Abstract
Establishing if an adult’s resting energy expenditure (REE) is high or low for their body size is a pervasive question in nutrition research. Early workers applied body mass and height as size measures and formulated the Surface Law and Kleiber’s Law, although each has limitations when adjusting REE. Body composition methods introduced during the mid-twentieth century provided a new opportunity to identify metabolically homogeneous “active” compartments. These compartments all show improved correlations with REE estimates over body mass-height approaches, but collectively share a common limitation: REE-body composition ratios are not “constant” but vary across men and women and with race, age, and body size. The now-accepted alternative to ratio-based norms is to adjust for predictors by applying regression models to calculate “residuals” that establish if a REE is relatively high or low. The distinguishing feature of statistical REE-body composition models is a “non-zero” intercept of unknown origin. The recent introduction of imaging methods has allowed development of physiological tissue-organ based REE prediction models. Herein we apply these imaging methods to provide a mechanistic explanation, supported by experimental data, for the non-zero intercept phenomenon and in that context propose future research directions for establishing between subject differences in relative energy metabolism.
Keywords: Energy Metabolism, Body Composition, Mathematical Model
INTRODUCTION
The study of human energy metabolism is central to diverse fields of inquiry ranging from the basic biological sciences to clinical weight control management. A fundamental question in this field is if a subject’s resting energy expenditure (REE) is appropriate for their body size1–8. High or low REE values can signal underlying metabolic disease9, predisposition to weight gain or loss2, or metabolic processes that are unrelated to body size. This core question forms the basis of a research thread that extends for well over one century and continues to be debated today6–8. Herein, we review the evolution of concepts in this field and we supplement our report with new data analyses that reveal underlying current model limitations and that direct future potential research efforts.
REE Measurement
Resting energy expenditure is usually taken as a subject’s endogenous metabolic activity separate from the metabolic effects of food and activity. In this report we use the term REE in this context and recognize similar metrics such as resting, basal, and sleeping metabolic rates10.
Lavoisier and La Place were the first to measure animal heat production in 1780 using a “direct” ice calorimeter and five years later extended their research to humans11. Today human energy expenditure, the source of endogenous heat production, is typically measured in the laboratory with indirect calorimetry1 and REE is usually evaluated at thermoneutrality in the supine subject without movement at least 4 hours after the last meal and is reported as a 24 hour value12. Rigorous adherence to measurement conditions, including ensuring subjects avoid exercise, stressful situations, or stimulants at defined intervals prior to the test will ensure the quality of data collected. Classical indirect calorimetry involves measurement of oxygen consumption and carbon dioxide and urinary urea production rates, although most investigators now use an abbreviated approach that eliminates the need for urinary urea analyses.
REE PREDICTION
Body Size Models
The major REE determinant is body size, an observation first studied systematically in the late nineteenth century9. Methods for accurately evaluating body composition did not appear until the middle of the twentieth century13 and so early approaches for referencing REE focused on two measurable features of body size, weight and height.
Surface Area Law
Scientific advances during the late eighteenth century provided a physical framework for understanding natural phenomena. Sarrus & Rameaux in an 1838 French Royal Academy presentation14 and later Rameux’s 1858 publication15 advanced the concept that food intake varies as a power function of body weight14. Energy intake, according to the author’s hypothesis, must satisfy the need for energy or heat production, the latter varying as a function of body surface area (SA). Human body volume and thus mass (M) was considered to be a cubic function of height (H), with surface area considered to be proportional (∝) to H2. According to this construct, SA∝M2/3 or M0.66. Sarrus & Rameaux gave birth to the concept that REE∝SA or M0.66. Heavy subjects would therefore need to eat less relative to their body weight than do subjects who have a smaller body mass16.
Newton’s Law of Cooling provides a physical basis for the biological construct of Sarrus & Rameux: the body cools at a rate approximately proportional to the temperature difference between the heated human body at temperature T and the surrounding environment Ts
where A is surface area and α the coefficient of heat transfer17. According to this construct the amount of heat, or energy, produced must balance surface-area related losses in order to keep core temperature stable.
Rubner in 188318 first formally articulated the Surface Area Law based on his observation in fasting dogs that REE depends on surface area and is otherwise independent of body weight. These observations were later expanded to other species in 1901 by Voit19 and in 1938 by Benedict20. Dubois & Dubois derived the first widely used human surface area equation in 191621 and REE standards based on this and other surface area equations were developed and applied in clinical settings during the first half of the twentieth century. Rubner’s hypothesis was that neural signals in skin, which is proportional to surface area, stimulate cellular metabolic processes and heat production16.
Von Hoesslin in 1888 was among the first to question the validity of the Surface Area Law by showing that the rate of increase in heat production did not meet predictions when dogs were placed in reduced ambient temperatures22. Others also raised concerns with the Surface Area Law theoretical and empirical basis, but it was not until Kleiber and Brody’s seminal publications23, 24 that biologists began to look for alternative explanations for intra- and interspecies differences in REE, or more typically the metabolically similar basal metabolic rate.
Kleiber’s Law
Krogh in 1916 suggested that energy metabolism be expressed as kMp, with k and p constants25. By applying regression analysis methods to inter-species mammalian data, Kleiber23 and Brody in 193224 showed that REE ∝ kMp where p ranges from 0.70 to instead of p = 0.66 as would be predicted from Sarrus and Rameaux’s Surface Area Law. The inter-species “three-quarters power law” or “Kleiber’s Law” has since been the subject of extensive research and speculation related to mammalian biology26. A consequence of mammalian REE scaling as M0.75 is that smaller animals have a greater mass-specific REE (i.e., REE/M) than their larger counterparts and this observation is referred to as quarter-power scaling: REE/M∝M−0.25.
Limitations of Body Surface Area Approach
A logical outgrowth of these collective early observations is that between-subject human REE comparisons should be based on REE/Mp(0.66 ≤ p ≤ 0.75 or REE/SA. This approach assumes that adjusting for the right body mass metric will eliminate the metabolic effects of body size differences that are present between individuals and thus provide the investigator with an unbiased measure of relative REE. A critical analysis of this approach begins with the question how does REE scale to adult human body mass? Importantly, even if interspecies comparisons conform to the Surface Area Law or Kleiber’s Law, a REE model for humans developed solely from these laws may not capture inter-individual variance attributed to age, sex, gender, or race in humans10. An ideal REE model for humans should include the aforementioned potential covariates with the capacity for scaling by body size to a power.
To examine the question of REE scaling to adult body size, Heusner was among the first16, and he did so by analyzing data reported on the 238 subjects who participated in the classic 1919 Harris-Benedict REE study4. Heusner found that REE scaled to M with powers (X±SE) of 0.61±0.04 and 0.38±0.05 in men and women, respectively. The author then analyzed a cohort of 169 obese men and women reported by White and Alexander27 and respective M powers were 0.64±0.09 and 0.68±0.04.
