Abstract
Background & Aims
GATA4, a zinc finger domain transcription factor, is critical for jejunal identity. Mice with an intestine-specific GATA4 deficiency (GATA4iKO) are resistant to diet-induced obesity and insulin resistance. Although they have decreased intestinal lipid absorption, hepatic de novo lipogenesis is inhibited. Here, we investigated dietary lipid-dependent and independent effects on the development of steatosis and fibrosis in GATA4iKO mice.
Methods
GATA4iKO and control mice were fed a Western-type diet (WTD) or a methionine and choline-deficient diet (MCDD) for 20 and 3 weeks, respectively. Functional effects of GATA4iKO on diet-induced liver steatosis were investigated.
Results
WTD-but not MCDD-fed GATA4iKO mice showed lower hepatic concentrations of triglycerides, free fatty acids, and thiobarbituric acid reactive species and had reduced expression of lipogenic as well as fibrotic genes compared with controls. Reduced nuclear sterol regulatory element-binding protein-1c protein levels were accompanied by lower lipogenic gene expression. Oil red O and Sirius Red staining of liver sections confirmed the observed reduction in hepatic lipid accumulation and fibrosis. Immunohistochemical staining revealed an increased number of jejunal glucagon-like peptide 1 (GLP-1) positive cells in GATA4iKO mice. Consequently, we found enhanced phosphorylation of hepatic AMP-activated protein kinase and acetyl-CoA carboxylase alpha.
Conclusions
Our results provide strong indications for a protective effect of intestinal GATA4 deficiency on the development of hepatic steatosis and fibrosis via GLP-1, thereby blocking hepatic de novo lipogenesis.
Abbreviations: DNL, de novo lipogenesis; TG, triglycerides; NAFLD, non-alcoholic fatty liver disease; WTD, Western-type diet; ACC, acetyl-CoA carboxylase alpha; MCDD, methionine and choline-deficient diet; GLP-1, glucagon-like peptide-1; IIS, ileal interposition surgery; GATA4iKO, intestine-specific GATA4 deficiency; ALT, alanine aminotransferase; AST, aspartate transaminase; LDH, lactate dehydrogenase; FFA, free fatty acids; TBARS, thiobarbituric acid reactive substances; AMPK, AMP-activated protein kinase; p, phosphorylated; p38, p38 mitogen-activated protein kinase; Pparg, peroxisome proliferator-activated receptor gamma; α-SMA, alpha-smooth muscle actin; SREBP-1c, sterol regulatory element-binding protein-1c; HSC, hepatic stellate cells
Keywords: GATA4, Non-alcoholic fatty liver disease, GLP-1, Ileal interposition surgery, De novo lipogenesis
Introduction
Metabolic syndrome is a state of metabolic deregulation characterized by obesity, hyperlipidemia, atherosclerosis, glucose intolerance, and hepatic steatosis [1]. A key mechanism contributing to the development of metabolic syndrome is the rate of hepatic de novo lipogenesis (DNL) [2]. Hepatic DNL contributes only 5% of liver triglycerides (TG) under healthy circumstances but up to 30% in case of non-alcoholic fatty liver disease (NAFLD) [3]. The derangement of DNL in murine models of high fat diet-induced hepatic steatosis underscores the intestinal contribution in regulating hepatic lipid metabolism. Western-type diet (WTD), which is generally used to induce atherosclerosis, insulin resistance, and metabolic syndrome in mice, also causes non-alcoholic steatohepatitis [4,5]. WTD contains 43% of calories in the form of carbohydrates and dietary carbohydrates are known to activate hepatic DNL via acetyl-CoA carboxylase (ACC) [6]. Methionine and choline-deficient diet (MCDD) is widely used as a model of hepatic steatosis and fibrosis by inhibiting the release of very low density lipoproteins and by decreasing mitochondrial fatty acid oxidation [7].
