8 Pole, Warner Instruments, Hamden, CT), digitized, and acquired through Digidata 1320A and AxoScope 10 software (Axon-Molecular Devices, Union City, CA).
Analysis of single -channel open probability (Po), mean open and closed time constants (τo and τc, respectively) were calculated using pClamp 9 software. Total n = 9 independent BLM measurements were performed in the absence or presence of EGCG titrated from 10 nM to 1 μM.
Analysis of SERCA2 Activity.
Activity of the thapsigargin-sensitive SERCA2 in isolated cardiac SR membranes was measured using a coupled enzyme assay that monitors the rate of oxidation of NADH at 340 nm as described previously (Ta et al., 2006). In brief, 1.5 ml of assay buffer consisted of 7 mM HEPES, pH 7.0, 143 mM KCl, 7 mM MgCl2, 0.085 mM EGTA, 0.43 mM sucrose, 0.0028 mM phosphoenolpyruvate, 1 mM Na2ATP, coupling enzyme mixture (700 units of pyruvate kinase II and 1000 units of lactate dehydrogenase), 0.048 mM free Ca2+, 10 nM rotenone (Cherednichenko et al., 2004), and 100 μg/ml cardiac membrane protein at 37°C. Thapsigargin (TG) (0.2 μM) was added to the negative control to inhibit the SERCA2 component of ATPase activity. Cardiac membrane protein was incubated in the absence or presence of EGCG (0.1–1 μM) for 3 min before 0.4 μM NADH was added to initiate measurement of Ca2+ (Mg2+)-ATPase activity. A total of four independent measurements were made under these assay conditions in the presence or absence of EGCG.
Measurement of Na+-K+-ATPase Activity.
The Na+-K+-ATPase activity was measured using a modified version of the Fiske and SubbaRow method (Fiske and Subbarow, 1925). Whole cardiac membrane preparations (0.1 mg/ml protein) were prepared in a pH 7.4 medium containing 40 mM Tris HCl, 1 mM EDTA, 5 mM MgCl2, 15 mM KCl, 5 mM NaN3, 133 mM NaCl, 1 mM dithiothreitol, 20 nM rotenone, and 200 nM thapsigargin. The Na+-K+-ATPase activity was determined by measuring the Pi released from the cardiac membranes into the solution by addition of ATP in the presence or absence of ouabain (100 μM) to inhibit all ouabain-sensitive ATPase activity (Na+-K+-ATPase). Ouabain-sensitive Na+-K+-ATPase constituted 60% of the total ATPase activity in the whole cardiac preparations. EGCG (1–10 μM) was incubated for 10 min at 37°C before addition of ATP to start the reaction. After a 15-min incubation at 37°C, the enzymatic activity was stopped, and Pi was determined by the addition of equivalent amount of colorimetric reagent. Coloring reagent contained equal amounts of 10% ascorbic acid, 2.5% ammonium molybdate, and 15% H2SO4. After another 15 min of incubation for color development, the absorbance was read at 810 nm using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA). To verify the results obtained with cardiac preparations, the above experiments were repeated using purified Na+-K+-ATPase from porcine cerebral cortex (Sigma-Aldrich, St. Louis, MO).
Reagents.
[3H]Ryanodine was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA); nonradioactive ryanodine was from Abcam (Cambridge, MA). High-purity EGCG (>95%, the chemical structure of (EGCG is shown in Fig. 1, inset) was purchased from Sigma-Aldrich. Stock solutions for EGCG were freshly made immediately before experiments with nanopure H2O and kept on ice until use. Caffeine, phenylmethylsulfonyl fluoride, phosphocreatine, antipyrylazo, creatine phosphokinase, CsCl, NADH, ruthenium red, benzyl-p-toluene sulfonamide, and thapsigargin were purchased from Sigma-Aldrich. Phosphatidylethanolamine-phosphatidylserine-phosphatidylcholine were purchased from Avanti Polar Lipids (Alabaster, AL). Sucrose, KCl, NaCl, and HEPES were from Thermo Fisher Scientific. Sodium pyrophosphate, MgATP, and leupeptin were purchased from MP Biomedicals (Solon, OH). Lactate dehydrogenase was purchased from Calbiochem (San Diego, CA). Fura-2 AM was purchased from Invitrogen (Carlsbad, CA).
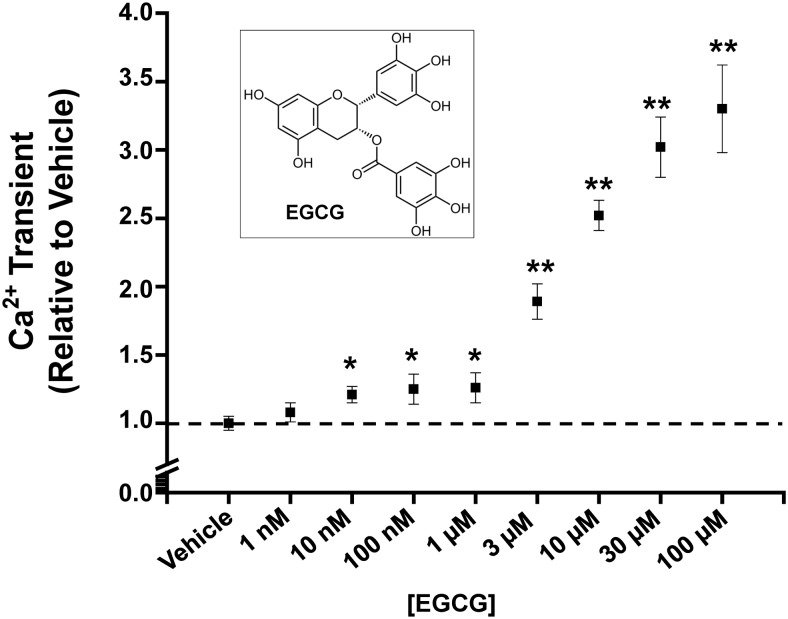
Fig. 1.
