Abstract
There is significant progress toward understanding catalysis throughout the essential MEP pathway to isoprenoids in human pathogens; however, little is known about pathway regulation. The present study begins by testing the hypothesis that isoprenoid biosynthesis is regulated via feedback inhibition of the fifth enzyme cyclodiphosphate IspF by downstream isoprenoid diphosphates. Here, we demonstrate recombinant E. coli IspF is not inhibited by downstream metabolites and isopentenyl diphosphate (IDP), dimethylallyl diphosphate (DMADP), geranyl diphosphate (GDP) and farnesyl diphosphate (FDP) under standard assay conditions. However, 2C-methyl-d-erythritol 4-phosphate (MEP), the product of reductoisomerase IspC and first committed MEP pathway intermediate, activates and sustains this enhanced IspF activity, and the IspF-MEP complex is inhibited by FDP. We further show that the methylerythritol scaffold itself, which is unique to this pathway, drives the activation and stabilization of active IspF. Our results suggest a novel feed-forward regulatory mechanism for 2Cmethyl-d-erythritol 2,4-cyclodiphosphate (MEcDP) production and support an isoprenoid biosynthesis regulatory mechanism via feedback inhibition of the IspF-MEP complex by FDP. The results have important implications for development of inhibitors against the IspF-MEP complex, which may be the physiologically relevant form of the enzyme.
Keywords: cyclodiphosphate synthase, IspF, methylerythritol phosphate, MEP pathway regulation
INTRODUCTION
Isoprenoids represent a large class of natural products and metabolites that include but are not limited to perfumes, drugs, lipids, and hormones.1 Despite the impressive structural diversity of this natural product class, isoprenoids are all derived from dimethylallyl diphosphate (DMADP) and isopentenyl diphosphate (IDP) which are products of two independent metabolic pathways, the mevalonate (MVA) pathway and the 2C-methyl-d-erythritol 4-phosphate (MEP) pathway. The MVA pathway, first identified in the late 1950s,2 was thought to be the sole source of IDP and DMADP until the MEP pathway was discovered in the early 1990s.3–5 The MEP pathway is utilized by higher plants, algae, bacteria and many pathogenic organisms including Mycobacterium tuberculosis and the apicomplexan parasites Plasmodium falciparum and Taxoplasma gondii. In contrast, mammals utilize the MVA pathway as the exclusive source of isoprenoids; thus, the MEP pathway enzymes have gained attention as promising targets for development of anti-infective agents and herbicides.6–8
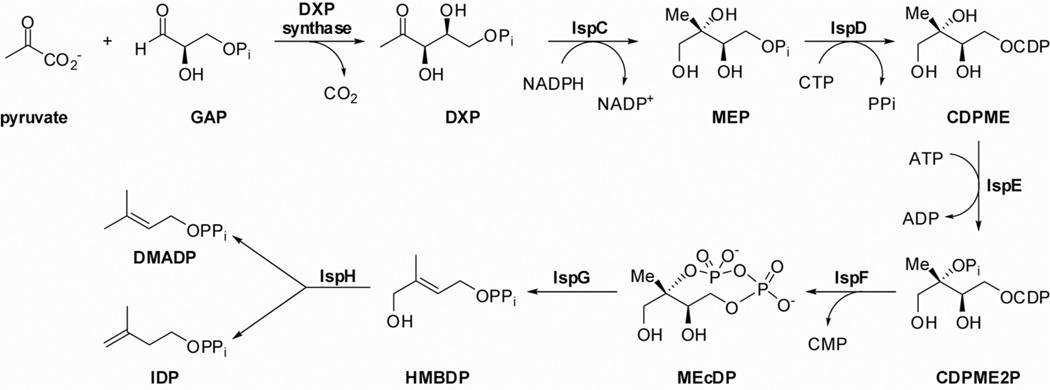
The MEP pathway to IDP and DMADP is comprised of seven enzymes starting from 1-deoxy-d-xylulose 5-phosphate (DXP) synthase (Figure 1), which catalyzes formation of DXP from pyruvate and d-glyceraldehyde 3-phosphate (GAP). Formation of 2C-methyl-d-erythritol 4-phosphate (MEP) from DXP is catalyzed by reductoisomerase IspC and represents the first committed step in non-mammalian isoprenoid biosynthesis. MEP then undergoes cytidylation (IspD) and phosphorylation (IspE) to form 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (CDPME2P). Cyclodiphosphate synthase IspF, which is the focus of this study, catalyzes the conversion of CDPME2P to the cyclic diphosphate 2Cmethyl-d-erythritol 2,4-cyclodiphosphate (MEcDP) with concomitant release of CMP. MEcDP undergoes reductive ring opening catalyzed by IspG to form linear diphosphate (E)-4-hydroxy-3-methylbut-2-enyl diphosphate (HMBDP) which is finally converted into IDP and DMADP by the action of the reductase IspH.
Figure 1.
The methylerythritol phosphate (MEP) pathway.
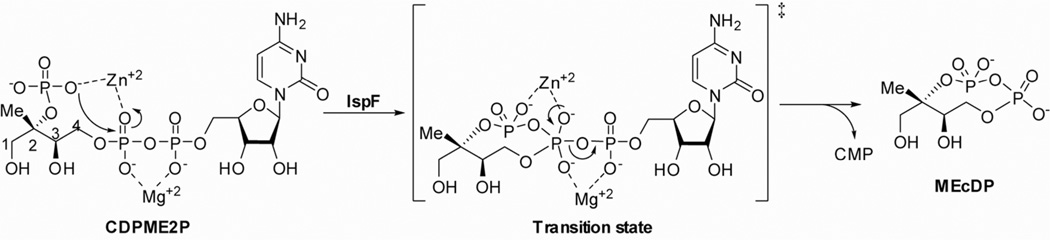
The fifth enzyme in the MEP pathway, IspF, catalyzes a unique cyclization reaction which has generated interest as a potential point of inhibition of isoprenoid biosynthesis toward the development of new anti-infective agents. Accordingly, this enzyme has been shown to be indispensable in M. tuberculosis and B. subtilis in studies to validate it as a drug target.9–11 In this reaction, the 2-phosphate group of CDPME2P displaces CMP to form cyclic diphosphate MEcDP (Figure 2). X-ray crystallography and mechanistic studies have demonstrated that IspF is active in its trimeric form,12 with three active sites at the monomer interfaces. The enzyme requires Zn2+ and Mg2+ to position the substrate and stabilize the developing charge in the pentavalent transition state.11, 13–17
Figure 2.
Proposed IspF reaction mechanism depicting substrate positioning and charge stabilization by Zn2+ and Mg2+.
Several structural studies have now confirmed the presence of a hydrophobic cavity along the threefold symmetry axis of the IspF homotrimer14, 16–18 that appears to accommodate downstream isoprenoid intermediates (IDP/DMADP, GDP or FDP), suggesting the possibility of feedback regulation of the MEP pathway via inhibition of IspF. However, to our knowledge, the biochemical evaluation of IDP/DMADP, FDP or GDP as inhibitors of IspF has not been reported. In the present study to understand regulation of isoprenoid biosynthesis and identify new mechanisms of inhibition of this unique enzyme, we show that IspF is not inhibited by IDP, DMADP, GDP or FDP under standard assay conditions. Contrary to our expectations, we have observed apparent enhancement of enzyme activity and subtle stabilizing effects on IspF activity by several downstream isoprenoid diphosphates. This unexpected outcome led to analysis of all MEP pathway intermediates as possible modulators of IspF activity, revealing the greatest enhancement of IspF activity and most striking activity-sustaining effects by the first committed pathway intermediate, methylerythritol phosphate (MEP). Here, we report the biochemical characterization of MEP-induced activation/stabilization of IspF, and show that the 2Cmethyl-d-erythritol scaffold itself drives this effect. Our studies come full circle to demonstrate biochemical inhibition of IspF by FDP, only in the presence of MEP. This work is of interest because it reveals a possible feed-forward regulatory mechanism for MEcDP production via MEP-induced activation/stabilization of IspF, and supports an isoprenoid biosynthesis regulatory mechanism via feedback inhibition of the IspF-MEP complex by downstream isoprenoids.