Ileum of the small intestine is an important endocrine organ, which signals the dietary status to other organs including the liver by the release of hormones such as glucagon-like peptide-1 (GLP-1) [8,9]. A recent meta-analysis of 15 studies has investigated the effect of various bariatric procedures on NAFLD. The study has concluded that bariatric surgery ameliorates steatosis in 92%, steatohepatitis in 81% and leads to complete resolution in 70% of patients [10]. Ileal interposition surgery (IIS) is one of the bariatric procedures that mitigate the metabolic syndrome [11]. IIS in rats improves glucose tolerance and increases synthesis and release of GLP-1 and peptide YY [12,13]. Kohli et al. have recently reported that the cycling of bile acids is increased in rats that have undergone IIS and that these mice are protected from obesity-associated co-morbidities [14]. These studies provide evidence that postsurgical changes in intestinal anatomy and function, especially earlier exposure of the ileum to nutrients together with alterations in the secretion of enteric hormones, contribute to improve glucose tolerance and lipid homeostasis after IIS. However, the long-term effects this surgical procedure may exert on hepatic lipid metabolism, steatosis, fibrosis, and inflammation are still unknown.
GATA4, a zinc finger domain transcription factor expressed throughout the small intestine except the distal ileum, plays an important role in maintaining jejuno-ileal differences in absorptive enterocyte gene expression [15,16]. Using a Villin-Cre approach, Battle et al. generated intestine-specific GATA4 knockout (GATA4iKO) mice and showed that 47% of ileal genes were ectopically expressed in the jejunum of these mice [15]. This jejuno-ileal transition resembles IIS. We have previously demonstrated that GATA4iKO mice were resistant to diet-induced obesity and insulin resistance owing to reduced intestinal lipid absorption and increased GLP-1 release [17].
In the present study, we have investigated the effect of intestinal GATA4 deficiency on hepatic steatosis and fibrosis. Using two dietary conditions, we provide evidence that decreased hepatic DNL leads to protection from diet-induced NAFLD in GATA4iKO mice.
Materials and methods
Animals and diets
Generation of Gata4loxP/nullVillin-Cre knockout and Gata4loxP/+ Villin-Cre control mice have previously been described [15]. All experiments were performed using male mice. Mice had free access to food and water under a 12 h light/12 h dark cycle in a temperature-controlled environment. Individually housed knockout and control littermates were fed a normal chow diet (11.9% caloric intake from fat, Ssniff®, Soest, Germany) or switched to WTD (TD88137 mod.; Ssniff®, Soest, Germany) for 20 or MCDD (A02082002B, Research Diets, Inc. New Brunswick, NJ) for 3 weeks. WTD contained 21% (weight/weight) crude fat and 0.15% (weight/weight) cholesterol with ≈ 4.53 kcal/g (42% of calories from crude fat, 15% from protein, and 43% from carbohydrate). Features of metabolic syndrome along with steatohepatitis are reported to develop after long-term WTD feeding [4]. MCDD is reported to induce severe steatohepatitis in a relatively shorter time period from 3 to 6 weeks [7]. Appropriate feeding regimens were chosen accordingly. Animal experiments were performed in accordance with the standards established by the Austrian Federal Ministry of Science and Research, Division of Genetic Engineering and Animal Experiments (Vienna, Austria).
Biochemical analyses
Blood was drawn via retro-orbital puncture and plasma was isolated for analysis. Levels of alanine aminotransferase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH) were routinely measured in 4 mice per genotype.
Oral fat tolerance test
Blood was drawn via retro-orbital puncture before and after an oral gavage of 200 μl of corn oil at pre-established time points and plasma was isolated. Plasma TG (n = 8/group) was estimated according to manufacturer’s protocol (DiaSys, Holzheim, Germany).
Hepatic TG and free fatty acid (FFA) estimation
Hepatic TG and FFA concentrations were determined from Folch extracts using enzymatic kits according to manufacturer’s protocols (DiaSys, Holzheim, Germany; Wako Chemicals GmbH, Neuss, Germany). Briefly, liver samples from GATA4iKO and control mice fed WTD (n = 5/group), MCDD (n = 4/group) or chow diet (n = 5/group) were weighed and homogenized, and lipids were extracted in chloroform:methanol (2:1) in a volume 20 times the weight of the sample. Lipid extracts were then solubilized in freshly prepared 0.2% Triton X-100 in chloroform, dried under a gentle stream of nitrogen, and resuspended in 200 μl sterile phosphate-buffered saline.