EGCG concentration-response relationship of Ca2+ transients in intact murine myocytes field-stimulated at 1 Hz. Note the biphasic response to EGCG. The chemical structure of EGCG is shown in the inset. n = 8 to 40/group *, p < 0.05 versus vehicle; **, p < 0.0001 versus vehicle.
Statistics.
Differences between the groups were analyzed using Student's t test. p < 0.05 was considered statistically significant.
Results
Nanomolar EGCG Enhances Myocyte Contractility, Ca2+ Transients, and SR Ca2+ Content.
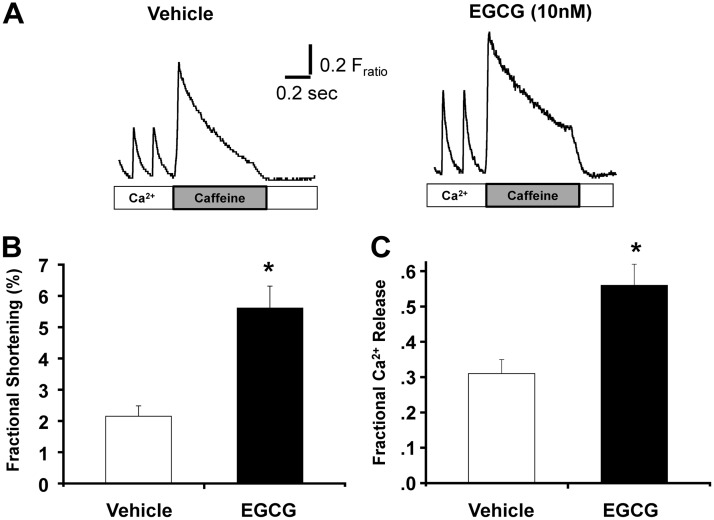
We first investigated the concentration-response relationship of EGCG-positive inotropic action in intact murine myocytes stimulated at 1 Hz. Consistent with a previous report (Lorenz et al., 2008), EGCG concentrations >1 μM progressively increased myocyte contractility and Ca2+ transient amplitude (Fig. 1). However, we noted that submicromolar EGCG already caused a robust increase in Ca2+ transient amplitude, resulting in a biphasic concentration-response relationship (Fig. 1). This result suggests that nanomolar EGCG has a different molecular target that contributes to its positive inotropic action. Thus, we next investigated the action of nanomolar EGCG in more detail. Representative traces are shown in Fig. 2A. EGCG (10 nM) significantly increases fractional shortening in intact myocytes (percentage fractional shortening, vehicle: 2.15 ± 0.33 versus EGCG: 5.61 ± 0.70; p < 0.05) (Table 1; Fig. 2B). The increase in contractility is explained by the significantly increased amplitude of Ca2+ transients compared with that of the cells exposed to vehicle (Fratio, vehicle: 0.26 ± 0.03, EGCG: 0.64 ± 0.15; p < 0.05). However, EGCG did not alter the decay kinetics of the Ca2+ transient nor the end-diastolic Ca2+ level between stimuli. Table 1 summarizes the effect of 10 nM EGCG on myocyte contractility and Ca2+ handling parameters. Next, we measured SR Ca2+ content by rapid caffeine (10 mM) application. EGCG significantly increased Ca2+ content compared with that of vehicle-treated myocytes (Fratio, vehicle: 0.63 ± 0.1 versus EGCG: 0.92 ± 0.1; p < 0.01). The decay of the caffeine-evoked transient was 25% slower in EGCG-treated cells than in the vehicle control (2.31 ± 0.17 versus 1.72 s−1; p < 0.05). Of interest, EGCG also significantly increased the fraction of SR Ca2+ released during each beat (p < 0.002) (Fig. 2C). In cardiac muscle, Ca2+ influx via L-type Ca2+ channels triggers Ca2+ release from the SR (Näbauer et al., 1989). Thus, we next tested whether EGCG-induced increased Ca2+ influx into the cell contribute to increased Ca2+ transients and SR Ca2+ content. EGCG (10 nM) had no effect on Ca2+ transients in myocytes incubated with 50 μM ryanodine and 10 μM thapsigargin (SR block; Supplemental Fig. 1, A and B). Next, we measured the effect of EGCG L-type Ca2+ channel activity using Ba2+ as the charge carrier, which does not activate RyR2 channels and therefore does not cause SR Ca2+ release (Ferreira et al., 1997). EGCG did not change Ba2+ currents (Supplemental Fig. 1, C and D) in myocytes. Taken together, our data suggested that nanomolar EGCG increased contractility of myocytes via directly enhancing SR Ca2+ release and increasing the Ca2+ content of the SR without changing L-type Ca2+ currents.