RESULTS
Characterization of IspF activity by HPLC and NMR
The biochemical evaluation of IspF is challenging because of the difficulties in accessing the substrate, CDPME2P, coupled with the instability of the substrate under a variety of assay conditions. In addition, the IspF product MEcDP (Figure 2) does not bear a chromophore and therefore is not detectable by HPLC-UV analysis. Thus, IspF-catalyzed formation of MEcDP from CDPME2P was confirmed using a two-dimensional H–P–P NMR method developed in our lab19, 20 (Figure S2a,b). Formation of CMP in this reaction was confirmed by HPLC comparison to an authentic sample. An HPLC-based assay monitoring enzyme-catalyzed CMP formation from CDPME2P was previously developed for characterization of IspF.13 Here, we have optimized assay conditions to prevent degradation of the CDPME2P and ensure reproducible HPLC retention times for substrate and product (Figure S2c). First, we have determined that use of HEPES, Tris, MOPS, TEA, and Tricine buffers promotes degradation of the substrate CDPME2P to CDP at both 37°C and 20°C. Substrate degradation in these buffers is enhanced with increasing BSA or MgCl2 concentrations. However, 50 mM phosphate buffer causes only minor substrate degradation at 37°C, and no degradation is observed at 20°C. Thus, the standard assay conditions reported here include the use of phosphate buffer at 20°C.
Second, quenching of the enzyme reaction with EDTA or organic solvent (MeOH) results in unreliable retention times and peak areas of the product, CMP. This problem is resolved through the use of 0.1% SDS to quench reactions.21–24 Under these optimized assay conditions, a pH/rate analysis indicates the E. coli IspF enzyme exhibits an apparent maximal rate at pH 7.4 in 50 mM phosphate buffer (Figure S3).
Detailed kinetic analysis of IspF-catalyzed formation of CMP suggests that IspF follows hyperbolic kinetics, and kinetic parameters (KmCDPME2P = 339 ± 32 µM and kcat = 61 ± 3 min−1; Table 1 and Figure S4) are comparable to those previously reported.25 Various inhibitors of IspF have been reported25–27 including CDP.26 For further validation of this HPLC-based assay, we tested inhibition of IspF by CDP. Under optimized conditions and in the presence of 100 µM CDPME2P, an IC50 of 768 µM was determined for CDP (Figure S5).
Table 1.
A summary of the kinetic parameters of E. coli cyclodiphosphate synthase IspF at 20°C in the absence or presence of 2C-methyl-d-erythritol 4-phosphate (MEP) or 2C-methyl-d-erythritol (ME).
| Km (µM) | kcat (min−1) |
kcat/ Km (µM−1 min−1) |
AC50 (µM) | |
|---|---|---|---|---|
| IspF | 339 ± 32 | 60.6 ± 3 | 0.18 | --- |
| IspF + MEP | 93.8 ± 11 | 80.7 ± 5 | 0.86 | 133 ± 33 |
| IspF + ME | 119 ± 18 | 87.4 ± 7 | 0.74 | 106 ± 14 |
Evaluation of IDP/DMADP, GDP and FDP as inhibitors of IspF
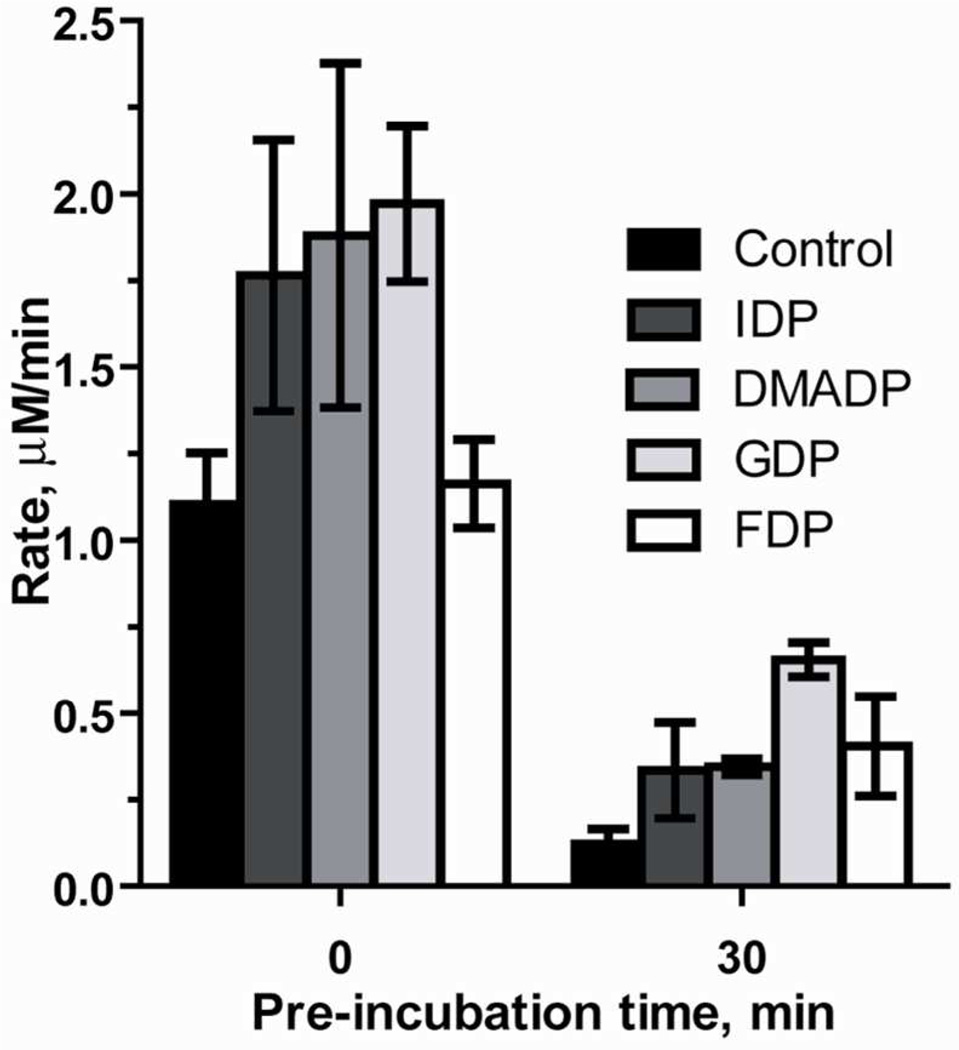
Crystallographic studies of IspF by several groups have reported the presence electron density in the hydrophobic inter-subunit cavity of the enzyme.14, 18 Subsequent mass spectrometry experiments identified the downstream isoprenoid products IDP/DMADP, GDP and, FDP bound to IspF in an approximate ratio of 1:4:2 respectively.18 These findings suggested the possibility of feedback regulation of the MEP pathway through inhibition of IspF by downstream isoprenoids. However, in the present study, we are unable to demonstrate biochemical inhibition of IspF by IDP, DMADP, GDP or FDP at concentrations up to 500 µM (Figure 3), under our standard assay conditions. To examine the possibility of a feedback inhibition mechanism requiring a particular composition of downstream isoprenoids,18 we tested the inhibition of IspF in the presence of isoprenoid mixtures containing a 1:4:2 ratio of IDP, GDP and FDP. Again, inhibition of IspF was not observed, even in the presence of 200:800:400 µM IDP:GDP:FDP (Table S1). Interestingly, under standard assay conditions an apparent enhancement of IspF reaction rate, albeit subtle, was observed in the presence of IDP (1.6-fold increase in initial rate at 500 µM), DMADP (1.7-fold increase in initial rate at 500 µM) and GDP (1.8-fold increase in initial rate at 500 µM), compared to control (Figure 3). In the absence of any observed inhibitory effects by downstream metabolites, we reasoned that inhibition of IspF by these isoprenoids might instead be time-dependent. Thus, we reevaluated IDP, DMADP, GDP and FDP as inhibitors under conditions where these downstream metabolites were pre-incubated with IspF for 30 minutes, and initial reaction rates were measured after initiation with the substrate CDPME2P (Figure 3). In the absence of added isoprenoids, only 11% activity is retained following pre-incubation at 20°C. In the presence of 500 µM IDP or DMADP, 19% and 18% activity is retained, respectively, following a 30-minute pre-incubation. Interestingly, pre-incubation of IspF with 500 µM, GDP, or FDP prevents loss of IspF activity to some degree. In the presence of GDP, ~33% activity is retained following pre-incubation, representing a 5.4-fold enhancement in the rate of CMP formation, compared to control after the same pre-incubation. In the presence of FDP, 35% activity is retained, and the rate of CMP formation is 3.3-fold higher than control after the same pre-incubation. Sustained activity was also evident following a 10 minute pre-incubation, and rate enhancements at this time point fall within expected trends.
