Abstract
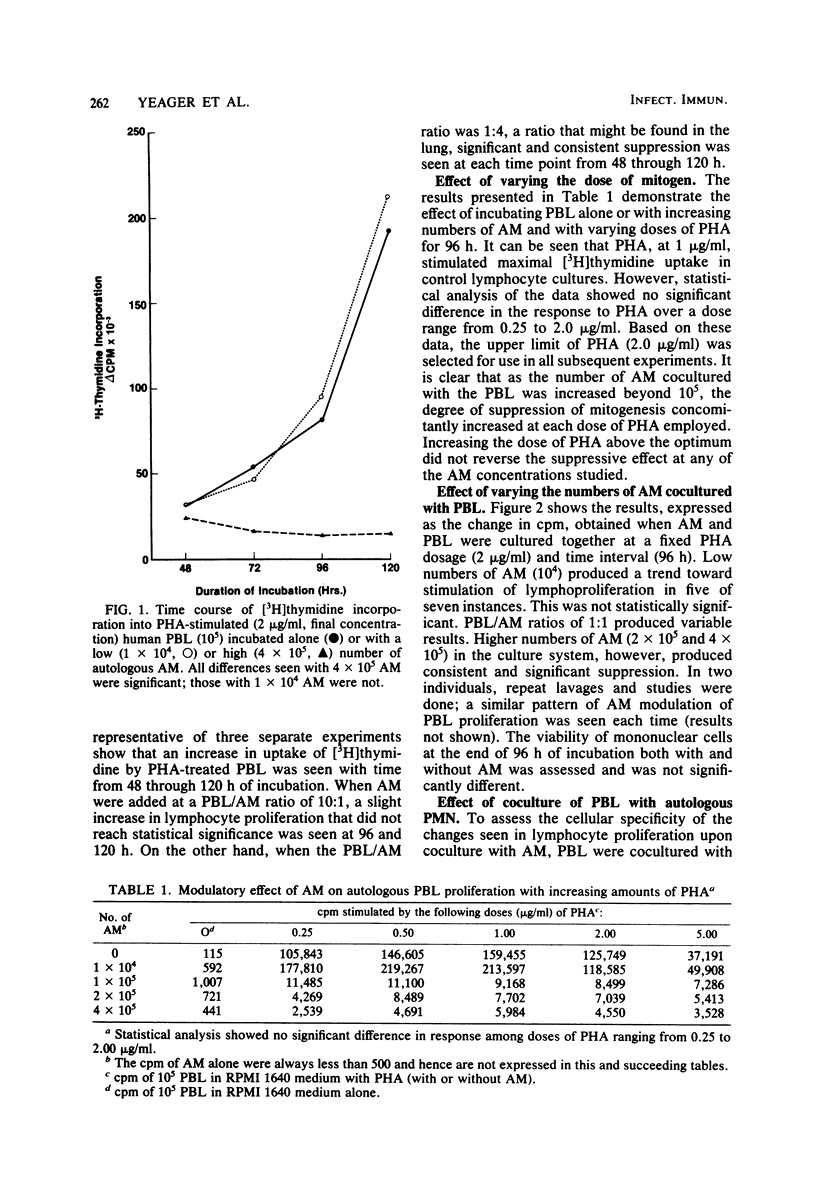
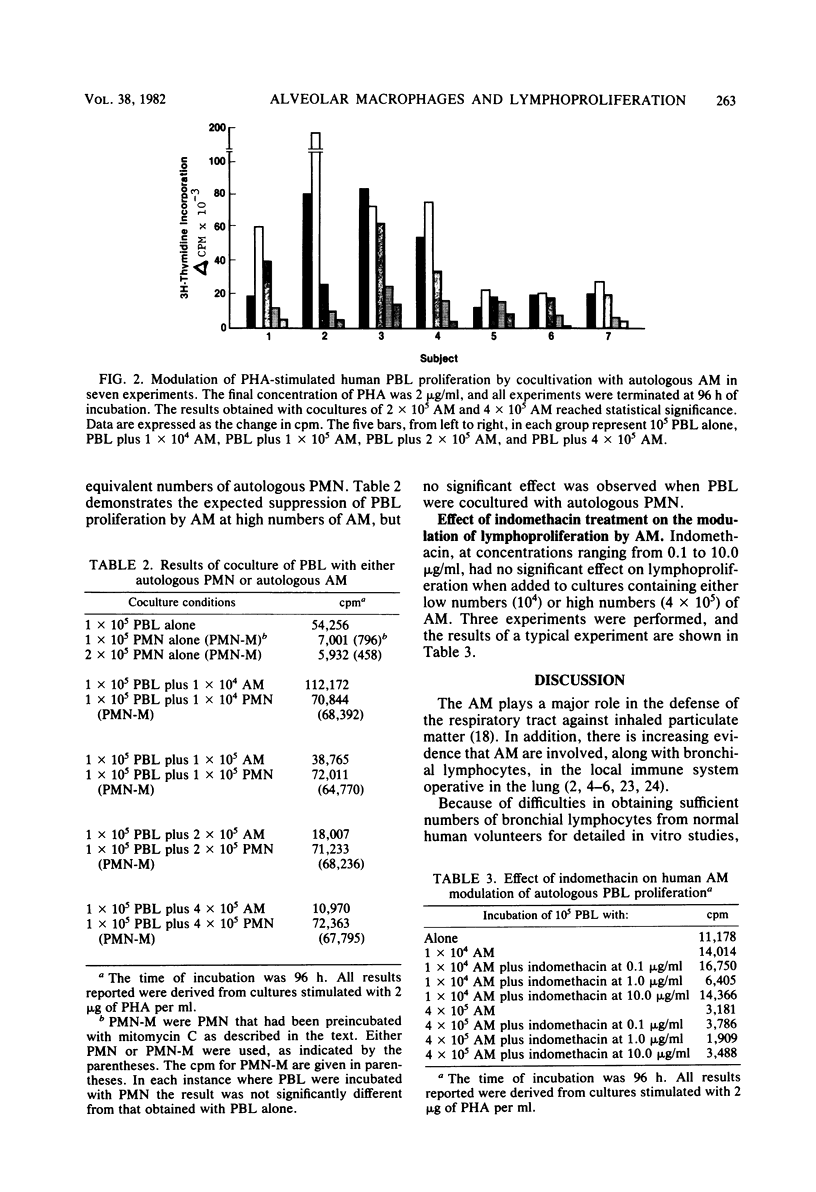
Experiments were carried out to determine the effect of cocultivation of T-cell-enriched human peripheral blood lymphocytes with autologous alveolar macrophages on mitogen-induced proliferation as determined by [3H]thymidine uptake. Cells obtained by fiberoptic bronchoscopy and saline bronchial lavage from 14 normal volunteers were enriched for macrophages by adherence in plastic dishes for 1 h in RPMI 1640 medium supplemented with 10% fetal calf serum. Nonadherent mononuclear cells were prepared from heparinized venous blood after Ficoll-Hypaque sedimentation by passage over nylon wool columns. T-cell-enriched populations were incubated with and without alveolar macrophages, either in the presence or absence of phytohemagglutinin. In these experiments, the number of lymphocytes was held constant (105 per well), while the number of alveolar macrophages was varied (0.1 × 105 to 4.0 × 105 per well). Alveolar macrophages generally tended to stimulate phytohemagglutinin-induced lymphoproliferation at lymphocyte/macrophage ratios of 10:1 but consistently and significantly suppressed proliferation at ratios which approach those usually observed in recovered human bronchial lavage fluid, namely, 1:4. The suppressive effect of alveolar macrophages was observed as early as 48 h after culture initiation, while the magnitude of suppression increased with time. Suppression did not appear to be due to alteration in lymphocyte viability, nor was it sensitive to indomethacin. These results indicate that human alveolar macrophages can modulate the in vitro proliferative response of autologous peripheral blood lymphocytes. This observation may have relevance to interactions between alveolar macrophages and bronchial lymphocytes in the human lung in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979 Feb;122(2):542–548. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clancy R. L., Pucci A., Jelihovsky T., Bye P. Immunologic "memory" for microbial antigens in lymphocytes obtained from human bronchial mucosa. Am Rev Respir Dis. 1978 Mar;117(3):513–518. doi: 10.1164/arrd.1978.117.3.513. [DOI] [PubMed] [Google Scholar]
- Clancy R., Bienenstock J. The proliferative response of bronchus-associated lymphoid tissue after local and systemic immunization. J Immunol. 1974 Jun;112(6):1997–2001. [PubMed] [Google Scholar]