Fig. 2.
EGCG (10 nM) increases cardiomyocyte Ca2+ transients and contractility. A, examples of traces of Ca2+ fluorescence recordings from field-stimulated (1 Hz) cardiomyocytes after a 5-min exposure to EGCG (10 nM) or vehicle (water). Rapid caffeine application (10 mM) was used to estimate SR Ca2+ content. Fractional Ca2+ release was calculated as the ratio between the amplitude of the field-stimulated Ca2+ transient and caffeine-induced Ca2+ transient. B and C, comparison of average cardiomyocyte shortening (B) and fractional Ca2+ release (C). Data are from three independent myocyte preparations. n = 25/group, *, p < 0.05 versus vehicle.
TABLE 1.
Effect of EGCG (10 nM) on Ca2+ kinetics and sarcomere shortening in ventricular myocytes
n = 25.
| Vehicle (n = 31) | EGCG (n = 21) | |
|---|---|---|
| Ca2+ transient | ||
| Diastolic signal (Fratio) | 1.31 ± 0.04 | 1.35 ± 0.01 |
| Peak height (Fratio) | 0.26 ± 0.03 | 0.64 ± 0.15* |
| Time to peak (ms) | 26 ± 2 | 45 ± 4* |
| Time to 50% peak (ms) | 9 ± 1 | 10 ± 1 |
| τ (ms) | 340 ± 27 | 284 ± 25 |
| Caffeine peak height (Fratio) | 0.63 ± 0.1 | 0.92 ± 0.1* |
| Caffeine τ (s) | 1.72 ± 0.10 | 2.31 ± 0.17* |
| Cell shortening | ||
| Diastolic sarcomere length (μm) | 1.72 ± 0.01 | 1.75 ± 0.02 |
| % fractional shortening | 2.15 ± 0.33 | 5.61 ± 0.70* |
| Time to peak (ms) | 151 ± 12 | 119 ± 7* |
| Time to 50% peak (ms) | 43 ± 4 | 38 ± 4 |
p < 0.05 versus vehicle.
Nanomolar EGCG Has Negligible Effects on SR Ca2+-ATPase and Na+-K+-ATPase.
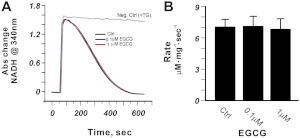
We next investigated the molecular mechanism(s) responsible for the EGCG-induced increase in Ca2+ transients and SR Ca2+ content. Previous studies in isolated myocytes have already shown that nanomolar EGCG does not alter myocyte Ca2+ influx via L-type Ca2+ channels (Kang et al., 2010); hence, we focused our studies on key Ca2+ handling proteins involved in SR Ca2+ regulation. The activity of SERCA2 in cardiac SR membranes was measured using a coupled enzyme assay that monitors the rate of oxidation of NADH at 340 nm as described previously (Ta et al., 2006). TG (0.2 μM) was included as the negative control, which indicated that >98% of the ATPase activity in the SR membrane preparations was attributable to SERCA2. EGCG (≤1 μM) had no influence on SERCA2 activity (Fig. 3). Together with the finding that the decay rate of whole-cell Ca2+ transients, a marker of SERCA2 activity in intact myocytes, was not changed by EGCG, these results show that altered SERCA2 function was not responsible for the EGCG-induced myocyte contractility.
Fig. 3.
EGCG <1 μM does not alter TG-sensitive SERCA2 activity in cardiac SR vesicles. A, sample traces. B, average data from n = 4 determinations. Abs, absorbance; Ctrl, control.
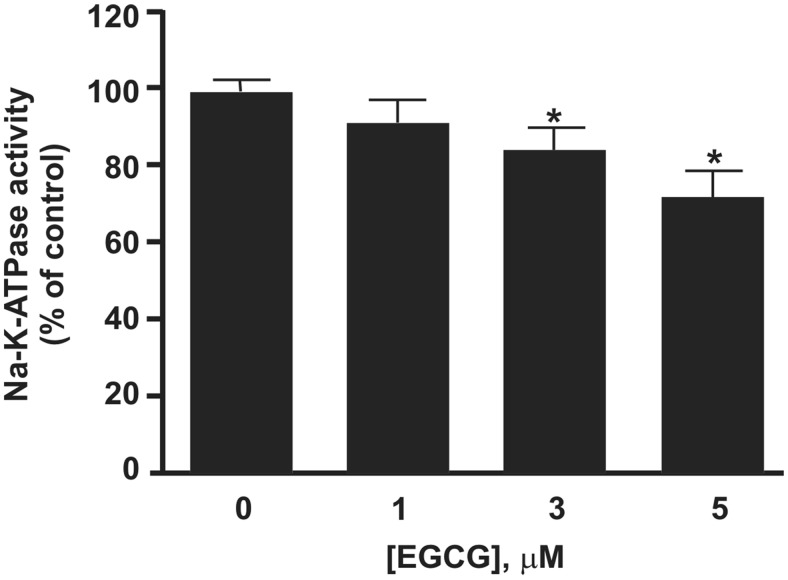
Na+-K+-ATPase activity importantly regulates intracellular [Na+]. Na+-K+-ATPase inhibition, e.g., by cardiac glycosides, increases intracellular [Na+] and thereby inhibits Ca2+ efflux via the NCX, which is a well established mechanism for increasing SR Ca2+ content and cardiac contractility (Demiryürek and Demiryürek, 2005). Previous work has demonstrated that micromolar EGCG inhibits Na+-K+-ATPase activity in human red blood cell membranes (Rizvi and Zaid, 2005). Thus, we next tested the effect of EGCG on Na+-K+-ATPase activity of whole cardiac membranes. EGCG had negligible effects at concentrations <3 μM, demonstrating that Na+-K+-ATPase is not a relevant target of nanomolar EGCG (Fig. 4).