Figure 3.
Rate of IspF-catalyzed CMP formation when enzyme reactions are initiated by addition of IspF (0 min) or CDPME2P (30 min) following a 30 minute pre-incubation of IspF with 500 µM IDP, DMADP, GDP, or FDP.
Evaluation of activity-enhancing and activity-sustaining effects of DXP, MEP, CDPME and HMBDP
The moderate enhancement of IspF activity by downstream isoprenoids and the apparent stabilization effects of GDP and FDP on enzyme activity through a 30-minute pre-incubation prompted the analysis of the activity-enhancing and/or activity-sustaining effects of other MEP pathway intermediates. Upstream MEP pathway intermediates DXP and CDPME, products of DXP synthase and IspD, respectively, neither increase initial rate nor prevent the loss of IspF activity (Figure 4a). The product of IspG, HMBDP, exhibits subtle enhancing effects (1.4-fold rate increase at 500 µM HMBDP) and sustains IspF activity comparably to IDP and DMADP with 18% residual activity of IspF after a 30-minute pre-incubation (Figure 4a). However, MEP, the product of reductoisomerase IspC and first dedicated intermediate in non-mevalonate isoprenoid biosynthesis, demonstrates the most striking effects of any MEP pathway intermediate tested in this study. MEP enhances the IspF-catalyzed rate of CMP formation by 2-fold (at 500 µM MEP) and, notably, this activity is sustained for >24 hours. Interestingly, analysis by size exclusion chromatography indicates the quaternary structure of IspF appears unchanged over time (data not shown), in the presence or absence of MEP, and inclusion of the reducing agent TCEP in the enzyme assay buffer has no effect on the time-dependent deactivation of IspF (data not shown). Thus, changes in quaternary structure or oxidation state of IspF neither explain the observed time-dependent instability in the absence of MEP nor account for the stabilization effects of MEP. In addition, the inactivated form of IspF cannot be reactivated by MEP (following a 30 minute pre-incubation at 20°C), suggesting MEP induces rate enhancement and prevents loss of activity of a more physiologically relevant form of IspF.
Figure 4.
(a) Rate of CMP formation in the presence of 500 µM DXP, CDPME, HMBDP, DX, EP, MEP, and ME. (b) Structures of d-erythritol 4-phosphate (EP), 2C-methyl-d-erythritol (ME), and deoxyxylulose. *No appreciable rate after 24 h.
Methylerythritol scaffold is essential to enhance and sustain IspF activity
The absence of IspF enhancing effects of the monophosphate-containing bioprecursor DXP suggests the methylerythritol scaffold itself contributes to the particular effects exhibited by MEP. We prepared 2C-methyl-d-erythritol (ME) and d-erythritol 4-phosphate (EP) (Figure 4b)28 to assess the contribution of either the phosphoryl or 2C-methyl group, respectively, in the activity-enhancing and stabilizing properties of MEP. Under the same reaction conditions, ME exhibits a comparable activating effect to MEP (2.0-fold at 500 µM, Figure 4b), and IspF activity in the presence of ME, like MEP, is retained for >24 hours. Interestingly, the 2C-desmethyl analog EP only subtly activates IspF (1.3-fold at 500 µM, Figure 4b) and does not sustain IspF activity despite sharing a similar structure to MEP. These results suggest these effects are specific and driven by the 2C-methylerythritol scaffold that is unique to this pathway. The lack of activating or stabilizing properties of other poly-hydroxylated compounds, deoxyxylulose (DX) and the widely-used enzyme stabilizers glycerol or DDM (Figure S6), further supports this specific effect of MEP.
Characterization of MEP and ME effects on IspF turnover efficiency
The activity-enhancing properties of MEP and ME were characterized by measuring kinetic parameters of IspF in the presence of MEP or ME and by determining the AC50 value for each, the concentration of the additive that elicits 50% of IspF maximal activity.29, 30
In the presence of 100 µM CDPME2P, an AC50MEP of 133 ± 33 µM was determined (Table 1, Figure S7), and the maximal initial rate under these conditions was found to be ~2-fold the initial rate measured in the absence of MEP. It was reasoned that MEP may enhance turnover efficiency as a consequence of changes in KmCDPME2P, kcat, or both. To gain further insight into MEP-mediated IspF activation, we determined the kinetic parameters of IspF in the presence of 500 µM MEP, conditions producing a near-maximal rate enhancement. In the presence of MEP, KmCDPME2P = 93.8 ± 11 µM (Table 1, Figure S4), representing a 3.6-fold increase in affinity of the substrate (KmCDPME2P = 339 ± 32 µM in the absence of MEP. A comparable kcat (1.3-fold) was also observed in the presence of MEP (kcat = 80.7 ± 5 min−1). The addition of MEP therefore results in a 4.8-fold increase in the efficiency of turnover (kcat/Km), consistent with the idea that MEP induces a more active form of IspF (Table 1).
Similarly, an AC50ME of 106 ± 14 µM was measured (Table 1, Figure S7). Further, ME appears to induce an IspF conformation displaying a 2.9-fold increase in affinity for the substrate (KmCDPME2P = 119 ± 18 µM in the presence of 500 µM ME, Table 1, Figure S4), comparable to the effect observed in the presence of MEP. The kcat in the presence of ME (80.7 ± 5 min−1, 1.4-fold) is also comparable to that of MEP reaction. Thus, the efficiency of turnover (kcat/Km) increases 4.1-fold in the presence of ME, a comparable increase to that observed in the presence of MEP (Table 1, Figure S4).