- Daniele R. P., Altose M. D., Rowlands D. T., Jr Immunocompetent cells from the lower respiratory tract of normal human lungs. J Clin Invest. 1975 Oct;56(4):986–995. doi: 10.1172/JCI108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Dauber J. H., Altose M. D., Rowlands D. T., Jr, Gorenberg D. J. Lymphocyte studies in asymptomatic cigarette smokers. A comparison between lung and peripheral blood. Am Rev Respir Dis. 1977 Dec;116(6):997–1005. doi: 10.1164/arrd.1977.116.6.997. [DOI] [PubMed] [Google Scholar]
- Goldyne M. E., Kennedy M. S., Stobo J. D. Human and animal monocyte heterogeneity expressed through the synthesis of prostaglandins and related lipids. Adv Prostaglandin Thromboxane Res. 1980;8:1655–1659. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenberg D. J., Daniele R. P. The alveolar macrophage: its capacity to act as an accessory cell in mitogen-stimulated proliferation of guinea pig lymphocytes. Cell Immunol. 1978 Mar 1;36(1):115–127. doi: 10.1016/0008-8749(78)90255-1. [DOI] [PubMed] [Google Scholar]
- Greineder D. K., Rosenthal A. S. Macrophage activation of allogeneic lymphocyte proliferation in the guinea pig mixed leukocyte culture. J Immunol. 1975 May;114(5):1541–1547. [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscowitz H. B., Conrad R. E., Pennline K. J. Alveolar macrophage--induced suppression of the immune response. Adv Exp Med Biol. 1979;121(A):459–484. doi: 10.1007/978-1-4684-3593-1_41. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. Peritoneal macrophages in the immune response to SRBC in vitro. Immunology. 1970 Jun;18(6):791–797. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology. 1979 Jun;37(2):437–445. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. IV. Interspecies differences in activity in proliferating lymphocyte cultures. Cell Immunol. 1980 Mar 1;50(1):210–215. doi: 10.1016/0008-8749(80)90020-9. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Inhibitory activity of unstimulated alveolar macrophages on T-lymphocyte blastogenic response. Am Rev Respir Dis. 1978 Oct;118(4):791–793. doi: 10.1164/arrd.1978.118.4.791. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kagan E., Solomon A., Cochrane J. C., Kuba P., Rocks P. H., Webster I. Immunological studies of patients with asbestosis. II, Studies of circulating lymphoid cell numbers and humoral immunity. Clin Exp Immunol. 1977 May;28(2):268–275. [PMC free article] [PubMed] [Google Scholar]