Fig. 4.
EGCG concentration-response relationship on Na+-K+-ATPase activity measured in whole cardiac membranes. EGCG concentrations ≥3 μM were required to partially inhibit Na+-K+-ATPase activity (p < 0.05). Bars represent mean Na+-K+-ATPase activity of the cardiac membrane preparation relative to the control measured in the presence of dimethyl sulfoxide vehicle. n = 9 for each concentration.
Nanomolar EGCG Inhibits NCX.
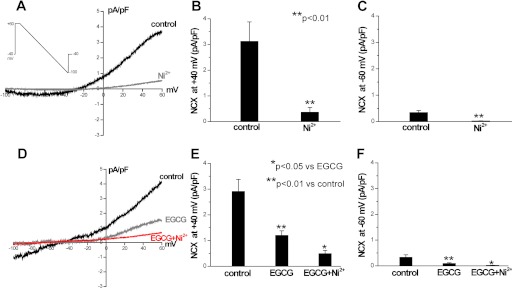
Because the decay of Ca2+ in the continued presence of caffeine is determined by Ca2+ extrusion via the NCX (Bers, 2000), the finding that EGCG significantly slows the decay of caffeine-induced Ca2+ transients (Table 1) suggests that EGCG inhibits NCX. To test this hypothesis directly, we measured NCX currents in voltage-clamped myocytes. NCX currents are quantified as the Ni2+-sensitive current in response to a voltage ramp (Woo and Morad, 2001; Reppel et al., 2007b). Application of 5 mM NiCl2 blocked NCX at all membrane potentials (Fig. 5, A–C). Next, we determined the effect of EGCG on NCX currents. Exposure to EGCG (10 nM) for 15 min significantly reduced both inward and outward NCX currents (Fig. 5, D–F) in myocytes. Addition of NiCl2 in the presence of EGCG caused a further reduction of NCX. The average effect of EGCG on Ni2+-sensitive NCX currents is summarized in Fig. 5F. Taken together, these results suggest that EGCG increases SR Ca2+ content by directly inhibiting NCX-mediated Ca2+ extrusion from the cell.
Fig. 5.
Nanomolar EGCG reduce NCX currents. A to C, examples and average data of NiCl2 (5 mM)-sensitive NCX currents in mouse ventricular myocytes. The voltage-clamp protocol is shown as an inset. Inward and outward NCX currents were compared at the membrane potentials of −60 and +40 mV, respectively. D to F, effect of EGCG (10 nM) on NCX currents. n = 4 myocytes/group. *, p < 0.05; **, p < 0.01.
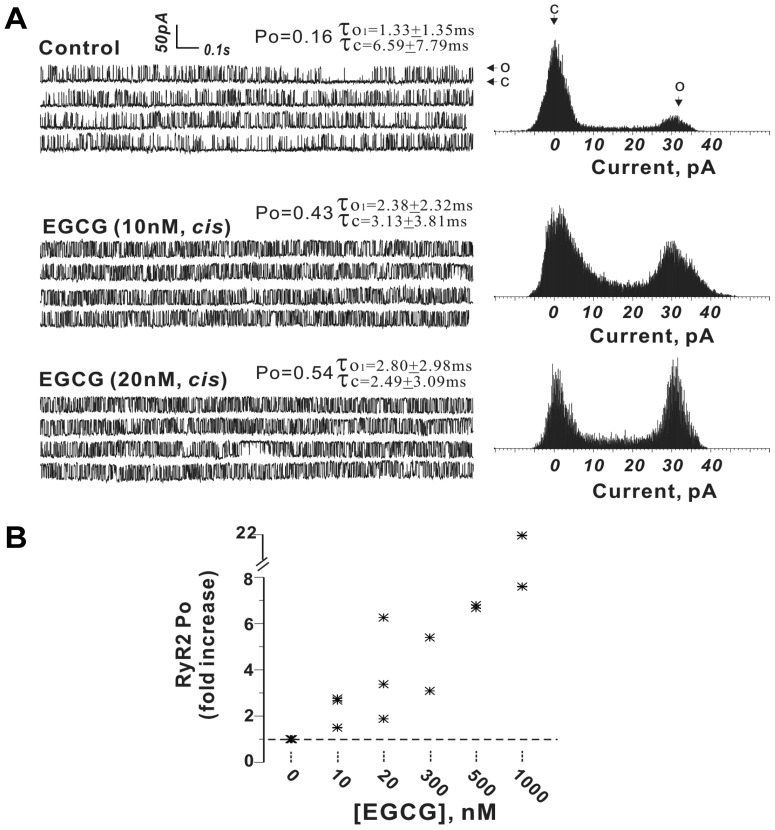
Nanomolar EGCG Is a Potent Activator of RyR2.