Inhibition of the IspF-MEP complex
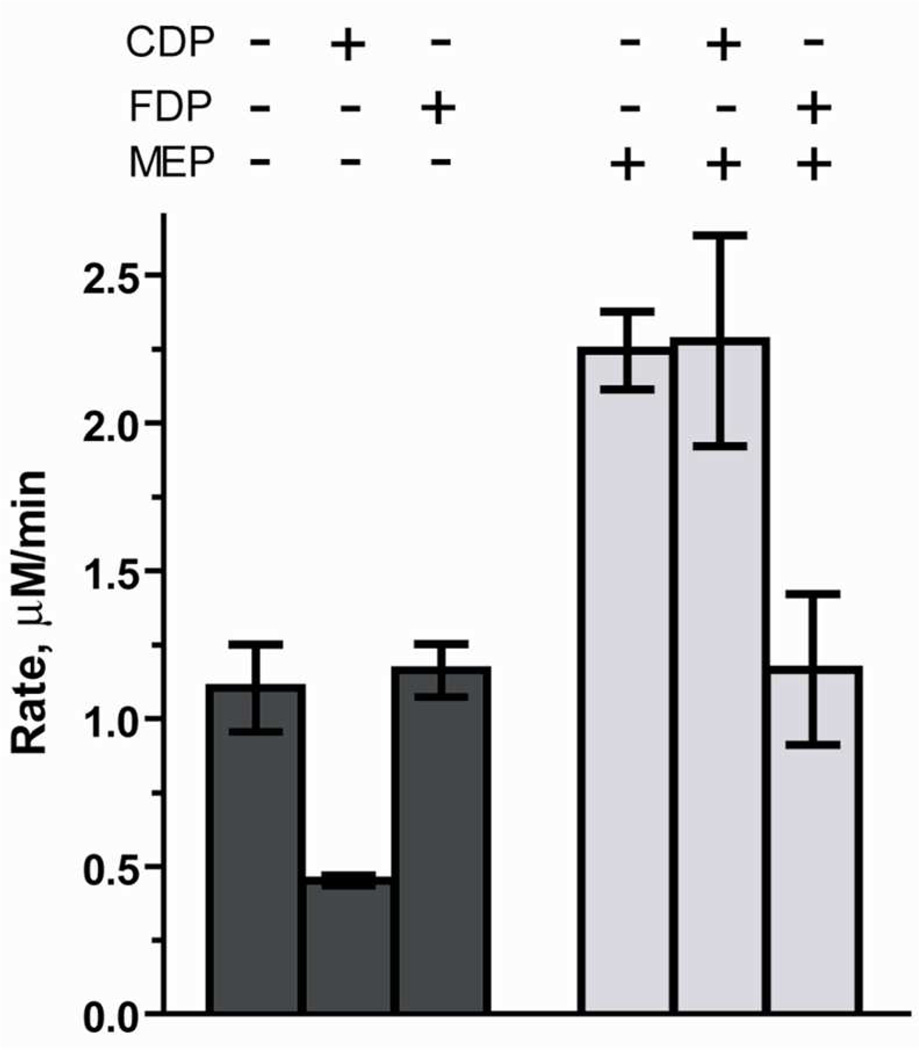
The idea that MEP enhances activity of IspF and prevents enzyme deactivation raises questions about the influence of IspF inhibitors on the IspF-MEP complex and, in particular, the relevance of the IspF-MEP complex in a feedback inhibition mechanism to regulate isoprenoid biosynthesis. To ascertain the effects of MEP on inhibition of IspF, we re-evaluated the known inhibitor CDP as well as downstream metabolites that could act as feedback inhibitors (HMBDP, IDP, DMADP, GDP and FDP). In the absence of MEP, CDP inhibits IspF with an IC50 of 768 µM (Figure S5). However, in the presence of 500 µM MEP, comparable initial rates of CMP formation were observed in the presence or absence of 700 µM CDP (Figure 5), suggesting the putative IspF-MEP complex is less sensitive to the effects of CDP.
Figure 5.
Evaluation of inhibitory effects of CDP (700 µM) and FDP (500 µM) in the presence or absence of MEP (500 µM).
In the absence of MEP, downstream metabolites HMBDP, IDP, DMADP and GDP are not inhibitors of IspF (Figure 3). Likewise, these downstream metabolites do not inhibit the IspF-MEP complex (Table S2). However, while FDP shows no IspF inhibitory activity in the absence of MEP (Figure 3), inhibition of the IspF-MEP complex (43%) by FDP is observed (Figure 5, Table S2), suggesting a MEP-stabilized conformation may be more sensitive to the effects of FDP.
DISCUSSION
The MEP pathway has drawn tremendous interest as a target for drug discovery since it is absent in humans and is the sole source of essential isoprenoid building blocks in many pathogenic organisms.3–8 In addition, the pathway has the potential to be manipulated through bioengineering methods to enable increased and sustained production of isoprenoids with medicinal value.31–34 Understanding enzyme function and regulation in the MEP pathway is critical in the endeavor to target these enzymes in the development of new anti-infective agents or optimize isoprenoid production in bioengineering efforts. However, relatively little is known about regulation of the MEP pathway in pathogenic organisms.35, 36 Studies carried out by Brown, et al. demonstrated that overexpression of the dxs gene (encoding DXP synthase) in M. tuberculosis leads to accumulation of downstream MEP pathway product HMBDP, via upregulation of IspC (Dxr) and IspG (GcpE).35 Their findings suggest that DXP synthase (Dxs) is involved in transcriptional control and therefore controls flux at the starting point of the pathway. In addition, the authors proposed IspG (GcpE) as a rate limiting step of the pathway. Other studies have focused on the interesting IspDF bifunctional enzyme36–39 as a potential regulatory mechanism in isoprenoid biosynthesis. IspDF is known to catalyze the first and third steps in the conversion of MEP to MEcDP in several bacterial species and early reports proposed this organization of enzymes could enhance flux through the MEP pathway by substrate channeling through a IspDF/IspE complex;38 however, studies by Lherbert, et al. 40 could not provide evidence of enhanced metabolic flux through this complex, suggesting the IspDF bifunctional enzyme may serve some other regulatory role.
The present study was motivated by the hypothesis that isoprenoid biosynthesis is regulated through feedback inhibition of IspF. 14, 17, 18 Our initial results suggested that IspF is not inhibited by downstream isoprenoids IDP, DMADP, GDP or FDP under standard assay conditions, in the absence of any other additives. Contrary to our initial expectations, the rate of formation of CMP is slightly enhanced in the presence of downstream isoprenoids; further, whereas IspF activity is dramatically decreased over time in the absence of additives, addition of downstream isoprenoid metabolites to the pre-incubation mixture appears to prevent the loss of IspF activity by varying degrees. It is unknown whether this subtle effect occurs through binding of isoprenoid diphosphates in the hydrophobic cavity of the IspF homotrimer.
Subsequent investigation of all MEP pathway intermediates as potential modulators of IspF activity has revealed the most striking effects by the first committed intermediate in non-mammalian isoprenoid biosynthesis, MEP. In the presence of MEP, the rate of CMP formation is increased to ~2-fold and this activity is sustained for >24 hours. Upstream metabolites DXP, CDPME neither enhance nor prevent the loss of IspF activity, and HMBDP displays subtle effects similar to IDP and DMADP, suggesting that the activating/stabilizing effects are specific to MEP within the context of this pathway. Additional structure-activity relationship studies demonstrate the methylerythritol scaffold itself drives the effects of MEP on IspF, and highlights the particular requirement for the 2C-methyl substituent which is unique to the MEP pathway.
An analysis of initial rates of CMP formation at varying MEP concentrations indicates an AC50 of 133 ± 33. Zhang, et al. recently reported MEP levels in E. coli to be 0.055 fg/cell (corresponding to ~394 µM, assuming a cell volume of 0.65 µm3),41, 42 suggesting the concentration of MEP required to activate IspF is physiologically relevant. Further, detailed kinetic analysis of IspF in the presence of MEP reveals a 4.8-fold increase in kcat/Km, which is primarily a consequence of increased affinity of IspF for its substrate CDPME2P and is comparable to the effects of other known small molecule activators.29 Interestingly, ME induces a similar increase in turnover efficiency. While the results of these biochemical analyses suggest MEP and/or ME-induced activation of IspF is possible in vivo, further studies are required to evaluate the effects of MEP depletion on cellular IspF activity and determine the role, if any, of ME in vivo.