Livingston and Kohlstadt28 evaluated data from the Harris-Benedict4 and Owen et al.29, 30 studies along with measurements of their own to develop an allometric REE prediction model and additionally explored the developed model using data published as a supplement to the Institute of Medicine’s (IOM) Macronutrient report31. The authors found a small sex difference in how REE scaled to M and their composite equations on 655 adults are: 202xM0.4722 with an R2 of 0.64 and an age (A)-adjusted version, 261M0.4456-(6.52xA), R2=0.68, both p<0.001.
Heymsfield et al.32 published REE scaling powers based on metabolic data from men and women reported in the IOM Macronutrient report and the New York Obesity Research Center (NYORC). The models with mass scaling powers were 4.92+M0.69-A0.14 for 429 IOM men 5.68+M0.44-A0.084 for 455 IOM women. The corresponding results for 147 NYORC men were 5.56+M0.53-A0.12 and for 197 NYORC women 5.70+M0.46-A0.11. These collective human studies reveal that the group mean powers observed when REE is scaled to body mass in adults, even after controlling for age, are highly variable and range widely, much more than the inter-specific Surface Area Law and Kleiber’s Law mass powers of 0.66 and 0.75, respectively. Expressing REE as a ratio to body mass alone raised to a power of 0.66 or 0.75 or to surface area may not appropriately adjust metabolism for between-subject differences in body size. In other words, adjusting energy metabolism as REE/M0.66–0.75 will create a size-dependent measure if REE actually scales to body size as M~0.45 as with Livingston-Kohlstadt28 or Heymsfield et al.32. A question that arises is why are the powers observed when REE is scaled to body mass so variable and in some cases far smaller in magnitude than predicted from either the Surface Area Law or Kleiber’s Law? This question is examined in a later section of our review.
Rather than taking a simple ratio to body mass, a pragmatic solution for adjusting REE to body size is to calculate a “predicted” REE from a well-defined reference group. A clinical subject or research group’s REE can then be compared to that of the reference group as a residual, Z, %, or any number of other comparative statistics1–3, 8,3, 33, 34. Many cross-sectional data sets were developed with the aim of referencing REE for surface area (i.e., weight and height), age, and sex35. Numerous statistical prediction formulas are used to reference REE for age, sex, and race, and countless research reports over the past century have used these formulas to see if a subject or group’s REE is high or low relative to body size.
Body Composition Models
Molecular and Cellular Levels
The next period of energy metabolism-body size research had roots in the late 19th century when animal and fetal chemical composition were first established, eventually leading to the discovery that the body contains tissues of varying metabolic activity. Ludwig Pfeiffer in 188736 first noted that the variability in animal body water content could be reduced if expressed on a fat-free basis. The concept of fat-free tissue was further elaborated on by Voit in 190119 and Rubner in 190237 who spoke of an “active” protoplasmic mass. Adolf Magnus-Levy reported in 190638 that ideally tissue composition be reported on a fat-free basis, giving rise to the concept of a fat-free mass (FFM). By 1937 Hastings and Eichelberger39 further refined the FFM concept by demonstrating that water is not bound by neutral fats, nitrogen, or electrolytes. Out of this initial work arose the important division of body mass into two-compartments, fat mass and FFM.
Prior to World War II, however, there were few approaches that could provide reliable estimates of total body fat and FFM in humans. Then, just prior to the war, Talbot in 1938 reported a relationship between skeletal muscle mass and 24-hour urinary excretion of creatinine40. Behnke and his colleagues a few years later in 1942 first described the now-classical two-compartment underwater weighing method that partitions body mass into fat and FFM41. Not long after, Pace and Rathbun in 1945 described the hydration constancy of FFM that provided the basis of the two-compartment hydrometry model42. Anderson and his colleagues in 1961 reported a fat estimation method based upon whole-body 40K counting43 and Moore and his colleagues in 1963 reported the use of total body potassium and exchangeable potassium for estimation of “body cell mass”44. The stage was thus set for moving beyond body weight and height as a means of adjusting REE for between-individual differences in body size.
Miller and Blyth in 1953 were among the first to search for body composition methods that could be used as a “suitable reference standard for the expression of basal oxygen consumption”—in other words, for REE45. Good correlations were observed in normal college students between “lean body mass” (i.e., largely FFM) measured by underwater weighing, urinary creatinine excretion, and whole-body basal oxygen consumption. The authors concluded from their findings that “lean body mass” appeared “characterized by a constant basal rate of oxygen consumption”.
During the same year in 195346 Keys and BroŽek advanced the concept of metabolically “active tissue” as the difference between body mass and primarily fat, extracellular fluid, and bone. This concept is similar to that articulated earlier by Voit19 and Rubner37 of an “active” protoplasmic mass. The most variable of the three “inactive” components, fat, was emphasized by Keys and Brozek as practical to measure and their experimental body composition observations gave impetus to the use of FFM as a means of adjusting REE for differences in body size. Keys and Brozek noted that the lower REE expressed per unit body weight or surface area in women compared to men would likely resolve if REE were to be expressed per unit FFM or ideally active tissue mass46. Behnke similarly expressed the view in 195347 that sex differences in the “standard metabolic rate” would resolve if lean body mass replaced body weight or surface area as a means for adjusting metabolic measurements for body size.
Keys and Brozek in their 1953 review lament that “no aspect of basal metabolism is more confused than the problem of the correct method of expressing basal metabolic rate in obesity”46. Subnormal values are observed when REE in the obese is referenced to either body weight or surface area and the authors speculated that the “problem would be simpler if the energy metabolism is correctly attributed to the cellular mass of the body”.
Von Dobeln first tested these hypotheses in 195648 by evaluating basal whole body oxygen consumption and FFM by underwater weighing in healthy young adult men and women. The author confirmed that men have a significantly greater VO2/kg body weight or VO2/M2 surface area compared to women. The women, however, had a greater VO2/kg FFM compared to the men (p<0.1). Seemingly contradictory observations such as these ushered in the modern era of human metabolic rate assessment.
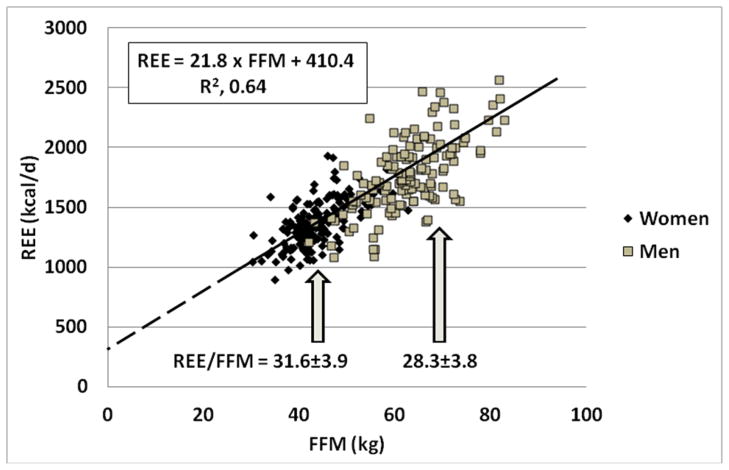
Von Dobeln’s observations six decades ago can be understood in the context of what is now known about the relationship between REE and FFM. As noted, REE should ideally be highly correlated with the body size-composition measure and the adjusted value of REE should be independent of body size. Consider the simple regression model REE = mFFM+b, with m slope and b y-axis (REE) intercept. For the ratio REE/FFM to be independent of FFM, the y-axis intercept must be zero so that REE=mFFM and thus REE/FFM is a stable value (i.e., m) across all healthy adults. In fact, when REE is regressed against FFM the b intercept term is positive and differs significantly in magnitude from zero1–3. Thus, women who on average have a smaller FFM than men have a larger REE/FFM ratio. This phenomenon is shown in Figure 1 with data taken from a previously published study by our group49. Resting energy expenditure for men and women is plotted against FFM and the resulting regression line has a significant intercept term of 410.4 kcal/d. According to this construct and as Von Dobeln showed, women have a larger REE/FFM than do men by about 10% (X±SD, 31.6±3.9 vs. 28.3±3.8 kcal/kg per day). But do women indeed have a relatively “greater” REE than do men after taking more than FFM into account?