One possible explanation for the increased Ca2+ transients (Fig. 2) is that EGCG acts directly on RyR2 channels to enhance Ca2+ release. To test this hypothesis, RyR2 channels were reconstituted into bilayer lipid membranes (BLMs). The gating activity of RyR2 channels rapidly increased after addition of 10 nM EGCG to the cis chamber (cytoplasmic side of the channel). For example, during a continuous recording period of ∼3 min in the presence of 1 μM Ca2+ cis-100 μM trans, the RyR2 channel displayed a stable gating mode with an Po of 0.14 (Fig. 6). Upon addition of 10 nM EGCG to the cis solution, Po increased 2.3-fold (Po = 0.32) and subsequently increasing EGCG to a final concentration of 20 nM further increased Po to 0.47 (Fig. 6A). EGCG titrated from 10 nM to 1 μM caused a concentration-dependent increase in RyR2 channel activity (Fig. 6B).
Fig. 6.
Nanomolar EGCG enhances RyR2 channel open probability. A, representative current traces and corresponding current histogram showing channel gating behavior before and after sequential addition of 10 and 20 nM EGCG to the cis chamber. B, summary data from n = 9 independent channels.
EGCG Enhances the Sensitivity of RyR2 Channel to Ca2+ Activation.
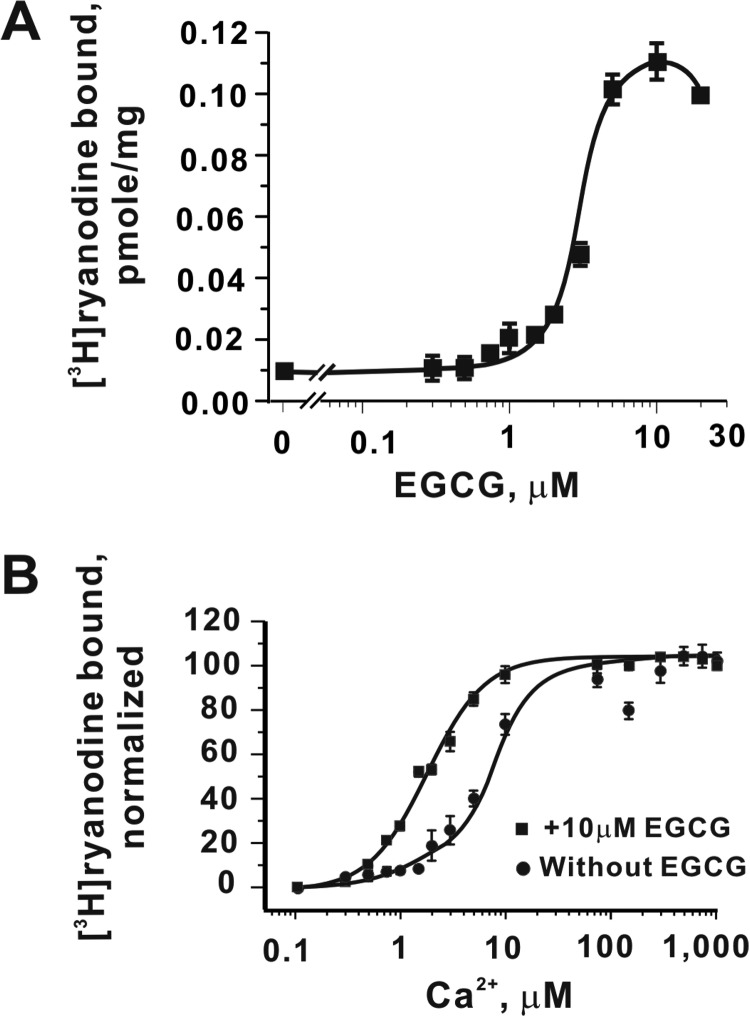
We next used high-affinity specific [3H]Ry binding as a biochemical tool to measure the dose-response relationship of EGCG to RyR2. EGCG increases the amount of [3H]Ry binding to cardiac SR preparations in a concentration-dependent manner, achieving an maximal effect at ≤10 μM compared with the control when measured in the presence of 1 μM free Ca2+ in the assay medium (Fig. 7A). To assess how EGCG influences the sensitivity of RyR2 to activation by Ca2+, we measured [3H]Ry binding in an assay buffer with free Ca2+ adjusted from 100 nM to 1 mM in the absence or presence of a saturating concentration of EGCG (10 μM). EGCG shifts Ca2+-dependent activation ∼3.5-fold to the left (EC50 1.8 ± 0.1 versus 6.2 ± 2.2 μM) (Fig. 7B).
Fig. 7.
EGCG sensitizes RyR2 channels to activation by cytosolic Ca2+. A, concentration-effect curve of EGCG on specific binding of [3H]ryanodine to cardiac SR membranes. B, EGCG (10 μM) significantly increases the sensitivity of [3H]ryanodine binding to Ca2+ in the assay buffer (EC50 = 6.2 ± 2.2 and 1.8 ± 0.1 for vehicle control and EGCG, respectively; p < 0.01). Data are means± S.D. of n = 3 determinations, each performed in duplicate.