The finding that MEP increases turnover efficiency and prevents the loss of IspF activity suggests a feed-forward mechanism to maximize MEcDP production, and raises interesting questions about the regulatory role of MEP in the cellular requirement for MEcDP. MEcDP is known to accumulate to high levels in many organisms in response to oxidative stress43–49, suggesting MEcDP may play a role as anti-stressor through regulation of metal-dependent enzymes by cation chelation or by regulation of antioxidants. More recently, Grieshaber, et al.50 have demonstrated that MEcDP facilitates release of DNA from the histone-like protein Hc1 and thus from chlamydial chromatin during differentiation of elementary bodies (infectious extracellular form) to reticulate bodies (intracellular replicate form). Similarly, Goncharenko, et al. have also shown that addition of MEcDP to resting (“nonculturable”) forms of M. smegmantis reactivates growth, suggesting MEcDP may play a role in mycobacterial transition to and from latency51 through a mechanism involving regulation of chromatin condensation-decondensation. Thus, MEP-induced stabilization of IspF activity would ensure sustained levels of MEcDP for purposes apart from isoprenoid biosynthesis; MEP may act as regulator of MEcDP levels in pathogenesis.
The observation that the IspF-MEP complex is inhibited by the downstream isoprenoid metabolite, FDP, suggests MEP induces a conformational change in IspF, and the IspF-MEP complex is more sensitive to inhibition by FDP. Inhibition of the more active IspF-MEP complex by FDP supports the notion that feedback inhibition is a potential regulatory mechanism for isoprenoid biosynthesis under conditions that favor high levels of MEcDP.
The biochemical mechanisms for IspF activation by MEP and inhibition of the IspF-MEP complex by FDP are not well-understood. On the basis that MEP induces enhancement in IspF turnover efficiency by decreasing KmCDPME2P, and shares structural components with the natural substrate, it is tempting to speculate that MEP may act as a feed-forward activator by binding to one of the three active sites. Given that CDPME2P itself exhibits hyperbolic kinetics in the absence of MEP, it is possible that MEP could adopt a binding mode distinct from substrate in at least one of the active sites to increase affinity of CDPME2P and induce a more active form of the enzyme that is also more stable. On the basis of previous reports14, 17, 18 and the hydrophobic environment at the trimer interface, it is also reasonable to speculate that FDP may inhibit the IspF-MEP complex through binding in the hydrophobic cavity. However, further biochemical and structural studies are required to shed light on these potential mechanisms of regulation.
The possibility that MEP plays a role in regulating MEcDP levels has important implications for anti-infective drug development. First, if activation and stabilization of IspF is indeed specific to MEP, it follows that inhibition of upstream enzymes to deplete cellular levels of MEP may also expedite loss of IspF activity. It is possible that fosmidomycin, a potent inhibitor of IspC52 and weak inhibitor of IspD,41 may also attenuate IspF activity through depletion of enzyme-stabilizing MEP. Thus, inhibitor combinations targeting IspC and IspF may result in potent inhibition of isoprenoid biosynthesis and attenuation of pathogenesis in bacterial pathogens.
Lastly, in terms of developing inhibitors of IspF, our results suggest that careful consideration should be given to the conditions under which potential IspF inhibitors are evaluated. In addition to re-characterizing the inhibitory effects of FDP in the presence of MEP, we have evaluated the previously characterized IspF inhibitor CDP in the presence or absence of MEP. Whereas CDP exhibits weak inhibitory activity in the absence of MEP, this effect is diminished in the presence of MEP, providing further evidence that MEP induces a conformational change in IspF that alters its susceptibility to inhibitors. To date, several studies toward development of IspF inhibitors have been reported. 26. It will be of interest to see how inhibitory activities are altered in the presence of MEP and whether compounds that reverse the activating/stabilizing effects of MEP can be identified as possible inhibitors of isoprenoid biosynthesis.
METHODS
Reagents
HMBDP, IDP, DMADP, GDP, and FDP were obtained from commercial sources. DXP and MEP were prepared according the procedures of Taylor, et al.53 and Urbansky, et al.28 respectively. CDPME was prepared enzymatically from MEP following the procedures of Illarionova, et al.54 and Narayanasamy, et al.55
Overexpression and purification of E. coli cyclodiphosphate synthase IspF
E. coli BL21 (DE3) competent cells harboring ispF-pET24b were grown to OD600 ~ 1.2 and induced with isopropyl β-d-thiogalactoside (IPTG, 100 µM) at 37°C, and shaking was continued for an additional 5.5 hours. The cells were harvested by centrifugation (2000 × g, 20 min) and stored at −20°C. The cell pellet was thawed on ice and re-suspended in protein purification buffer (2 mL of buffer per gram of cell pellet) containing 125 mM Tris pH 8.0, 157 mM NaCl and 10% v/v glycerol. Cells were lysed by French Press and centrifuged at 4°C (30 min, 15, 000 rpm) to pellet cell debris and insoluble protein. The supernatant was incubated with Ni2+ resin supplemented with 2 mM imidazole for 4.5 hours at 4°C. C-His6 IspF was eluted from the resin in 5 mL fractions over a stepwise gradient of 5–500 mM imidazole. The fractions were analyzed by 15% SDS-PAGE (stained with Coomassie Brilliant Blue G.), and those containing pure protein were pooled. IspF was dialyzed overnight against a 2 L buffer containing 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA and 10% v/v glycerol. A second dialysis was carried out against a 2 L buffer containing 50 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM TCEP and 10% v/v glycerol. Enzyme concentration was determined by Bradford Protein Assay with bovine serum albumin (BSA) as a standard. The C-His6 IspF stock solution was flash frozen in liquid nitrogen and stored at −80°C. Yield: 63.4 mg IspF/L of culture (Figure S1).