- Laughter A. H., Martin R. R., Twomey J. J. Lymphoproliferative responses to antigens mediated by human pulmonary alveolar macrophages. J Lab Clin Med. 1977 Jun;89(6):1326–1332. [PubMed] [Google Scholar]
- Lipscomb M. F., Lyons C. R., O'Hara R. M., Jr, Stein-Streilein J. The antigen-induced selective recruitment of specific T lymphocytes to the lung. J Immunol. 1982 Jan;128(1):111–115. [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Campbell D. A., Jr, Herberman R. B. In vitro inhibition of lymphoproliferative responses to tumor associated antigens and of lymphoma cell proliferation by rat splenic macrophages. Cell Immunol. 1977 Feb;28(2):355–370. doi: 10.1016/0008-8749(77)90118-6. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Conrad R. E., Gerber H. R., Herscowitz H. B. Suppressive effect of alveolar macrophages on the in vitro immune response of rabbit lymphocytes. J Reticuloendothel Soc. 1979 May;25(5):495–512. [PubMed] [Google Scholar]
- Pennline K. J., Herscowitz H. B. Dual role for alveolar macrophages in humoral and cell-mediated immune responses: evidence for suppressor and enhancing functions. J Reticuloendothel Soc. 1981 Sep;30(3):205–217. [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. I. INTERRELATIONSHIPS OF ACRIDINE ORANGE PARTICLES AND CYTOPLASMIC REDDENING. J Cell Biol. 1963 Aug;18:237–250. doi: 10.1083/jcb.18.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Orser M., Kaplan M. E. Human monocyte and macrophage modulation of lymphocyte proliferation. Cell Immunol. 1979 Apr;44(1):131–143. doi: 10.1016/0008-8749(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M. R., Todd L. S. Accessory cell function of rabbit alveolar macrophages. Am Rev Respir Dis. 1981 Jan;123(1):53–57. doi: 10.1164/arrd.1981.123.1.53. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Zimmet S. M., Schwartz S. L. Pinocytosis by human alveolar macrophages. Comparison of smokers and nonsmokers. J Clin Invest. 1974 Aug;54(2):247–251. doi: 10.1172/JCI107759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim S. Enhancing and suppressive effects of macrophages on T-lymphocyte stimulation in vitro. Cell Immunol. 1979 Jul;45(2):377–388. doi: 10.1016/0008-8749(79)90398-8. [DOI] [PubMed] [Google Scholar]