Discussion
Green tea catechins are receiving increasing attention for their potential palliative properties in lowering the risk of cardiovascular disease (Chacko et al., 2010) and as potential therapeutic intervention in cardiovascular diseases (Wolfram, 2007; Babu and Liu, 2008; Mak, 2012). Here, we report a novel mechanism of action for EGCG, the major catechin of green tea: EGCG modulates the function of Ca2+ handling proteins in cardiac muscle, RyR2 Ca2+ release channels, and NCX. By enhancing Ca2+ release from SR intracellular Ca2+ stores, nanomolar EGCG enhance myocyte contractility and increase electrically evoked Ca2+ transients (Fig. 2; Table 1). The EGCG effects are selective and occur at concentrations that are probably relevant for human consumption of green tea. Inhibition of Na+-K+-ATPase activity only contributes to the positive inotropic effects observed at EGCG concentrations >1 μM.
Mechanism of Positive Inotropy of EGCG.
Our results in murine ventricular myocytes are consistent with the positive inotropic effects of EGCG reported previously using higher EGCG concentrations (Hotta et al., 2006; Lorenz et al., 2008). EGCG (10 μM) significantly increased left ventricular developed pressure in isolated guinea pig hearts and increased Ca2+ transient amplitude in guinea pig myocytes (Hotta et al., 2006). In rat cardiac myocytes, low micromolar EGCG increased fractional shortening and enhanced intracellular systolic Ca2+ releases (Lorenz et al., 2008). However, the conclusions reached regarding the molecular targets responsible for the observed EGCG effects diverged. A recent study indicates that EGCG concentrations of 30 μM or higher cause a negative inotropic effect by binding to troponin C and reducing myofilament Ca2+ sensitivity (Tadano et al., 2010). In the present study, we identify the cardiac SR Ca2+ release channel, RyR2, as one of the novel and selective targets of EGCG. Our single channel experiment clearly demonstrates that nanomolar EGCG directly enhance RyR2 activity (Fig. 6). EGCG primarily increases the RyR2 channel Po by prolonging open dwell time and decreasing closed dwell times, without promoting subconductance behavior. [3H]Ryanodine binding analysis indicates that a prominent effect of EGCG is to sensitize RyR2 to activation by Ca2+ (Fig. 7). EGCG has a very strong affinity for forming hydrogen bonds with phospholipid headgroups (Sirk et al., 2009). It is therefore not unexpected that the apparent potency of EGCG observed in enhancing the binding of [3H]Ry to SR membrane preparations, which have a high lipid content, is significantly lower compared with its apparent potency enhancing single-channel Po in the BLM preparation. In a recent study, nanomolar EGCG was also shown to sensitize RyR1 channel activity, and its actions were fully reversible (Feng et al., 2010).
In our study, we found that EGCG at a concentration ≤1 μM significantly activated RyR2 channels but had negligible effect on SERCA2 activity. This finding is consistent with another independent report, demonstrating that no significant effect on SERCA2 activity was observed with EGCG at a concentration <4.8 μM (Kargacin et al., 2011). Ca2+ influx via L-type Ca2+ channels triggers Ca2+ release from the SR (Näbauer et al., 1989). Pan et al. (2002) showed that EGCG had no effect on Ca2+ currents in bovine chromaffin cells. Likewise, EGCG concentrations of 30 μM or higher were required to inhibit L-type Ca2+ currents in guinea pig ventricular myocytes (Kang et al., 2010). Green tea catechins have high affinity for phospholipids and high concentration (≥30 μM) cause lipid vesicles to leak their contents (Caturla et al., 2003; Tamba et al., 2007; Sun et al., 2009). However, 0.01 to 10 μM EGCG clearly influences RyR2 activity without detectable disruption of BLM permeability (Fig. 6). Experiments in guinea pig hearts showed that EGCG (4 μM) had no effect on intracellular cAMP or cGMP and did not alter phosphorylation of phospholamban (Lorenz et al., 2008). Furthermore, our data suggest that EGCG elicits positive inotropic effects on ventricular myocytes at nanomolar concentrations that do not influence the activities of SERCA2, Na+-K+-ATPase, Ca2+ influx, and Na+-H+ exchanger (NHE) (Rizvi and Zaid, 2005).