Preparation of 4-diphosphocytidyl-2C-methyl-d-erythritol-2-phosphate (CDPME2P)
CDPME2P was prepared following a modified procedure of Illarionova, et al.54 A mixture of MEP (5 mM), CTP (5 mM), ATP (500 µM), phosphoenol pyruvate (7 mM), MgCl2 (5 mM) E. coli IspD (2 µM), inorganic pyrophosphatase (7 units), pyruvate kinase (3.5 units), BSA (1 mg/mL), and E. coli IspE (2 µM) in 100 mM Tris (pH 8.0) was incubated at 37°C for 1.75 hours in a total volume of 8 mL. The reaction was quenched with 4 mL of cold acetonitrile, and the mixture was vortexed for 30 seconds to precipitate proteins. The cloudy mixture was centrifuged at 4000 rpm for 20 minutes at 4°C, filtered, and the supernatant containing product was subjected to purification by reversed-phase ion–pair HPLC using a Varian Dynamax C18 250 × 21.4 mm prep column. The column was developed with a linear gradient of 0 to 30% B at a flow rate of 10 mL/min (where A = 100 mM ammonium acetate buffer, 5 mM tetrabutyl ammonium bisulfate, pH 6.0, and B = acetonitrile containing 5 mM tetrabutyl ammonium bisulfate). Fractions containing the desired compound (λmax = 272 nm, from 37.20 to 44.21 minutes) were combined and concentrated under reduced pressure to remove organic solvent. The resultant mixture was diluted with cold (4°C) ddH2O and lyophilized. Dilution with ddH2O and lyophilization was repeated twice to ensure complete removal of ammonium acetate buffer. The resulting white powder was dissolved in ddH2O and subjected to ion exchange chromatography to convert the product to the ammonium form, using 8 g of NH4+-form DOWEX WX8–200 resin. The resultant compound, 12.2 mg (91%), was identical to previously reported CDPME2P.19
HPLC-based IspF assay
IspF reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 5 mM MgCl2, CDPME2P, 50 nM IspF and 50 µg/mL BSA in a total volume of 160 µL. The reactions at were initiated by addition of IspF to the mixture at 20°C. Sample preparation and analysis: To terminate the IspF reaction, 40 µL of reaction mixture was added to 80 µL of cold 0.1% SDS at 2, 4 and 6 minutes. Quenched mixtures were briefly vortexed and incubated on ice for 15 minutes. To remove proteins prior to HPLC analysis, the quenched reaction mixture was passed through 3K MWCO (molecular weight cut off) Nanosep® centrifugal devices from Pall® Corporation. Samples (90 µL) were injected onto a Beckman HPLC equipped with low-retention PEEK tubing to reduce sample-to-metal interaction56–58 and analyzed by reversed-phase ion-pair HPLC using an Altima C18 3 µ, 53 × 7 mm rocket column. The column was developed with a linear gradient of 0 to 100% B at a flow rate of 3 mL/min (where A = 100 mM phosphate buffer, 5 mM tetrabutyl ammonium bisulfate, pH 6.0 and B = 100 mM phosphate buffer, 5 mM tetrabutyl ammonium bisulfate in 30% acetonitrile (Retention times: CMP = 1.10 minutes & CDPME2P = 3.53 minutes, Figure S2c). The CMP and CDPME2P peak areas were measured, and the concentration of CMP was calculated as a fraction of the total peak area. The data were analyzed using GraFit version 7 software.
Determination of IspF kinetic parameters
IspF reactions contained 50 mM phosphate buffer, pH 7.4, 5 mM MgCl2, 4-diphosphocytidyl-2C-methyl-d-erythritol-2-phosphate (25 – 1000 µM in the absence of MEP or 25 – 700 µM in the presence of 500 µM MEP or 500 µM ME), 50 nM IspF and 50 µg/mL BSA in a total volume of 160 µL. Samples were then analyzed as described above (Figure S4).
IspF inhibition assay
IspF reactions contained 50 mM phosphate buffer, pH 7.4, 5 mM MgCl2, 100 µM CDPME2P, varying concentrations of inhibitor, 50 nM IspF and 50 µg/mL BSA in a total volume of 160 µL. For evaluation of CDP as an inhibitor (Table S2, Figure S5), a concentration range of 0.1 – 1000 µM CDP was used (Figure S4). For the initial evaluation of downstream isoprenoids as feedback inhibitors, each compound was tested at a final concentration of 500 µM (Table S1). For reactions involving isoprenoid mixtures, IDP, GDP and FDP were added to the reaction to a final ratio of 1:4:2 respectively (Table S1). Reactions were initiated by addition of IspF to the mixture at 20°C. Initial rates were measured using the HPLC-based assay described above. For determination of IC50CDP (Figure S5), initial rates were plotted as a function of CDP concentration, and the IC50 was determined using GraFit version 7 software.
Assays to evaluate time-dependent effects
IspF was pre-incubated with the compound under evaluation for 30 minutes or 24 h at 20°C, and the reaction was initiated with 4-diphosphocytidyl-2Cmethyl-d-erythritol 2-phosphate (100 µM). For evaluation of downstream isoprenoids as time-dependent inhibitors, DMADP, IDP, GDP, and FDP were pre-incubated with IspF at a final concentration of 500 µM. For evaluation of other MEP pathway intermediates, DXP, CDPME, MEP, and HMBDP were pre-incubated with IspF at a final concentration of 500 µM. Initial rates were then measured using the HPLC-based assay described above (Table S1).
MEP and ME AC50 determination
IspF reactions contained 50 mM phosphate buffer, pH 7.4, 5 mM MgCl2, 100 µM CDPME2P, 50 µg/mL BSA and varying concentrations of MEP (1 – 1500 µM) or ME (1 – 2000 µM) in a total volume of 160 µL. Initial rates were measured using the HPLC-based assay described above, and plotted as a function of MEP concentration to determine the AC50MEP or AC50ME (Figure S7). The data was analyzed by Kaleidagraph software version 4.03, using Equation 1:59
| Equation 1. |
Af is fractional activity at test concentration x, A0 is relative basal activity, AC50 is the concentration of activator (MEP) that gives 50% of maximal activity and s is the slope factor.
Inhibition of MEP-stabilized IspF
IspF reaction mixtures were prepared as described above for IspF inhibition assays. In addition, MEP (500 µM) was added to each mixture to promote formation of the MEP-stabilized form of IspF. CDP (tested at a concentration of 700 µM, Figure 5, Table S2), HMBDP, IDP, DMADP, GDP (Table S2), and FDP (tested at a final concentration of 500 µM, Figure 5, Table S2) were then evaluated as inhibitors of IspF using the protocol described above. Initial rates of CMP formation were measured using HPLC.
Supplementary Material
Acknowledgement
We acknowledge Y. Sun of The Johns Hopkins University School of Medicine and A. Majumdar of The Johns Hopkins University NMR facility for their help with 2D 1H – 31P NMR; S. Aripirala for assistance with IspF oligomeric state analysis. We thank M. Amzel, L. Brammer, D. Bolduc, P. Cole, D. Eyler, J. Liu, J. Stivers and D. Raben of The Johns Hopkins University School of Medicine for helpful discussions and critiques of this manuscript. This work was supported by funding from the NIH (GM084998) and Johns Hopkins Malaria Research Institute Pilot Grant.
Footnotes
Supporting Information Available. E. coli C-His6-IspF Ni2+ affinity purification, IspF reaction product characterization by 2-D NMR & HPLC, pH/rate profile, IspF inhibition by CDP, tabulated rates and assessment of IspF stabilization by glycerol and DDM. This material is available free of charge via World Wide Web at http://pubs.acs.org.