Figure 1.
Resting energy expenditure (REE) versus fat-free mass (FFM) in 131 men and 158 women reported in an earlier study49. The respective REE/FFM ratios±SD for men and women are also presented in the figure.
One consideration is that adipose tissue, the compartment in which most fat or triglyceride resides, has a substantially lower mass-specific energy expenditure than adipose-tissue free mass (i.e., the tissue-organ level counterpart of FFM)50. Women have a higher fraction of their body weight as adipose tissue compared to men and not taking this large compartment into account when interpreting the REE/FFM ratio is one factor that can lead to the impression that women have a “high” metabolic rate for their body size.
Now the standard approach to account for sex differences in REE after adjusting for FFM is to add sex as a prediction model covariate in addition to fat mass, age, and race1–3, 51. The results are typically variable with respect to the significance of these covariates and the reported prediction model will reflect sample size and characteristics along with the methods used to measure REE and body composition. However, even after appropriately adjusting for fat mass, age, and race, women still are often found to have a REE that differs significantly from men2.
But fat-free mass has remained the main compartment used by investigators over the past several decades to adjust REE for body size, although Keys and Brozek recognized the need to remove low metabolic rate compartments other than fat from body mass as a means of estimating “metabolically-active” mass46. Might these other compartments be ideal measures for adjusting REE for between-individual differences in body size? Information on the relationships between REE and compartments beyond FFM is scant.
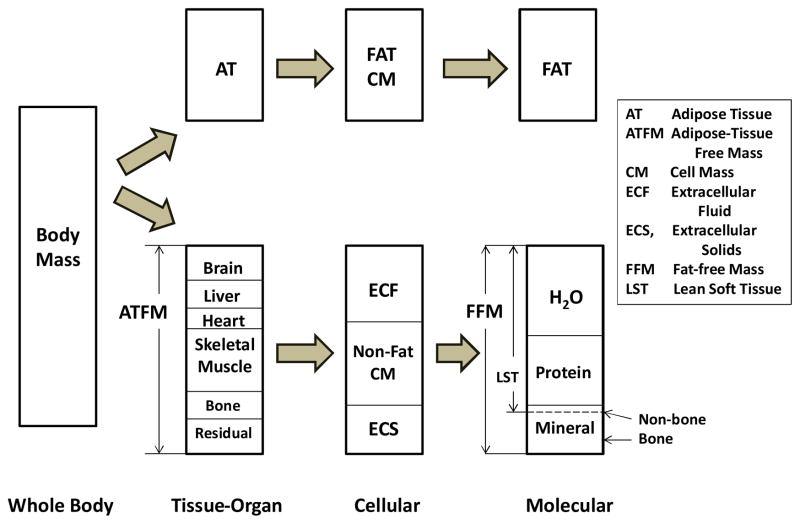
To explore this question the full scope of available body compartments needs to be defined first. Four body composition “levels” are relevant when considering REE: whole-body, tissue-organ, cellular, and molecular (Figure 2). Metabolic and body composition results from two previously published reports from our group52, 53 provided data for the descriptive examples that follow. In this work, subjects were healthy weight stable adults ranging widely in age and body mass index who participated in NYORC and Kiel body composition studies. Additional information on these groups and the measurement methods are provided in Supplementary Material.
Figure 2.
Major whole body compartments representing body mass across the tissue-organ, cellular, and molecular body composition levels as cited in this review.
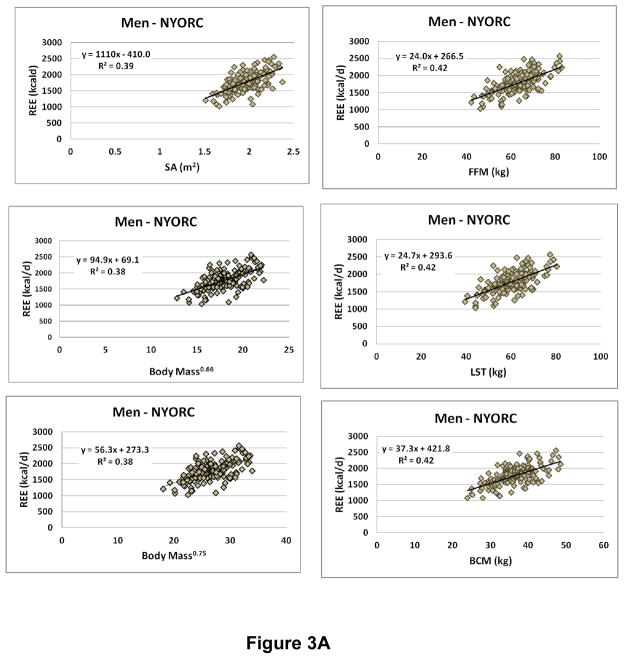
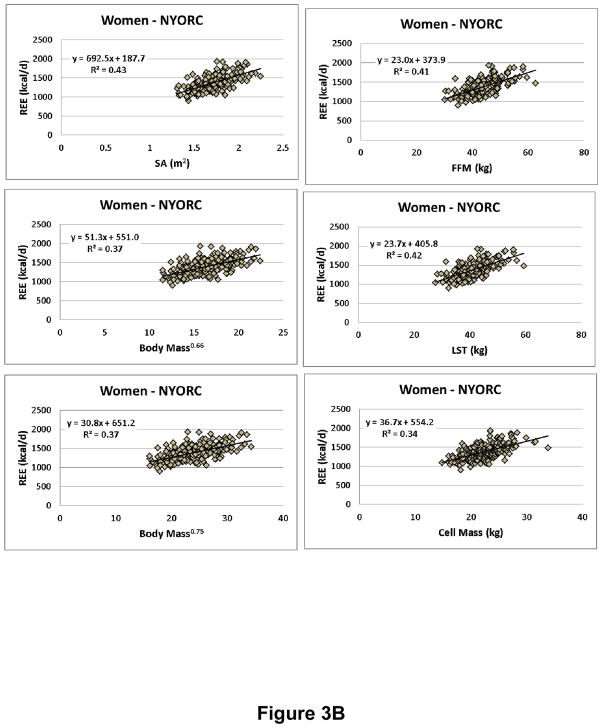
We evaluated the “metabolically active” compartment as suggested by Keys and Brozek at the molecular level as FFM and lean soft tissue (LST) mass, defined as the difference between FFM and the low energy expenditure compartment bone (i.e., bone mineral content, BMC). Both FFM and LST (i.e., FFM-BMC) were estimated with dual-energy x-ray absorptiometry (DXA). Cell mass (CM) at the cellular level was estimated from the whole-body potassium space using the 40K method as reported by Moore et al.44 and later refined by Wang et al.54. Moore considered the body’s cell mass as the actively metabolizing tissue devoid of fat, extracellular fluid, and bone minerals. We thus had three “metabolically-active” compartments for review (i.e., FFM, LST, and CM) in addition to the three provided by body mass and height, surface area, M0.66, and M0.75. The results for multiple regression analyses for REE vs. body size-composition are shown in Supplementary Table 1 and scatter plots are shown in Figure 3A (NYORC men) and 3B (NYORC women). Age was added as the only potential covariate to all models.