Previous studies used pharmacological means to assess the mechanisms by which EGCG produced its positive inotropic actions on isolated myocytes (Hotta et al., 2006; Lorenz et al., 2008). EGCG-enhanced Ca2+ transients were significantly reduced by the antagonist of the NHE, methyl-N-isobutyl amiloride, leading Lorenz et al. (2008) to conclude that the positive inotropic effects of EGCG involve activation of NHE and NCX. However, EGCG concentrations >10 μM were required to inhibit the NHE directly, making it unlikely that NHE inhibition contributes to the inotropic effect of EGCG (Rizvi and Zaid, 2005). In our experiments, we measured NCX activity directly and found that EGCG inhibits NCX at nanomolar concentrations that have clear positive inotropic actions on mouse ventricular myocytes (Fig. 5). Of interest, EGCG at concentrations >1 μM inhibited Na+-K+-ATPase activity in cardiac muscle membranes (Fig. 4). Na+-K+-ATPase inhibition will cause intracellular Na+ retention in myocytes and result in increased SR Ca2+ content, analogous to the positive inotropic effects of cardiac glycosides. Similar actions of micromolar EGCG on Na+-K+-ATPase activity have been reported in human red blood cells (Rizvi and Zaid, 2005). Furthermore, the end-diastolic Ca2+ level was significantly increased only at EGCG concentrations greater than 10 nM in myocytes (data not shown). This result raises the possibility that chronic exposure and/or accumulation of EGCG may exert Ca2+ overload and Na+ retention in myocytes. Thus, inhibition of Na+-K+-ATPase activity and progressive Ca2+ and Na+ accumulation probably are responsible for the second increase in inotropic effect that occurs at EGCG concentrations >1 μM (Fig. 1). Because EGCG also directly activates RyR2 channels, higher EGCG concentrations could lead to spontaneous Ca2+ release, which can trigger ventricular arrhythmias (Knollmann et al., 2006). Thus, patients taking EGCG in high doses could be at risk for developing cardiotoxicity from arrhythmias (Chopra et al., 2009).
In conclusion, our data suggested that nanomolar concentrations of EGCG elicit positive inotropic effects on ventricular myocytes via actions on RyR2 and NCX, whereas micromolar concentrations of EGCG exert inotropic effects via Na+-K+-ATPase inhibition. Free plasma EGCG concentrations in humans range from nanomolar values after recreational green tea consumption up to micromolar values during chronic EGCG administration in clinical trials (Shanafelt et al., 2009). Therefore, our findings could be relevant for pharmacological effects of EGCG in humans.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL88635, HL71670]; the National Institutes of Health National Institute of Health Sciences [Grant ES04699]; and the American Heart Association [Grants EIA0840071N, 09POST2240022].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- EGCG
- (−)-epigallocatechin-3-gallate
- RyR2
- ryanodine receptor type 2
- NCX
- Na+-Ca2+ exchanger
- SERCA2
- Ca2+-ATPase
- SR
- sarcoplasmic reticulum
- Fura-2 AM
- Fura-2 acetoxymethyl ester
- [3H]Ry
- [3H]ryanodine
- TG
- thapsigargin
- BLM
- bilayer lipid membrane
- NHE
- Na+/H+ exchanger.
Authorship Contributions
Participated in research design: Feng, Hwang, Yang, Pessah, and Knollmann.
Conducted experiments: Feng, Hwang, Yang, Kryshtal, Padilla, Tiwary, and Puschner.
Performed data analysis: Feng, Hwang, Yang, Kryshtal, Padilla, Tiwary, and Pessah.
Wrote or contributed to the writing of the manuscript: Feng, Hwang, Yang, Pessah, and Knollmann.
References
- Babu PV, Liu D. (2008) Green tea catechins and cardiovascular health: an update. Curr Med Chem 15:1840–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. (2000) Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res 87:275–281 [DOI] [PubMed] [Google Scholar]
- Caturla N, Vera-Samper E, Villalaín J, Mateo CR, Micol V. (2003) The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med 34:648–662 [DOI] [PubMed] [Google Scholar]
- Chacko SM, Thambi PT, Kuttan R, Nishigaki I. (2010) Beneficial effects of green tea: a literature review. Chin Med 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Zima AV, Feng W, Schaefer S, Blatter LA, Pessah IN. (2004) NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ Res 94:478–486 [DOI] [PubMed] [Google Scholar]
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, et al. (2007) Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res 101:617–626 [DOI] [PubMed] [Google Scholar]
- Chopra N, Laver D, Davies SS, Knollmann BC. (2009) Amitriptyline activates cardiac ryanodine channels and causes spontaneous sarcoplasmic reticulum calcium release. Mol Pharmacol 75:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. (2005) Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res 11:4627–4633 [DOI] [PubMed] [Google Scholar]
- Demiryürek AT, Demiryürek S. (2005) Cardiotoxicity of digitalis glycosides: roles of autonomic pathways, autacoids and ion channels. Auton Autacoid Pharmacol 25:35–52 [DOI] [PubMed] [Google Scholar]
- Feng W, Cherednichenko G, Ward CW, Padilla IT, Cabrales E, Lopez JR, Eltit JM, Allen PD, Pessah IN. (2010) Green tea catechins are potent sensitizers of ryanodine receptor type 1 (RyR1). Biochem Pharmacol 80:512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Tu J, Pouliquin P, Cabrales E, Shen X, Dulhunty A, Worley PF, Allen PD, Pessah IN. (2008) Dynamic regulation of ryanodine receptor type 1 (RyR1) channel activity by Homer 1. Cell Calcium 43:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng WY. (2006) Metabolism of green tea catechins: an overview. Curr Drug Metab 7:755–809 [DOI] [PubMed] [Google Scholar]