Assay for hepatic lipid peroxidation levels
Hepatic levels of thiobarbituric acid reactive substances (TBARS) were determined using the Cayman TBARS Assay kit (Cayman Chemicals, Ann Arbor, MI). Briefly, 25 mg of liver tissue from WTD-fed control and GATA4iKO mice (n = 5/group) was homogenized in 250 μl of radio-immunoprecipitation assay buffer supplemented with protease inhibitors (Roche, Indianapolis, IN) and sonicated on ice for 15 s at medium power. Supernatants were separated by centrifugation at 3500 rpm for 10 min at 4 °C. One hundred μl of each sample and standard was mixed with 4 ml color reagent containing 100 μl of sodium dodecyl sulfate solution. Samples were boiled for 1 h, and the reaction was stopped by placing samples on ice for 10 min. Thereafter, samples were centrifuged at 3500 rpm for 10 min at 4 °C. The absorbance was measured at 530 nm. Results were expressed as μM of malondialdehyde-adduct per g liver weight.
Assay for hepatic 4-hydroxyproline levels
Hepatic levels of 4-hydroxyproline were measured as previously described [18]. Briefly, liver tissues were homogenized in 2 N NaOH and autoclaved at 120 °C for 25 min for alkaline hydrolysis. The hydrolysates were cooled to RT and filtered through Whatmann filter paper No. 1 to remove particulate matter. Standard dilutions of 4-hydroxyproline (Sigma Aldrich, GmbH, Germany), samples and blanks in acetate-citrate buffer pH 6.5 were mixed in freshly prepared chloramine T and allowed to react at room temperature for 25 min. Color development was achieved by the addition of fresh Ehrlich’s aldehyde reagent and incubation at 65 °C for 20 min. Absorbances were measured at 550 nm. Results were plotted as mg collagen/g liver using the following formula:
Histochemistry and immunohistochemistry
For conventional light microscopy, livers of WTD-fed control and GATA4iKO mice (n = 3/group) and MCDD-fed control and GATA4iKO mice (n = 4/group) were fixed in 4% neutral buffered formaldehyde solution for 24 h and embedded in paraffin. Five μm sections were deparaffinized and subjected to routine haematoxylin and eosin, periodic acid Schiff and Sirius Red staining. For histochemical staining with Oil Red O, 7 μm cryosections were prepared and stained according to protocol; nuclei were counter-staining with Mayer’s haematoxylin. For the detection of GLP-1 positive cells in the jejunum of control and GATA4iKO mice, harvested jejunum was longitudinally exposed and luminal contents were washed in PBS. The tissue was immediately fixed in 10% formaldehyde for 24 h and embedded in paraffin. Five μm sections were processed for staining. Sections were deparaffinized and blocked in 2% pre-immune goat serum. Sections were incubated with anti-GLP-1 antibody (1:500; Phoenix Pharmaceuticals Inc., Burlingame, CA) overnight at 4 °C, washed, incubated with biotinylated goat anti-rabbit secondary antibody followed by signal enhancement using the ABC reagent (Vector Laboratories Inc., Burlingame, CA). Horse radish peroxidase was detected using the 3,3′-diaminobenzidine with metal enhancer reagent (Sigma Aldrich, St. Louis, MO). Images were acquired using an Olympus FSX100 at indicated magnifications.
RNA isolation and quantitative real-time PCR
Total RNA from tissues of mice was isolated using the TriFast reagent according to the manufacturer’s protocol (Peqlab Biotechnologies GmbH, Erlangen, Germany). Two μg of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed on a Roche LightCycler 480 instrument (Roche Diagnostics, Palo Alto, CA) using the QuantiFast™ SYBR® Green PCR Kit (Qiagen, Valencia, CA). Samples were measured in triplicate for each experiment and normalized to cyclophilin A mRNA expression as an internal control. Expression profiles and associated statistical parameters were analyzed using the public domain program Relative Expression Software Tool – REST 2010 (http://www.gene-quantification.com/download.html) [19]. Primer sequences are available upon request.