Figure 3.
Resting energy expenditure (REE) versus body mass, stature, and composition measures in A. men (n=154) and B. women (N=208) from the New York Obesity Research Center (NYORC) study (Supplementary Material). CM, cell mass; FFM, fat-free mass; LST, lean soft tissue; SA, surface area. Data are fit with simple power functions and all are statistically significant at p<0.001.
Within each sex and center (NYORC and Kiel) group the correlations (i.e., R-values) between REE and surface area, weight0.66, weight0.75, FFM, LST, and CM were approximately the same. Most of the models included age as a significant covariate and all had significant intercept terms (Supplementary Table 1). Thus, none of these measures meets the criteria for serving as an ideal REE reference since all relate to REE with a significant intercept. Qualitatively, the presence of a significant regression model intercept leads to the concern noted by von Dobeln48 and many others since55: compared to small subjects, “large” subjects have a lower magnitude REE/FFM. Thus, taking the ratio of REE to FFM, LST, or even CM raises the possibility that subjects who have a large body size have a relatively lower REE55.
The presence of a non-zero intercept requires the use of statistical REE adjustment approaches to establish if a subject or group has a “low” or “high” metabolic rate. The question arises, if we indeed can measure the “metabolically active” compartment of the body with great precision, why should there be a non-zero intercept when REE is regressed against FFM, LST, or CM?
Tissue-Organ Level
The resolution to this question may lie in the observation that body composition is more heterogeneous than a division into FM and FFM or even CM; a simple two-component model, even with covariates, does not account for the different metabolic activities of tissues and organs. For example, the brain is known to consume about one-fifth of a person’s REE, whereas bone is essentially metabolically inert50 even though both are components of FFM. This implies that to remove the intercept, one should ideally measure energy expenditure at the tissue-organ level.
The first major advance in measuring tissue and organ volumes occurred in 1971 with the introduction of computerized axial tomography (CT) by Hounsfield56. Accurate estimates of all major organ and tissue volumes were made possible by combining contiguous high resolution cross-sectional images50. The reconstructed volume estimates were then converted to mass with an assumed tissue density (Table 1). Magnetic resonance imaging (MRI) was introduced in the early nineteen seventies by Lauterbur, Mansfield, and Damadian56. As with CT, MRI provides high resolution cross sectional images that can be used to reconstruct tissue and organ volumes50.
Table 1.
Tissue and organ basal mass-specific metabolic rates (kcal/kg/d) and density*.
The availability of tissue-organ level (Figure 2) estimates provided a new opportunity to develop physiological REE prediction formulas based upon established heat production rates of major body tissues. The concept underlying these models is simple: tissue and organ mass can be estimated with CT or MRI; the energy expended by each tissue and organ can be calculated as the product of mass and mass-specific metabolic rate, the latter based on values compiled from in vivo and in vitro observations50; and REE can then be calculated as the sum of all tissue and organ metabolic rates.
A challenging aspect of these models is derivation of the tissue and organ mass-specific metabolic rates. The relative contributions of tissues and organs to energy expenditure was first studied, largely in intact animals or in isolated preparations, by Barcroft in 190857 and later in 1939 by Field et al.58 The 1940s ushered in a new era in measurement of human tissue and organ oxygen consumption as typified by the classic study of Kety and Schmidt in 194559. The authors were the first to estimate brain mass-specific oxygen consumption in unanesthetized healthy young men (3.7 ml/100 g/min) using the nitrous oxide method to measure cerebral blood flow59. Assuming that one liter of consumed oxygen translates to 4.9 kcal60, 61, brain mass-specific energy expenditure in these subjects was calculated as 260 kcal/kg/day. Data for other tissues and organs appeared in the literature during this period and by 1950 authors such as Drabkin were tabulating estimated oxygen consumption values for brain, heart, liver, kidneys, and skeletal muscle62. Brozek and Grande reported human mass-specific oxygen consumption values for these five compartments with additional refinements in 195563. Holliday et al. in 1967 extended these efforts by calculating organ mass-specific metabolic rates to produce the first physiological tissue-organ REE model for humans60. Elia in 1992 derived mass-specific organ metabolic rates for liver, brain, heart, kidneys, skeletal muscle, adipose tissue, and residual mass, calculated as the difference between body mass and the sum of the measured tissues and organs64. Gallagher et al. in 199850 evaluated Elia’s physiological model with tissue and organ volumes measured with MRI in healthy young non-obese adults and found excellent agreement between “calculated” and measured REE50. Gallagher’s approach was to calculate the REE of each organ as the product of MRI-measured mass and assumed mass-specific metabolic rate and to then sum the seven estimated whole-body REE components (Table 1). These observations have since been confirmed and expanded upon by Wang et al.65, 66, Later et al.67, and Muller et al.68 and the mass-specific metabolic rates reported by Elia have been found applicable across men and women69 but values are slightly lower in obese65 and elderly subjects (Table 1)66.
Although the relative contribution of tissues and organs to REE was thus known and appreciated for almost one century, the major breakthrough with respect to physiological REE model development occurred when MRI became available as a means of safely quantifying body composition across the entire human lifespan.
The most striking feature of viewing REE from the tissue-organ perspective is the large contributions of four FFM components (brain, liver, heart, and kidneys) to whole body REE. While <6% of representative body mass, these organs contribute to 60–70% of REE50, 63. Lungs, spleen, and other organs that are relatively small in mass also likely have high mass-specific metabolic rates. Physiological REE model terms for brown fat and the microbiome are not yet available.
In light of this emerging information on human energy metabolism it is worth reconsidering early observations on the relationships between REE and body size and composition. Not only might accounting for organ energy expenditure reduce between-subject variability in REE, but it also might allow REE to form a ratio to body composition that is independent of body size55, 70. Indeed, a growing literature supports the hypothesis that tissue-organ proportions vary with body size and adult age71, across men and women72, and between race groups73. Several representative examples are presented here.