REFERENCE
- 1.Sacchettini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788–1789. doi: 10.1126/science.277.5333.1788. [DOI] [PubMed] [Google Scholar]
- 2.Chaykin S, Law J, Phillips AH, Tchen TT, Bloch K. Phosphorylated intermediates in the synthesis of squalene. Proc. Natl. Acad. Sci. U.S.A. 1958;44:998–1004. doi: 10.1073/pnas.44.10.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohmer M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. elucidation and distribution. Pure Appl. Chem. 2003;75:375–388. [Google Scholar]
- 4.Dubey VS, Bhalla R, Luthra R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003;28:637–646. doi: 10.1007/BF02703339. [DOI] [PubMed] [Google Scholar]
- 5.Hunter W, Bond C, Gabrielsen M, Kemp L. Structure and reactivity in the nonmevalonate pathway of isoprenoid biosynthesis. Biochem. Soc. Trans. 2003;31:537–542. doi: 10.1042/bst0310537. [DOI] [PubMed] [Google Scholar]
- 6.Dubey VS. Mevalonate-independent pathway of isoprenoids synthesis: A potential target in some human pathogens. Curr. Sci. 2002;83:685–688. [Google Scholar]
- 7.Moreno SNJ, Li Z. Anti-infectives targeting the isoprenoid pathway of toxoplasma gondii. Expert Opin. Ther. Targets. 2008;12:253–263. doi: 10.1517/14728222.12.3.253. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Concepcion M. The MEP pathway: A new target for the development of herbicides, antibiotics and antimalarial drugs. Curr. Pharm. Des. 2004;10:2391–2400. doi: 10.2174/1381612043384006. [DOI] [PubMed] [Google Scholar]
- 9.Buetow L, Brown A, Parish T, Hunter W. The structure of mycobacteria 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase, an essential enzyme, provides a platform for drug discovery. BMC Struct. Biol. 2007;7:68. doi: 10.1186/1472-6807-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell TL, Brown ED. Characterization of the depletion of 2-C-methyl-Derythritol-2,4-cyclodiphosphate synthase in escherichia coli and bacillus subtilis. J. Bacteriol. 2002;184:5609–5618. doi: 10.1128/JB.184.20.5609-5618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinbacher S, Kaiser J, Wungsintaweekul J, Hecht S, Eisenreich W, Gerhardt S, Bacher A, Rohdich F. Structure of 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase involved in mevalonate-independent biosynthesis of isoprenoids. J. Mol. Biol. 2002;316:79–88. doi: 10.1006/jmbi.2001.5341. [DOI] [PubMed] [Google Scholar]
- 12.Sgraja T, Kemp LE, Ramsden N, Hunter WN. A double mutation of escherichia coli 2C-methyl-d-erythritol-2,4-cyclodiphosphate synthase disrupts six hydrogen bonds with, yet fails to prevent binding of, an isoprenoid diphosphate. Acta Crystallogr. , Sect. F: Struct. Biol. Cryst. Commun. 2005;61:625–629. doi: 10.1107/S1744309105018762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohdich F, Eisenreich W, Wungsintaweekul J, Hecht S, Schuhr CA, Bacher A. Biosynthesis of terpenoids. Eur. J. Biochem. 2001;268:3190–3197. doi: 10.1046/j.1432-1327.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- 14.Richard SB, Ferrer J, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP. Structure and mechanism of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase. J. Biol. Chem. 2002;277:8667–8672. doi: 10.1074/jbc.C100739200. [DOI] [PubMed] [Google Scholar]
- 15.Herz S, Wungsintaweekul J, Schuhr CA, Hecht S, Luttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk MH, Bacher A, Rohdich F. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-d-erythritol 2,4-cyclodiphosphate. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp LE, Bond CS, Hunter WN. Structure of 2C-methyl-d-erythritol 2,4-cyclodiphosphate synthase: An essential enzyme for isoprenoid biosynthesis and target for antimicrobial drug development. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6591–6596. doi: 10.1073/pnas.102679799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni S, Robinson H, Marsing GC, Bussiere DE, Kennedy MA. Structure of 2Cmethyl-D-erythritol-2,4-cyclodiphosphate synthase from shewanella oneidensis at 1.6 A: Identification of farnesyl pyrophosphate trapped in a hydrophobic cavity. Acta Crystallogr. , Sect. D: Biol. Crystallogr. 2004;60:1949–1957. doi: 10.1107/S0907444904021791. [DOI] [PubMed] [Google Scholar]
- 18.Kemp LE, Alphey MS, Bond CS, Ferguson MAJ, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN. The identification of isoprenoids that bind in the intersubunit cavity of escherichia coli 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase by complementary biophysical methods. Acta Crystallogr. , Sect. D: Biol. Crystallogr. 2005;61:45–52. doi: 10.1107/S0907444904025971. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar A, Shah MH, Bitok JK, Hassis-LeBeau M, Freel Meyers CL. Probing phosphorylation by non-mammalian isoprenoid biosynthetic enzymes using1H-31P-31P correlation NMR spectroscopy. Mol. Bio. Syst. 2009;5:935–944. doi: 10.1039/b903513c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majumdar A, Sun Y, Shah M, Freel Meyers CL. Versatile1H-31P-31P COSY 2D NMR techniques for the characterization of polyphosphorylated small molecules. J. Org. Chem. 2010;75:3214–3223. doi: 10.1021/jo100042m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osheroff N, Zechiedrich EL. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: Trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987;26:4303–4309. doi: 10.1021/bi00388a018. [DOI] [PubMed] [Google Scholar]
- 22.Mulimani V, Lalitha J. An experiment on the denaturation of α-chymotrypsin by an anionic surfactant, sodium dodecyl sulfate (SDS) Biochem. Educ. 1996;24:52–54. [Google Scholar]
- 23.Wu T, Chow L, Lin J. Sechiumin, a ribosome-inactivating protein from the edible gourd, sechium edule swartz. Eur. J. Biochem. 1998;255:400–408. doi: 10.1046/j.1432-1327.1998.2550400.x. [DOI] [PubMed] [Google Scholar]
- 24.Cho H, Ramer SE, Itoh M, Kitas E, Bannwarth W, Burn P, Saito H, Walsh CT. Catalytic domains of the LAR and CD45 protein tyrosine phosphatases from escherichia coli expression systems: Purification and characterization for specificity and mechanism. Biochemistry. 1992;31:133–138. doi: 10.1021/bi00116a019. [DOI] [PubMed] [Google Scholar]
- 25.Geist JG, Lauw S, Illarionova V, Illarionov B, Fischer M, Grawert T, Rohdich F, Eisenreich W, Kaiser J, Groll M, Scheurer C, Wittlin S, Alonso-Gomez JL, Schweizer WB, Bacher A, Diederich F. Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from mycobacterium tuberculosis and plasmodium falciparum. Chem. Med. Chem. 2010;5:1092–1101. doi: 10.1002/cmdc.201000083. [DOI] [PubMed] [Google Scholar]
- 26.Crane CM, Kaiser J, Ramsden NL, Lauw S, Rohdich F, Eisenreich W, Hunter WN, Bacher A, Diederich F. Fluorescent inhibitors for IspF, an enzyme in the nonmevalonate pathway for isoprenoid biosynthesis and a potential target for antimalarial therapy. Angew. Chem. , Int. Ed. 2006;45:1069–1074. doi: 10.1002/anie.200503003. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner C, Eberle C, Diederich F, Lauw S, Rohdich F, Eisenreich W, Bacher A. Structure-based design and synthesis of the first weak non-phosphate inhibitors for IspF, an enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. Helv. Chim. Acta. 2007;90:1043–1068. [Google Scholar]
- 28.Urbansky M, Davis CE, Surjan JD, Coates RM. Synthesis of enantiopure 2-Cmethyl-d-erythritol 4-phosphate and 2,4-cyclodiphosphate from D-arabitol. Org. Lett. 2004;6:135–138. doi: 10.1021/ol0362562. [DOI] [PubMed] [Google Scholar]
- 29.Zorn JA, Wells JA. Turning enzymes ON with small molecules. Nat. Chem. Biol. 2010;6:179–188. doi: 10.1038/nchembio.318. [DOI] [PubMed] [Google Scholar]