Western blotting
Livers from WTD-fed control and GATA4iKO mice were homogenized in radio-immunoprecipitation assay buffer and whole cell lysates were prepared and solubilized in Laemmli sample buffer. Proteins were resolved on precast Mini-PROTEAN–TGX gels (BioRad, Hercules, CA), blotted onto nitrocellulose membranes, blocked with 5% bovine serum albumin and probed with the appropriate polyclonal primary antibody overnight. After incubation with horseradish peroxidase-conjugated secondary antibody (Invitrogen, Carlsbad, CA), the immunoreactive proteins were detected using the enhanced chemiluminescence detection reagent (GE Healthcare, Piscataway, NJ). Antibodies against AMP-activated protein kinase (AMPK), phosphorylated (p)AMPK(Y172), acetyl-CoA carboxylase alpha (ACC), pACC(S79), p38 mitogen-activated protein kinase (p38), and p-p38(T180/Y182) were purchased from Cell Signaling Technology, Danvers, MA. Antibody against alpha smooth muscle actin (α-SMA) was purchased from Abcam, Cambridge, MA. For detection of the nuclear form of sterol regulatory element-binding protein 1 c (SREBP1c), 30 μg of hepatic nuclear extract prepared by using NE-PER nuclear cytoplasmic extraction reagents (Thermo Fisher Scientific, Pittsburgh, PA) was blotted and detected using an anti-SREBP1 antibody (Abcam, Cambridge, MA). The antibody against LaminA/C (Santa Cruz Biotechnology, Santa Cruz, CA) was a gift from Dr. W. Sattler (Medical University of Graz, Austria). Relative densities compared with controls were calculated from the percent area under the curve using Image J.
Statistical analysis
Plasma parameters were compared using two parameter analysis of variance (Two-way ANOVA) followed by Bonferroni post-test. Hepatic TG, FFA, hepatic TBARS concentrations, and histochemical scorings were compared using the non-parametric Mann–Whitney test. Statistical significance for the oral fat tolerance was calculated using one way analysis of variance. Statistical analysis of mRNA expression levels REST 2010 was used [19]. Values are mean ± SE. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001.
Results
Reduced hepatic TG accumulation, SREBP-1c maturation, and lipogenic gene expression in GATA4iKO mice fed WTD
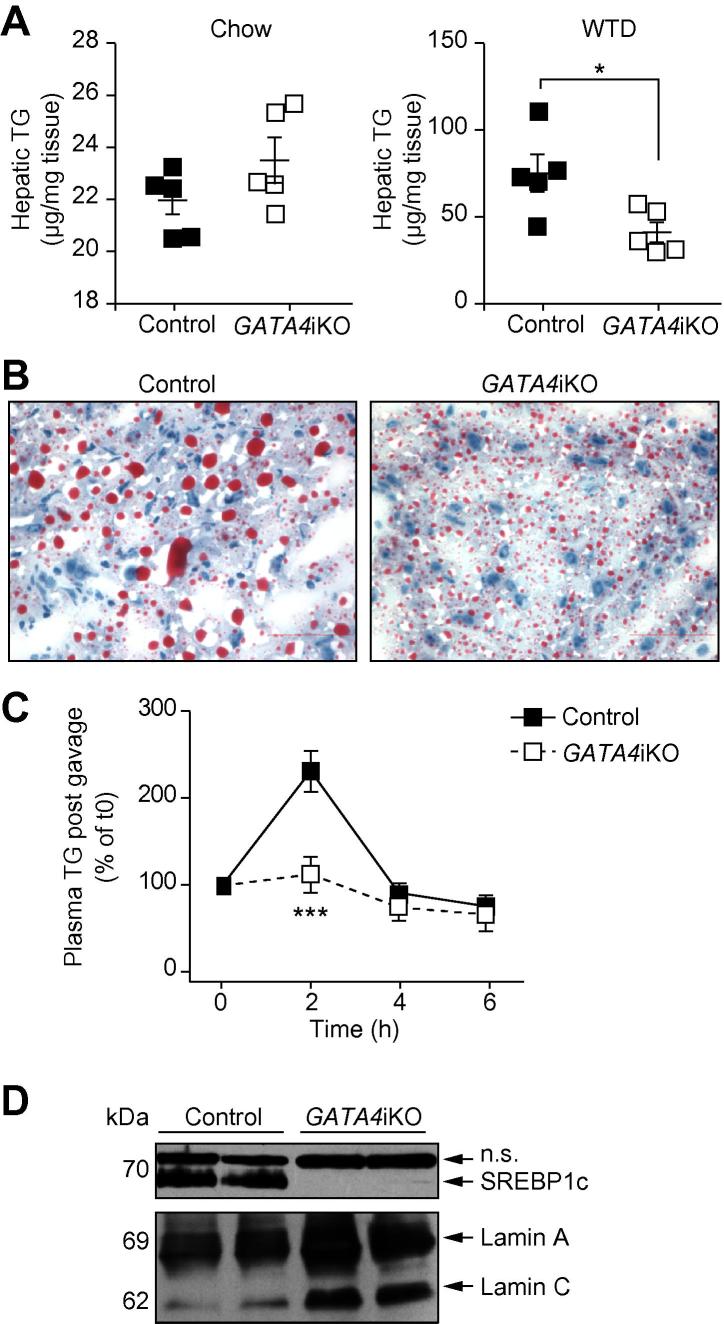