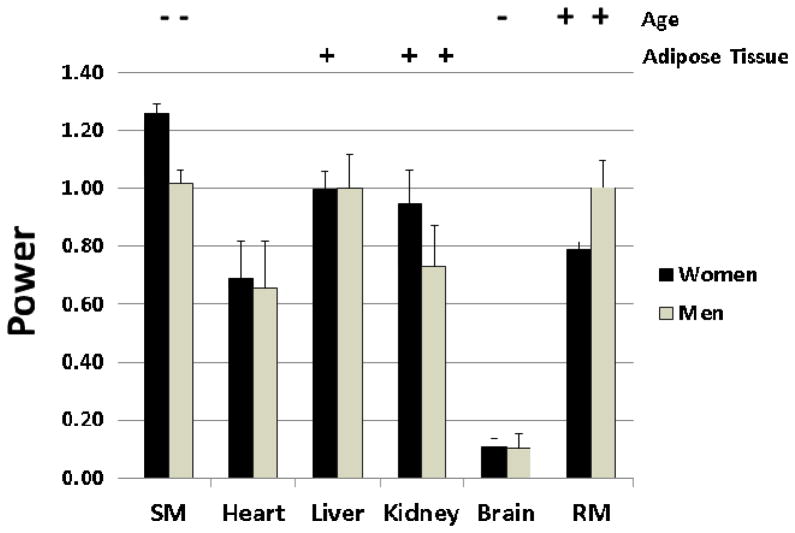
Brain mass, which accounts for about 20% of REE50, 71, is only weakly associated with adult body mass, stature, and FFM74. After controlling for FFM and age, tall subjects and women have a relatively smaller brain mass (i.e., as brain/M or FFM) than short subjects and men, respectively74. An example of how brain mass differs in relation to other major tissues and organs is shown for the Kiel subjects in Figure 4. Multiple regression analysis was used to establish how skeletal muscle, heart, liver, kidney, brain, and residual mass (weight minus the sum of other tissues, including adipose tissue, and organs) scale to FFM. Tissue-organ mass was set as the dependent variable and FFM, age, and adipose tissue mass as potential predictor variables in multiple regression models. A FFM power of 1.0 (i.e., tissue-organ ∝ FFM1) generally implies that the tissue and organ change in proportion to FFM. As shown in the figure, all tissues and organs scale to FFM with powers approaching 1.0 except brain, which has powers <0.2 for both men and women. The implication is that the fraction of FFM as brain scales inversely to FFM. In other words, the fraction of FFM as brain is smaller in subjects with a large FFM. Each tissue and organ is additionally associated with age and adipose tissue mass as other FFM predictor variables. This simple descriptive analysis begins to reveal the tissue-organ and thus mass-specific energy expenditure heterogeneity of FFM.
Figure 4.
Powers of Kiel Study (Supplementary Material) tissue-organ level components adjusted for significant age and adipose tissue mass effects observed when scaled to adipose-tissue free mass. The presence of a significant positive (+) or negative (−) age or adipose tissue covariate is noted in the figure. Results are shown ±SE. RM, residual mass; SM, skeletal muscle mass.
The tissue-organ model may also allow us to reduce the impact of or do away with FFM-based REE prediction model covariates such as age and race. For example, old subjects have a smaller fraction of FFM as skeletal muscle compared to young subjects75, while the fraction of FFM as skeletal muscle is greater in African Americans than Caucasians73.
Hence, the emerging view is that compartments such as “active protoplasmic mass” and FFM are not metabolically homogeneous but rather vary systematically in tissue-organ proportions with such factors as age, sex, and race.
One approach for establishing the appropriateness of an absolute REE measurement for body size and composition is to calculate a predicted value for REE using measured organ mass and the mass-specific metabolic rates presented in Table 1. The measured values can then be compared in control and active intervention groups to the predicted REE values50.
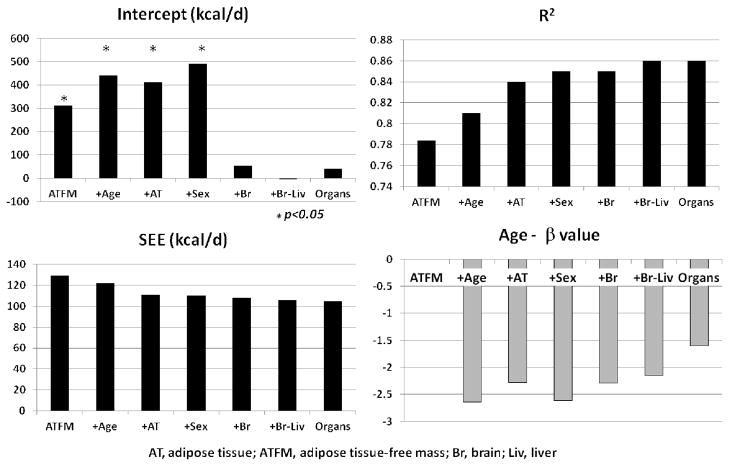
One explanation for the non-zero intercept for the REE-FFM function is that FFM is not a metabolically homogeneous compartment but instead FFM varies systematically in tissue-organ proportions as a function of body size70. The availability of tissue-organ and REE measurements from the Kiel Study allows us to test this hypothesis. Here we used the tissue-organ model approach (Figure 2) with adipose tissue and adipose-tissue free mass (ATFM) corresponding to molecular level fat and FFM, respectively. The first step was to derive the traditional REE model with ATFM alone as the predictor variable (model A: R2 = 0.78 and SEE = 129.4 kcal/d, Table 2 and Figure 5). Model R2 increased and SEE decreased with addition first of age (model B: R2, 0.81;SEE, 121.9) and the adipose tissue mass (model C: R2, 0.84;SEE, 112.2). Sex added as a borderline significant (model D: p=0.08) covariate to the REE predictor model with ATFM, age, and adipose tissue mass as significant covariates. All four of these models had statistically significant intercepts and they resemble the general form of traditional FFM-based REE prediction models1–3, 55.
Table 2.
Regression models developed for Kiel subjects for REE vs. tissue-organ level body composition.
| Model | R (R2) | SEE | P |
|---|---|---|---|
| A. 311.0†+24.4ATFM | 0.88 (0.78) | 129.4 | <0.001 |
| B. 442.2†+24.0ATFM-2.6A | 0.90 (0.81) | 121.9 | <0.001 |
| C. 411.0†+22.1ATFM+4.3AT-2.3A | 0.92 (0.84) | 112.2 | <0.001 |
| D. 491.4†+19.4ATFM+6.2AT+72.7S*-2.6A | 0.92 (0.85) | 110.0 | <0.001 |
| E. 54.4+19.8ATFM+5.3AT+303.7Br-2.3A | 0.92 (0.85) | 108.0 | <0.001 |
| F. −6.1+17.3ATFM+4.5AT+326.9Br+112.8Li-2.1A | 0.93 (0.86) | 106.4 | <0.001 |
| G. 40.6+5.1AT+294.2Br+141.5Li+20.3SM+13.7RM-1.6A | 0.93 (0.86) | 105.9 | <0.001 |
N= 37 men and 66 women.
p=0.08;
intercept p<0.05
Abbreviations: A, age; AT, adipose tissue; ATFM, adipose tissue-free mass; Br, brain; Li, liver; S, sex.
Figure 5.