- 30.Bishop A, Chen V. Brought to life: Targeted activation of enzyme function with small molecules. J. Chem. Biol. 2009;2:1–9. doi: 10.1007/s12154-008-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajikumar PK, Xiao W, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Isoprenoid pathway optimization for taxol precursor overproduction in escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in escherichia coli for production of terpenoids. Nat. Biotech. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 33.Ro D, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 34.Ye VM, Bhatia SK. Metabolic engineering for the production of clinically important molecules: Omega-3 fatty acids, artemisinin, and taxol. Biotechnol. J. 2012;7:20–33. doi: 10.1002/biot.201100289. [DOI] [PubMed] [Google Scholar]
- 35.Brown AC, Eberl M, Crick DC, Jomaa H, Parish T. The nonmevalonate pathway of isoprenoid biosynthesis in mycobacterium tuberculosis is essential and transcriptionally regulated by dxs. J. Bacteriol. 2010;192:2424–2433. doi: 10.1128/JB.01402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrielsen M, Rohdich F, Eisenreich W, Grawert T, Hecht S, Bacher A, Hunter WN. Biosynthesis of isoprenoids. Eur. J. Biochem. 2004;271:3028–3035. doi: 10.1111/j.1432-1033.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 37.Gabrielsen M, Bond CS, Hallyburton I, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN. Hexameric assembly of the bifunctional methylerythritol 2,4-cyclodiphosphate synthase and protein-protein associations in the deoxy-xylulose-dependent pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2004;279:52753–52761. doi: 10.1074/jbc.M408895200. [DOI] [PubMed] [Google Scholar]
- 38.Testa CA, Lherbet C, Pojer F, Noel JP, Poulter CD. Cloning and expression of IspDF from mesorhizobium loti characterization of a bifunctional protein that catalyzes nonconsecutive steps in the methylerythritol phosphate pathway. Biochim. Biophys. Acta, Proteins Proteomics. 2006;1764:85–96. doi: 10.1016/j.bbapap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Gil J, Bergua M, Boronat A, Imperial S. Cloning and functional characterization of an enzyme from helicobacter pylori that catalyzes two steps of the methylerythritol phosphate pathway for isoprenoid biosynthesis. Biochim. Biophys. Acta, Gen. Subj. 2010;1800:919–928. doi: 10.1016/j.bbagen.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Lherbet C, Pojer F, Richard SB, Noel JP, Poulter CD. Absence of substrate channeling between active sites in the agrobacterium tumefaciens IspDF and IspE enzymes of the methyl erythritol phosphate pathway. Biochemistry. 2006;45:3548–3553. doi: 10.1021/bi0520075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry. 2011;50:3570–3577. doi: 10.1021/bi200113y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubitschek HE. Cell volume increase in escherichia coli after shifts to richer media. J. Bacteriol. 1990;172:94–101. doi: 10.1128/jb.172.1.94-101.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogrel OD, Fegeding KV, Kharationa EF, Sudarikov AB, Ostrovsky D. The ability of a recombinant escherichia coli strain to synthesize 2-C-methyl-D-erythritol-2,4-cyclopyrophosphate correlates with its tolerance to in vitro induced oxidative stress and to the bactericidal action of murine peritoneal macrophages. Curr. Microbiol. 1996;32:225–228. doi: 10.1007/s002849900040. [DOI] [PubMed] [Google Scholar]
- 44.Ostrovsky D, Diomina G, Lysak E, Matveeva E, Ogrel O, Trutko S. Effect of oxidative stress on the biosynthesis of 2-C-methyl-d-erythritol-2,4-cyclopyrophosphate and isoprenoids by several bacterial strains. Arch. Microbiol. 1998;171:69–72. doi: 10.1007/s002030050680. [DOI] [PubMed] [Google Scholar]
- 45.Ostrovsky D, Shipanova I, Sibeldina L, Shashkov A, Kharatian E, Malyarova I, Tantsyrev G. A new cyclopyrophosphate as a bacterial antistressor? FEBS Lett. 1992;298:159–161. doi: 10.1016/0014-5793(92)80045-i. [DOI] [PubMed] [Google Scholar]
- 46.Ostrovsky D, Shashkov A, Sviridov A. Bacterial oxidative-stress substance is 2-Cmethyl-D-erythritol 2,4-cyclopyrophosphate. Biochem. J. 1993;295:901–902. doi: 10.1042/bj2950901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ershov Y. 2-C-methylerythritol phosphate pathway of isoprenoid biosynthesis as a target in identifying new antibiotics, herbicides, and immunomodulators: A review. Appl. Biochem. Microbiol. 2007;43:115–138. [PubMed] [Google Scholar]
- 48.Rivasseau C, Seemann M, Boisson A, Streb P, Gout E, Douce R, Rohmer M, Bligny R. Accumulation of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate in illuminated plant leaves at supraoptimal temperatures reveals a bottleneck of the prokaryotic methylerythritol 4-phosphate pathway of isoprenoid biosynthesis. Plant, Cell Environ. 2009;32:82–92. doi: 10.1111/j.1365-3040.2008.01903.x. [DOI] [PubMed] [Google Scholar]
- 49.Mongelard G, Seemann M, Boisson A, Rohmer M, Bligny R, Rivasseau C. Measurement of carbon flux through the MEP pathway for isoprenoid synthesis by 31P-NMR spectroscopy after specific inhibition of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate reductase. effect of light and temperature. Plant, Cell Environ. 2011;34:1241–1247. doi: 10.1111/j.1365-3040.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 50.Grieshaber NA, Fischer ER, Mead DJ, Dooley CA, Hackstadt T. Chlamydial histone-DNA interactions are disrupted by a metabolite in the methylerythritol phosphate pathway of isoprenoid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7451–7456. doi: 10.1073/pnas.0400754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goncharenko A, Ershov Y, Salina E, Wiesner J, Vostroknutova G, Sandanov A, Kaprelyants A, Ostrovsky D. The role of 2-C-methylerythritol-2,4-cyclopyrophosphate in the resuscitation of the "nonculturable" forms of mycobacterium smegmatis. Microbiology. 2007;76:147–152. [PubMed] [Google Scholar]
- 52.Kuzuyama T, Shimizu T, Takahashi S, Seto H. Fosmidomycin, a specific inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 1998;39:7913–7916. [Google Scholar]
- 53.Taylor SV, Vu LD, Begley TP, Schorken U, Grolle S, Sprenger GA, Bringer-Meyer S, Sahm H. Chemical and enzymatic synthesis of 1-deoxy-D-xylulose-5-phosphate. J. Org. Chem. 1998;63:2375–2377. [Google Scholar]
- 54.Illarionova V, Kaiser J, Ostrozhenkova E, Bacher A, Fischer M, Eisenreich W, Rohdich F. Nonmevalonate terpene biosynthesis enzymes as antiinfective drug targets: Substrate synthesis and high-throughput screening methods. J. Org. Chem. 2006;71:8824–8834. doi: 10.1021/jo061466o. [DOI] [PubMed] [Google Scholar]
- 55.Narayanasamy P, Eoh H, Crick DC. Chemoenzymatic synthesis of 4-diphosphocytidyl-2-C-methyl-D-erythritol: A substrate for IspE. Tetrahedron Lett. 2008;49:4461–4463. doi: 10.1016/j.tetlet.2008.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asakawa Y, Tokida N, Ozawa C, Ishiba M, Tagaya O, Asakawa N. Suppression effects of carbonate on the interaction between stainless steel and phosphate groups of phosphate compounds in high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Chromatogr., A. 2008;1198–1199:80–86. doi: 10.1016/j.chroma.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 57.Shi G, Wu J, Li Y, Geleziunas R, Gallagher K, Emm T, Olah T, Unger S. Novel direct detection method for quantitative determination of intracellular nucleoside triphosphates using weak anion exchange liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:1092–1099. doi: 10.1002/rcm.684. [DOI] [PubMed] [Google Scholar]
- 58.Veltkamp SA, Hillebrand MJX, Rosing H, Jansen RS, Wickremsinhe ER, Perkins EJ, Schellens JHM, Beijnen JH. Quantitative analysis of gemcitabine triphosphate in human peripheral blood mononuclear cells using weak anion-exchange liquid chromatography coupled with tandem mass spectrometry. J. Mass Spectrom. 2006;41:1633–1642. doi: 10.1002/jms.1133. [DOI] [PubMed] [Google Scholar]
- 59.Montgomery HJ, Bartlett R, Perdicakis B, Jervis E, Squier TC, Guillemette JG. Activation of constitutive nitric oxide synthases by oxidized calmodulin mutants. Biochemistry. 2003;42:7759–7768. doi: 10.1021/bi027097h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.