When fed a chow diet, GATA4iKO mice showed identical hepatic TG content (Fig. 1A). Twenty weeks of WTD feeding led to an increase in hepatic TG concentrations in both genotypes. GATA4iKO mice had a 44% reduction in hepatic TG levels compared with controls (Fig. 1A). These results were confirmed by Oil Red O staining of livers from WTD-fed control and GATA4iKO mice (Fig. 1B). Thus, the lack of intestinal GATA4 prevented diet-induced hepatic TG accumulation. To test for liver function, we measured serum concentrations of ALT, AST, and LDH and found them to be markedly reduced in GATA4iKO mice compared with controls (Table 1). WTD feeding also led to a decrease in body weight gain in GATA4iKO mice (Supplementary Fig. 1). To investigate the underlying mechanism of reduced hepatic TG accumulation, we first determined oral fat tolerance, which was prominently reduced in GATA4iKO mice (Fig. 1C). To compensate for the reduced intestinal uptake, we expected a proportional increase in hepatic DNL in GATA4iKO mice. Interestingly, hepatic Srebp-1c mRNA expression was reduced by 71% in WTD-fed GATA4iKO compared with control mice (Table 2). Livers from GATA4iKO animals lacked mature SREBP1c protein (Fig. 1D). Accordingly, hepatic mRNA expression of lipogenic targets of SREBP-1c was markedly decreased such as CD36 antigen, glycerol-3-phosphate acyltransferase 1, fatty acid synthase, acetyl-CoA carboxylase alpha, and stearoyl-CoA desaturase 1 in GATA4iKO compared with control mice (Table 2).
Fig. 1.
Reduced hepatic triglyceride (TG) concentrations in Western-type diet (WTD)-fed GATA4iKO mice. (A) Hepatic TG levels in Folch extracts of control and GATA4iKO mice fed chow and WTD (n = 5/group). (B) Representative Oil Red O stained images of liver sections from control and GATA4iKO mice fed WTD, showing TG (red) and nuclei (blue). (C) Plasma TG concentrations from three experiments (n = 5/group per experiment) of oral fat tolerance test expressed as percent of time 0. (D) Nuclear protein abundance of sterol response element-binding protein 1c (SREBP1c) and levels of lamin A/C as loading control. Values are means ± SE. ∗p <0.05, ∗∗∗p <0.001. n.s., not specific.
Table 1.
Plasma parameters of hepatic damage in WTD-fed GATA4iKO mice compared to controls.
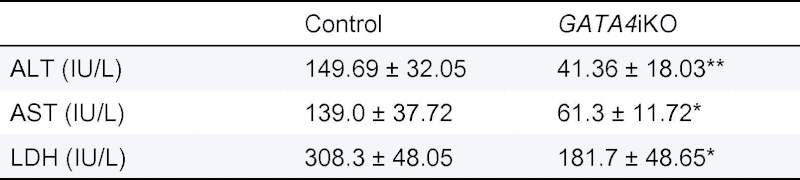 |
Values are means ± SE (n = 5/group). ∗p <0.05, ∗∗p <0.01.