Characteristics of multiple regression models predicting REE in Kiel Study (Table 2 and Supplementary Material) participants. The simplest model predicts REE from adipose-tissue free mass (ATFM) with subsequent addition of age, adipose-tissue (AT), sex, brain mass (Br), and liver mass (Liv) as potential covariates. The final model included brain, liver, kidneys, spleen, heart, skeletal muscle, bone, adipose tissue, and residual mass as potential covariates with brain, liver, skeletal muscle, bone, adipose tissue, residual mass, and age remaining as significant predictor variables. Of the added potential predictor variables, brain, liver, skeletal muscle, bone, adipose tissue, residual mass, and age added as significant covariates. The age panel depicts the β values for age observed across the seven models.
We next added brain mass to this last model, knowing from our earlier example that brain relates substantially differently to FFM than do other tissues and organs. Adding brain mass to the model further decreased the SEE (model E: 108.0) and substantially reduced the intercept from 411.0 to 54.4, which was then non-significant. This observation is consistent with similar findings on an independent database reported by Javed et al.76. When high metabolic rate liver mass was then added to the expanded ATFM-centered model, the R2 increased further (model F: 0.86), SEE decreased (106.4), and the intercept term remained non significant at −6.1. Sex did not add to either of these organ-supplemented statistical models although age remained a significant covariate. We lastly excluded the composite ATFM compartment and developed a REE prediction model with brain, liver, kidneys, spleen, heart, skeletal muscle, bone (as BMC), adipose tissue, and residual mass along with age and sex as potential predictor variables. Of these, brain, liver, skeletal muscle, bone, adipose tissue, residual mass, and age added as significant covariates. This last model (G) had the highest R2 (0.86) and lowest SEE (105.9) of the developed series of REE prediction models with a non-significant intercept of 40.6.
Several new observations emerge from this series of analyses. First, building cross-sectional REE prediction models around compartments such as FFM, and by inference, LST and CM, fails to capture the metabolic heterogeneity that ultimately yields an individual or groups REE. Simply adding brain mass to the “traditional” REE prediction model with ATFM, age, and sex largely eliminated the significant intercept term inherent in these models. Liver, another high metabolic rate organ, also added significantly with brain to the traditional ATFM-based model with elimination of the borderline significant sex term. An opportunity clearly exists to expand these kinds of cross-sectional statistical REE prediction models with larger and more diverse subject groups and to longitudinally monitored cohorts. We can pose questions such as why is liver so variable in mass between individuals such that its effect on REE is significant even after controlling first for ATFM? Does brain mass, which changes little with body size, explain the “non-zero” intercept phenomenon or are other factors involved? Very little is known about how organs change in size with weight gain or loss or with aging and if they remain stable proportions of ATFM.
A second observation is that even though we accounted for all major organs and tissues in our final example model, age remained a significant predictor of REE (Figure 5); older subjects are projected to have a lower REE even after controlling for the mass of all major measured organs and tissues as previously reported by Gonzalez et al.77. Herein lays a fundamental limitation of conventional CT or MRI: measured values reflect tissue volume and not composition. Taking liver as an example, we can estimate total liver volume but we cannot account for liver composition in our study. Fat infiltration, collagen deposition, and any number of other components might influence liver composition and thus mass-specific energy expenditure in a manner that cannot be accounted for by measured total liver volume. With the perspective of aging, we ideally would like to have a measure of liver fat-free cell mass. A related effect is that all lean organs and tissue have some lipid (fat), such as in cell membranes, and the fractional amount might vary under different conditions such as noted for liver. Among all organs, brain has the highest fractional lipid content and it is unclear if the reported proportion (Supplementary Table 2) is stable between individuals. Imaging methods, advancing at a rapid pace, might in the near future provide measures of fat-free organ mass.
Returning now to the classical question, why are such variable powers observed when REE is scaled to body mass in adult humans, in some cases far different in magnitude than predicted from either Surface Area of Kleiber’s Laws? Here we can gain insights into these associations through the newly acquired knowledge developed though our analyses of REE links to tissue-organ body composition. Our fundamental model is REE∝kMp and we can consider adult body mass developing along two planes, one as a function of height and the other related to adiposity. With greater adult height, Quetelet’s rule states that M∝H2 53. Body mass index (W/H2) can serve as a measure of adiposity and we can estimate REE for any given body mass, height, and age using the sex-specific Harris-Benedict equations4.
We first examine how REE varies across subjects differing in height. Beginning with an arbitrary fixed BMI (e.g., 24 kg/m2) and age (25 yrs), we can model how REE scales to M by systematically varying H (Supplementary Material). Our model reveals mass powers of 0.81 and 0.51 in men and women, respectively. If we instead lower BMI in our model to 20 kg/m2, respective powers increase to 0.83 and 0.54. For perspective, recall that adults differ in relative tissue-organ proportions across height53 and between men and women74. These underlying tissue-organ patterns begin to explain the differing empirically observed REE-body mass scaling patterns noted earlier in our review.
We next model REE as a function of adiposity by keeping height constant and systematically varying BMI by changing body mass. Resting energy expenditure now scales to body mass with powers of 0.61 and 0.43 in men and women, respectively. These substantially lower body mass powers presumably reflect a very different mix of low mass-specific adipose tissue50 and other higher metabolic rate organs and tissues than observed in our variable height model. If we combine our height and adiposity models across men and women we find that REE ∝ M0.54. Real populations are obviously far more complex with large variations in height, body mass, age, race, and many other factors. Varying adiposity while keeping height constant thus presents a distinct pattern of changes in tissue and organ proportions that has yet to be fully identified.
An obvious conclusion from this simple modeling exercise is that the scaling relationship between REE and body mass depends on the distribution of body mass across subjects varying in stature, adiposity, or a combination of the two. Stature and adiposity variation each brings differing tissue-organ relationships into play. The notion of a universally applicable scaling relationship between REE and body mass in humans is likely an elusive goal.
EMERGING CONCEPTS
Our historical overview and entry into the current era with technological advances point directly to the next potential wave of research aimed at establishing the basis for individual differences in REE after controlling for body size. We have now shown the complexity of body size-composition relations as defined by adult human energy expenditure. Clearly, these relations negate the likelihood of finding a simple and practical ratio-based means of universally adjusting REE for differences in body size and composition. However, if we return to the key question, if and how subjects differ in REE after controlling for body size and composition, our focus extends beyond the whole body to individual tissues and organs. We might ideally capitalize on emerging non-invasive technologies to measure individual organ mass-specific metabolic rates and cell mass. Rather than focus on the whole body and the associated complexities that come with that process, we might ask instead if brain, liver, or any other organ’s metabolic rate is appropriate for its cell mass. As noted earlier, we can now measure the volume of individual tissues and organs with great accuracy. A new line of research needs to extend this work to organ tissue composition, notably separating the metabolically active portions of cells from other less active or metabolically inactive components. A second line of research needs to focus on existing or new methods of non-invasively quantifying organ mass-specific metabolic rates, of which there are several examples78, 79. Armed with these new methods, studies can focus on how the energy expended by each organ change with perturbations in energy balance, including weight gain and loss brought about by different dietary measures and physical activity regimens.