- Ferreira G, Yi J, Ríos E, Shirokov R. (1997) Ion-dependent inactivation of barium current through L-type calcium channels. J Gen Physiol 109:449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CH, SubbaRow Y. (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–379 [Google Scholar]
- Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, et al. (2006) Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol 552:123–130 [DOI] [PubMed] [Google Scholar]
- Ikigai H, Nakae T, Hara Y, Shimamura T. (1993) Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta 1147:132–136 [DOI] [PubMed] [Google Scholar]
- Kang J, Cheng H, Ji J, Incardona J, Rampe D. (2010) In vitro electrocardiographic and cardiac ion channel effects of (−)-epigallocatechin-3-gallate, the main catechin of green tea. J Pharmacol Exp Ther 334:619–626 [DOI] [PubMed] [Google Scholar]
- Kargacin ME, Emmett TL, Kargacin GJ. (2011) Epigallocatechin-3-gallate has dual, independent effects on the cardiac sarcoplasmic reticulum/endoplasmic reticulum Ca2+ ATPase. J Muscle Res Cell Motil 32:89–98 [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, et al. (2006) Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116:2510–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M, Hellige N, Rieder P, Kinkel HT, Trimpert C, Staudt A, Felix SB, Baumann G, Stangl K, Stangl V. (2008) Positive inotropic effects of epigallocatechin-3-gallate (EGCG) involve activation of Na+/H+ and Na+/Ca2+ exchangers. Eur J Heart Fail 10:439–445 [DOI] [PubMed] [Google Scholar]
- Mak JC. (2012) Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol 39:265–273 [DOI] [PubMed] [Google Scholar]
- Näbauer M, Callewaert G, Cleemann L, Morad M. (1989) Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science 244:800–803 [DOI] [PubMed] [Google Scholar]
- Pan CY, Kao YH, Fox AP. (2002) Enhancement of inward Ca2+ currents in bovine chromaffin cells by green tea polyphenol extracts. Neurochem Int 40:131–137 [DOI] [PubMed] [Google Scholar]
- Pessah IN, Durie EL, Schiedt MJ, Zimanyi I. (1990) Anthraquinone-sensitized Ca2+ release channel from rat cardiac sarcoplasmic reticulum: possible receptor-mediated mechanism of doxorubicin cardiomyopathy. Mol Pharmacol 37:503–514 [PubMed] [Google Scholar]
- Pessah IN, Waterhouse AL, Casida JE. (1985) The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun 128:449–456 [DOI] [PubMed] [Google Scholar]
- Pessah IN, Zimanyi I. (1991) Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: evidence for a sequential mechanism in ryanodine action. Mol Pharmacol 39:679–689 [PubMed] [Google Scholar]
- Reppel M, Fleischmann BK, Reuter H, Sasse P, Schunkert H, Hescheler J. (2007a) Regulation of the Na+/Ca2+ exchanger (NCX) in the murine embryonic heart. Cardiovasc Res 75:99–108 [DOI] [PubMed] [Google Scholar]
- Reppel M, Reuter H, Sasse P, Hescheler J, Fleischmann BK. (2007b) NCX current in the murine embryonic heart: development-dependent regulation by Na+. Ann NY Acad Sci 1099:175–182 [DOI] [PubMed] [Google Scholar]
- Rizvi SI, Zaid MA. (2005) Impairment of sodium pump and Na/H exchanger in erythrocytes from non-insulin dependent diabetes mellitus patients: effect of tea catechins. Clin Chim Acta 354:59–67 [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, Secreto CR, Ghosh AK, Kabat BF, Lee MJ, et al. (2009) Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol 27:3808–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirk TW, Brown EF, Friedman M, Sum AK. (2009) Molecular binding of catechins to biomembranes: relationship to biological activity. J Agric Food Chem 57:6720–6728 [DOI] [PubMed] [Google Scholar]
- Stangl V, Dreger H, Stangl K, Lorenz M. (2007) Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc Res 73:348–358 [DOI] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. (1998) Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19:1771–1776 [DOI] [PubMed] [Google Scholar]
- Sun Y, Hung WC, Chen FY, Lee CC, Huang HW. (2009) Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys J 96:1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TA, Feng W, Molinski TF, Pessah IN. (2006) Hydroxylated xestospongins block inositol-1,4,5-trisphosphate-induced Ca2+ release and sensitize Ca2+-induced Ca2+ release mediated by ryanodine receptors. Mol Pharmacol 69:532–538 [DOI] [PubMed] [Google Scholar]
- Tadano N, Du CK, Yumoto F, Morimoto S, Ohta M, Xie MF, Nagata K, Zhan DY, Lu QW, Miwa Y, et al. (2010) Biological actions of green tea catechins on cardiac troponin C. Br J Pharmacol 161:1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamba Y, Ohba S, Kubota M, Yoshioka H, Yoshioka H, Yamazaki M. (2007) Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys J 92:3178–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Velotta JB, McDonough AA, Farley RA. (2001) All human Na+-K+-ATPase α-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol Cell Physiol 281:C1336–C1343 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho CT. (2009) Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem 57:8109–8114 [DOI] [PubMed] [Google Scholar]
- Wolfram S. (2007) Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr 26:373S–388S [DOI] [PubMed] [Google Scholar]
- Woo SH, Morad M. (2001) Bimodal regulation of Na+-Ca2+ exchanger by β-adrenergic signaling pathway in shark ventricular myocytes. Proc Natl Acad Sci USA 98:2023–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.