Table 2.
mRNA fold change and p value for various genes in WTD-fed GATA4iKO mice compared to controls.
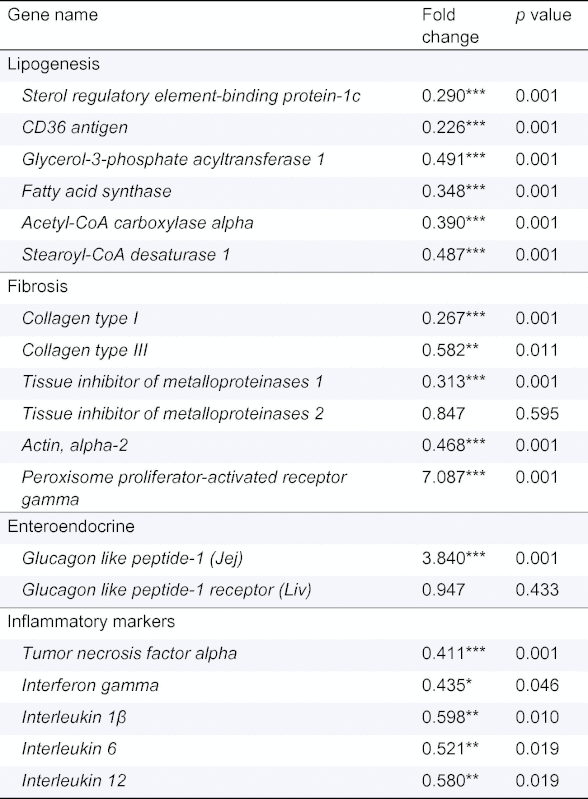 |
Jej, Jejunum; Liv, Liver.
Values are means ± SE (n = 5/group). ∗p <0.05 ∗∗p <0.01, ∗∗∗p <0.001.
Increased GLP-1 positive cells and AMPK activation in livers of GATA4iKO mice
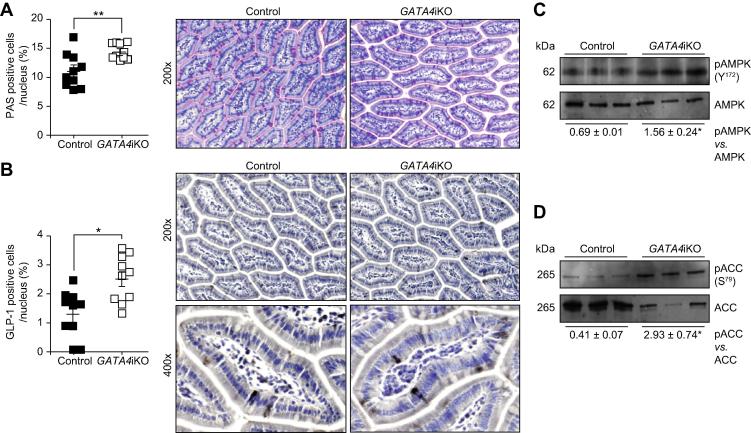
Next, we investigated the mechanism by which the lack of intestinal GATA4 suppressed hepatic maturation of SREBP-1c and lipogenic gene expression. Previous reports in inducible intestine-specific GATA4 knockout mice had shown that intestinal posteriorization led to a shift in cell type composition [15,20]. In agreement with these data, we observed a 1.4-fold increase in goblet cells in GATA4iKO jejuna [20] (Fig. 2A). To test whether intestinal posteriorization also led to an anterior increase in GLP-1 secreting L-cells, we stained for GLP-1, revealing 1.7-fold more GLP-1 positive cells in GATA4iKO compared with control jejuna (Fig. 2B). In accordance, jejunal transcript levels of Glp-1 were 3.8-fold upregulated in GATA4iKO mice while hepatic expression of Glp-1 receptor was comparable in GATA4iKO and control livers (Table 2). Since GLP-1 is known to suppress hepatic lipogenesis via activation of AMPK [21], we determined hepatic phosphorylation of AMPK and its target ACC. As shown in Fig. 2C and D, hepatic phosphorylation of both AMPK(Y172) and ACC(S79) was increased in GATA4iKO compared with control mice. Consistent with our finding of suppressed Acc transcript levels (Table 2), we also found a dramatic reduction of ACC protein expression in GATA4iKO livers (Fig. 2D).