While our review has focused on REE evaluation in humans, an intense parallel discussion is ongoing among investigators exploring aspects of energy balance components in animal models6–8. Many of the same critical issues of REE adjustment for body size are at play and the potential advances described in this report are directly applicable to animal research.
CONCLUSIONS
The question of how REE relates to adult human body size and composition has been of interest to workers in diverse research areas for well over one century. A new emphasis is appearing on if and how subtle REE variation can be accounted for by genetic and metabolic processes that are independent of body composition. As shown in our review, the field has evolved in stages driven by new physical theories and measurement technologies. We now stand at the horizon of the next potential advance beyond the whole body to evaluate the REE of individual tissues and organs. Developments in this area are well under way and this tissue-organ focus will likely finally resolve many lingering questions related to biological factors that influence an individual’s energy expenditure.
Supplementary Material
Acknowledgments
DMT is supported by a Herman and Margret Sokol Institute for Pharmaceutical Life Sciences Fellowship and NIH grants R15 DK090739, U01 DK094418.
Footnotes
Disclosures: None of the investigators report conflicts of interest for this study.
References
- 1.Bogardus C, Lillioja S, Ravussin E, et al. Familial dependence of the resting metabolic rate. N Engl J Med. 1986;315:96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- 2.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–75. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 4.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Carnegie Institution of Washington; 1919. [Google Scholar]
- 5.Kleiber M. Huntington, N.Y: R. E. Krieger Pub. Co; 1975. The fire of life: an introduction to animal energetics. Rev. edn. [Google Scholar]
- 6.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–9. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschop MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60:17–23. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lusk G. The elements of the science of nutrition. 2. Philadelphia and London: W. B. Saunders company; 1909. [Google Scholar]
- 10.Durnin FVGA. Report of a joint FAO/WHO/UNU Expert Consultation on Energy and protein requirements. University of Glasgow; Glasgow: 1981. Basal Metabolic Rate in Man. [Google Scholar]
- 11.Audrey HE. Concise encyclopedia of foods and nutrition. CRC Press; 1995. [Google Scholar]
- 12.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Nutrition, Energy, and Human Performance. Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 13.Forbes GB. Human body composition: growth, aging, nutrition, and activity. Springer-Verlag; New York: 1987. Human Body Composition; pp. 28–49. [Google Scholar]
- 14.Se Rameaux. Bulletin de l’Académie royale de medécine de Belgique. Académie royale de medécine de Belgique; Bruxelles: 1839. Rapport sur un Memoire adresse a l’ Academie royale de medecine; pp. 1094–100. [Google Scholar]
- 15.Rameaux J-F. Des lois suivant lesquelles les dimensions du corps, dans certaines classes d’animaux, déterminent la capacité et les mouvements functionnels des poumons et du coeur. Bruxelles: 1858. [Google Scholar]
- 16.Heusner AA. Body size and energy metabolism. Annu Rev Nutr. 1985;5:267–93. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- 17.Winterton RHS. Newton’s law of cooling. Contemporary Physics. 1999;40:205–12. [Google Scholar]
- 18.Rubner M. Ueber den Einfluss der Korpergrosse auf Stoff- und Kraftwechsel. In: Kuhne W, Voit C, editors. Zeitschrift für Biologie. The University of California; 1883. pp. 535–62. [Google Scholar]
- 19.Voit E. Die Bedeutung des Körperfettes für die Eiweifszersetzung des hungernden Tieres. Z Biol. 1901:502–49. [Google Scholar]
- 20.Benedict FG. Vital energetics; a study in comparative basal metabolism. Washington: 1938. [Google Scholar]
- 21.Du Bois D, Du Bois EF. Clinical Calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII:863–71. [Google Scholar]
- 22.Hoesslin Hv Physiologische Gesellschaft zu B. Archiv für Physiologie. Veit & Comp; 1888. Ueber die Ursache der scheinbaren Abhängigkeit des Umsatzes von der Grösse der Körperobernäche; pp. 323–79. [Google Scholar]
- 23.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–51. [Google Scholar]
- 24.Brody SRCP. Relation between basal metabolism and mature body weight in different species of mammals and birds. M Agric Exp Stn Res Bull. 1932;166:89–101. [Google Scholar]
- 25.Krogh A. The respiratory exchange of animals and man. London; New York: Longmans, Green; 1916. Katherine Golden Bitting Collection on Gastronomy (Library of Congress) [Google Scholar]
- 26.Glazier DS. A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev Camb Philos Soc. 2010;85:111–38. doi: 10.1111/j.1469-185X.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- 27.White RI, Alexander JK. Body oxygen consumption and pulmonary ventilation in obese subjects. J Appl Physiol. 1965;20:197–201. [Google Scholar]
- 28.Livingston EH, Kohlstadt I. Simplified Resting Metabolic Rate-Predicting Formulas for Normal-Sized and Obese Individuals[ast][ast] Obesity. 2005;13:1255–62. doi: 10.1038/oby.2005.149. [DOI] [PubMed] [Google Scholar]
- 29.Owen OE, Kavle E, Owen RS, et al. A reappraisal of caloric requirements in healthy women. The American Journal of Clinical Nutrition. 1986;44:1–19. doi: 10.1093/ajcn/44.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Owen OE, Holup JL, D’Alessio DA, et al. A reappraisal of the caloric requirements of men. The American Journal of Clinical Nutrition. 1987;46:875–85. doi: 10.1093/ajcn/46.6.875. [DOI] [PubMed] [Google Scholar]
- 31.Council NR, editor. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; 2005. [Google Scholar]
- 32.Heymsfield SB, Childers D, Beetsch J, Allison DB, Pietrobelli A. Body size and human energy requirements: reduced mass-specific resting energy expenditure in tall adults. J Appl Physiol. 2007;103:1543–50. doi: 10.1152/japplphysiol.00461.2007. [DOI] [PubMed] [Google Scholar]
- 33.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–52. [PubMed] [Google Scholar]
- 34.Poehlman ET, Toth MJ. Mathematical ratios lead to spurious conclusions regarding age- and sex-related differences in resting metabolic rate. Am J Clin Nutr. 1995;61:482–5. doi: 10.1093/ajcn/61.3.482. [DOI] [PubMed] [Google Scholar]
- 35.Bruen C. Variation of basal metabolic rate per unit surface area with age. The Journal of General Physiology. 1930;13:607–16. doi: 10.1085/jgp.13.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer L. Ueber den Fettgehalt des Körpers uud verschiedener Theile desselben. In: Kühne W, Voit C, editors. Z Biol. 1887. pp. 340–80. [Google Scholar]
- 37.Rubner M. Die Gesetze des Energieverbrauchs bei der Ernährung. Leipzig: F. Deuticke; 1902. [Google Scholar]
- 38.Magnus-Levy A. Physiologie des Stoffwechsels. In: Noorden KH, Czerny A, editors. Handbuch der pathologie des stoffwechsels: Unter mitwirkung von Adalbert Czerny [u a] A Hirschwald; 1906. pp. 1–16. [Google Scholar]