Fig. 2.
Glucagon-like peptide 1 (GLP-1)-AMP-activated protein kinase (AMPK) pathway is active in GATA4iKO mice. (A) Jejunal periodic acid Schiff (PAS) cells/nucleus expressed in percent. Representative images of sections stained with PAS to reveal secretory cell types (pink) and nuclei (blue) in control and GATA4iKO jejunum. (B) Jejunal GLP-1 positive cells/nucleus expressed in percent. Representative images of sections immunohistochemically stained for GLP-1 positive cells (black) and nuclei (blue) in control and GATA4iKO jejunum. (C) Protein expression of AMPK, phosphorylated pAMPK(Y172), (D) acetyl-CoA carboxylase alpha (ACC) and pACC(S79, inhibitory phosphate) in control and GATA4iKO livers. Numerical values below the blots represent mean relative densities. Values represent means ± SE. ∗p <0.05, ∗∗p <0.01.
Reduced hepatic FFA concentrations, lipid peroxidation, and fibrosis in WTD-fed GATA4iKO mice
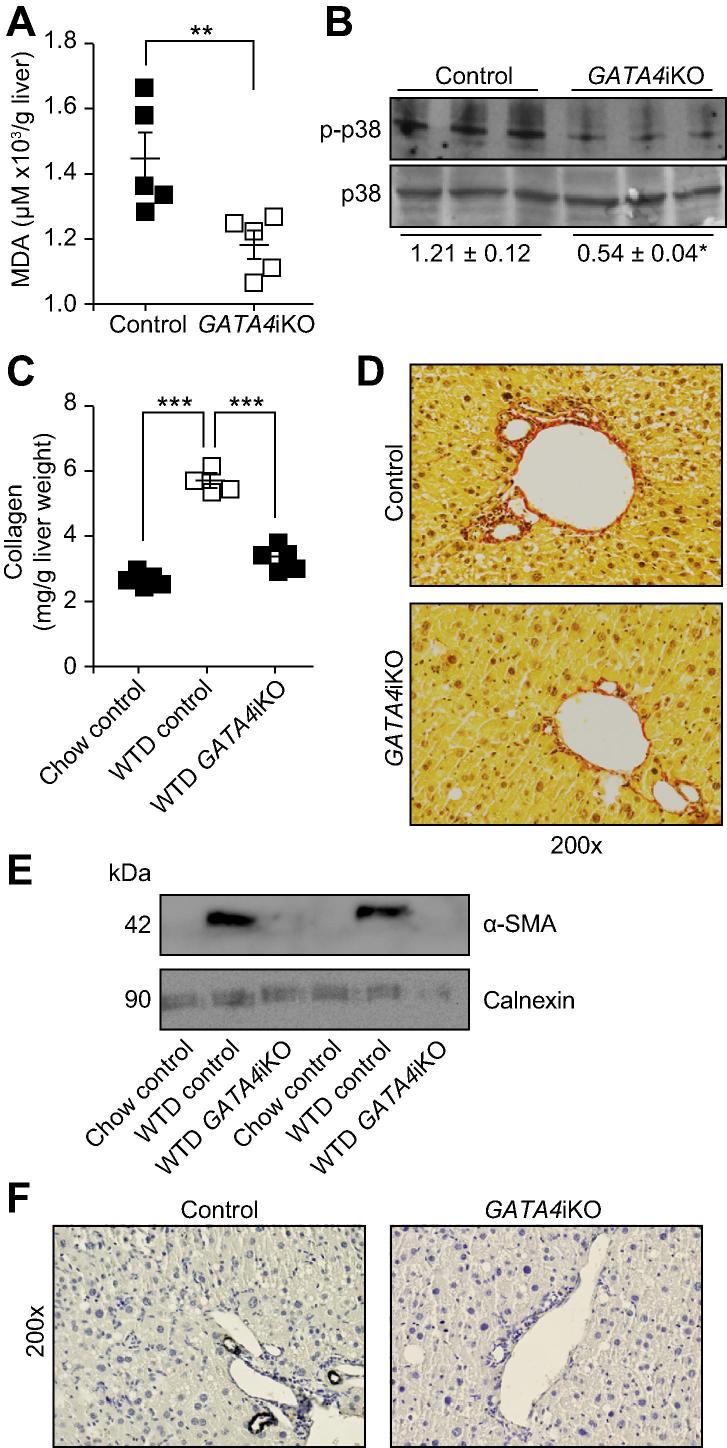
Hepatic accumulation of peroxidated lipids leads to oxidative damage contributing to hepatic injury [22]. We therefore determined the levels of FFA and TBARS in the liver. We found a 41% reduction in hepatic FFA concentrations (Supplementary Fig. 2) and a 19% decrease in hepatic lipid peroxidation (Fig. 3A) in WTD-fed GATA4iKO compared with control mice. As a result, GATA4iKO mice had lower levels of p38 phosphorylation (Fig. 3B).