- 39.Hastings AB, Eichelberger L. The exchange of salt and water between muscle and blood. J Biol Chem. 1937;117:73–93. [Google Scholar]
- 40.Talbot NB, Broughton F. Measurement of obesity by the creatinine coefficient. Am J Dis Child. 1938;55:42–50. [Google Scholar]
- 41.Behnke AR, Feen BG, Welham WC. The Specific Gravity of Healthy Men. Journal of the American Medical Association. 1942;118:495–98. [Google Scholar]
- 42.Pace N, Rathbun EN. Studies on Body Composition. J Biol Chem. 1945;158:685–91. [Google Scholar]
- 43.Anderson EC, Langham WH, Forbes GB, Hursh JB. Estimation of Total Body Fat from Potassium-40 Content. Science. 1961;133:1917–18. doi: 10.1126/science.133.3468.1917. [DOI] [PubMed] [Google Scholar]
- 44.Moore FD. The body cell mass and its supporting environment: body composition in health and disease. W.B. Saunders Co; 1963. [Google Scholar]
- 45.Miller AT, Blyth CS. Lean Body Mass as a Metabolic Reference Standard. J Appl Physiol. 1953;5:311–16. doi: 10.1152/jappl.1953.5.7.311. [DOI] [PubMed] [Google Scholar]
- 46.Keys A, BroŽek J. Body Fat in Adult Man. Physiol Rev. 1953;33:245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- 47.Behnke AR, Osserman EF, Welham WC. Lean body mass; its clinical significance and estimation from excess fat and total body water determinations. AMA Arch Intern Med. 1953;91:585–601. doi: 10.1001/archinte.1953.00240170011002. [DOI] [PubMed] [Google Scholar]
- 48.Von Dobeln W. Human standard and maximal metabolic rate in relation to fat-free body mass. Acta Physiol Scand Suppl. 1956;37:1–79. [PubMed] [Google Scholar]
- 49.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–8. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 51.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 52.Heymsfield SB, Thomas D, Nguyen AM, et al. Voluntary weight loss: systematic review of early phase body composition changes. Obes Rev. 2011;12:e348–61. doi: 10.1111/j.1467-789X.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 53.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, St-Onge MP, Lecumberri B, et al. Body cell mass: model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004;286:E123–8. doi: 10.1152/ajpendo.00227.2003. [DOI] [PubMed] [Google Scholar]
- 55.Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–56. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 56.Heymsfield SB. Development of imaging methods to assess adiposity and metabolism. Int J Obes (Lond) 2008;32 (Suppl 7):S76–82. doi: 10.1038/ijo.2008.242. [DOI] [PubMed] [Google Scholar]
- 57.Barcroft J. Zur Lehre vom Blutgnswechsel in den verschiedenen Organen. Ergeb Physiol. 1908;7:699–704. [Google Scholar]
- 58.Field J, Belding HS, Martin AW. An analysis of the relation between basal metabolism and summated tissue respiration in the rat. I. The post-pubertal albino rat. Journal of Cellular and Comparative Physiology. 1939;14:143–57. [Google Scholar]
- 59.Kety SS, Schmidt CF. The determination of cerebral blood flow in man by the use of nitrous oxide in low concentrations. American Journal of Physiology -- Legacy Content. 1945;143:53–66. [Google Scholar]
- 60.Holliday MA, Potter D, Jarrah A, Bearg S. The relation of metabolic rate to body weight and organ size. Pediatr Res. 1967;1:185–95. doi: 10.1203/00006450-196705000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Holliday MA. Metabolic Rate and Organ Size During Growth From Infancy to Maturity and During Late Gestation and Early Infancy. Pediatrics. 1971;47:169–79. [PubMed] [Google Scholar]
- 62.Drabkin DL, Jean R With the technical assistance of Priscilla Fourer JMFAMDCG. The distribution of the chromoproteins, hemoglobin, myoglobin, and cytochrome c, in the tissues of different species, and the relationship of the total content of each chromoprotein to body mass. J Biol Chem. 1950;182:317–34. [Google Scholar]
- 63.Brozek J, Grande F. Body composition and basal metabolism in man: correlation analysis versus physiological approach. Hum Biol. 1955;27:22–31. [PubMed] [Google Scholar]
- 64.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, editors. Energy metabolism: tissue determinants and cellular corollaries. Raven Press; New York: 1992. pp. 61–77. [Google Scholar]
- 65.Wang Z, Ying Z, Bosy-Westphal A, et al. Evaluation of specific metabolic rates of major organs and tissues: comparison between nonobese and obese women. Obesity (Silver Spring) 2012;20:95–100. doi: 10.1038/oby.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92:1369–77. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Later W, Bosy-Westphal A, Hitze B, et al. No evidence of mass dependency of specific organ metabolic rate in healthy humans. Am J Clin Nutr. 2008;88:1004–9. doi: 10.1093/ajcn/88.4.1004. [DOI] [PubMed] [Google Scholar]
- 68.Muller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat free mass (FFM) and resting energy expenditure (REE) in humans. Forum Nutr. 2003;56:301–3. [PubMed] [Google Scholar]
- 69.Wang Z, Ying Z, Bosy-Westphal A, et al. Evaluation of specific metabolic rates of major organs and tissues: Comparison between men and women. Am J Hum Biol. 2011;23:333–8. doi: 10.1002/ajhb.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinsier RL, Schutz Y, Bracco D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr. 1992;55:790–4. doi: 10.1093/ajcn/55.4.790. [DOI] [PubMed] [Google Scholar]
- 71.Gallagher D, Allen A, Wang Z, Heymsfield SB, Krasnow N. Smaller organ tissue mass in the elderly fails to explain lower resting metabolic rate. Ann N Y Acad Sci. 2000;904:449–55. doi: 10.1111/j.1749-6632.2000.tb06499.x. [DOI] [PubMed] [Google Scholar]
- 72.Hayes M, Chustek M, Wang Z, et al. DXA: potential for creating a metabolic map of organ-tissue resting energy expenditure components. Obes Res. 2002;10:969–77. doi: 10.1038/oby.2002.132. [DOI] [PubMed] [Google Scholar]
- 73.Jones A, Jr, Shen W, St-Onge MP, et al. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Clin Nutr. 2004;79:780–6. doi: 10.1093/ajcn/79.5.780. [DOI] [PubMed] [Google Scholar]
- 74.Heymsfield SB, Muller MJ, Bosy-Westphal A, Thomas D, Shen W. Human brain mass: Similar body composition associations as observed across mammals. Am J Hum Biol. 2012;24:479–85. doi: 10.1002/ajhb.22249. [DOI] [PubMed] [Google Scholar]
- 75.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–72. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 76.Javed F, He Q, Davidson LE, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr. 2010;91:907–12. doi: 10.3945/ajcn.2009.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alfonzo-Gonzalez G, Doucet E, Almeras N, Bouchard C, Tremblay A. Estimation of daily energy needs with the FAO/WHO/UNU 1985 procedures in adults: comparison to whole-body indirect calorimetry measurements. Eur J Clin Nutr. 2004;58:1125–31. doi: 10.1038/sj.ejcn.1601940. [DOI] [PubMed] [Google Scholar]
- 78.Zhu X-H, Qiao H, Du F, et al. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60:2107–17. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–57. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.