Fig. 3.
GATA4iKO mice have reduced hepatic lipid peroxidation and fibrosis upon 20 weeks of WTD feeding. (A) Hepatic thiobarbituric acid reactive species levels determined as μM malondialdehyde adduct (MDA)/g liver tissue of control and GATA4iKO mice (n = 5/group). (B) Phosphorylated (T180/Y182) and total levels of the stress-induced p38 mitogen-activated protein kinase (p38) in livers from control and GATA4iKO mice. Numerical values below blots represent mean relative densities ± SE. (C) 4-Hydroxyproline concentrations converted into mg collagen/g liver (for conversion formula refer to methods) in chow-fed control, WTD-fed control and WTD-fed GATA4iKO mice (n = 4–5/group). (D) Representative images of liver sections stained for Sirius Red revealing collagen fibers (red), cytoplasm (yellow) and nuclei (gray). (E) Protein expression of alpha-smooth muscle actin (α-SMA) in livers from chow and WTD-fed control and WTD-fed GATA4iKO mice. Protein expression of calnexin was determined as loading control. (F) Representative images of liver sections immunostained for α-SMA (black) and nuclei (blue). Values are means ± SE. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001.
To assess the fibrotic potential of WTD in our model, we determined 4-hydroxyproline concentrations (presented as mg collagen normalized to liver weight), Sirius Red staining and localization and protein expression levels of α-SMA. WTD led to a 2.2-fold increase in 4-hydroxyproline concentrations in control mice but levels were comparable in WTD-fed GATA4iKO and chow-fed controls (Fig. 3C). In agreement with these results, Sirius Red staining revealed reduced collagen fibers in GATA4iKO mice compared with controls (Fig. 3D). Protein expression of α-SMA was undetectable in liver homogenates of chow-fed controls, whereas WTD feeding caused a pronounced increase in α-SMA abundance in control but not GATA4iKO mice (Fig. 3E). Immunostaining for α-SMA was performed as a marker for myofibroblast and HSC activation. Staining was restricted around arterial walls and showed 16.3% positivity in WTD-fed GATA4iKO mice compared to 70.4% positivity in WTD-fed control mice (Fig. 3F middle and Supplementary Fig. 3). Thus, WTD led to a mild but significant hepatic fibrosis only in control mice. Hepatic transcript levels of collagen type I (73%), collagen type III (42%), tissue inhibitor of metalloproteinases 1 (69%), and actin, alpha-2 (53%) were downregulated in GATA4iKO compared with control mice (Table 2). The mRNA expression of peroxisome proliferator-activated receptor gamma (Pparg), a suppressor of HSC activation, was 7.1-fold upregulated in GATA4iKO mice (Table 2).
Haematoxylin and eosin staining indicated lower immune cell infiltration in WTD-fed GATA4iKO mice compared with controls (Supplementary Fig. 4). We also observed fewer CD45-positive cells in GATA4iKO mice (Supplementary Fig. 4). Transcript levels of the inflammatory cytokines tumor necrosis factor alpha (59%), interferon gamma (57%), interleukin 1β (40%), interleukin 6 (48%), and interleukin 12 (42%) were also downregulated in WTD-fed GATA4iKO mice (Table 2).
GATA4iKO fails to protect mice against MCDD-induced hepatic steatosis
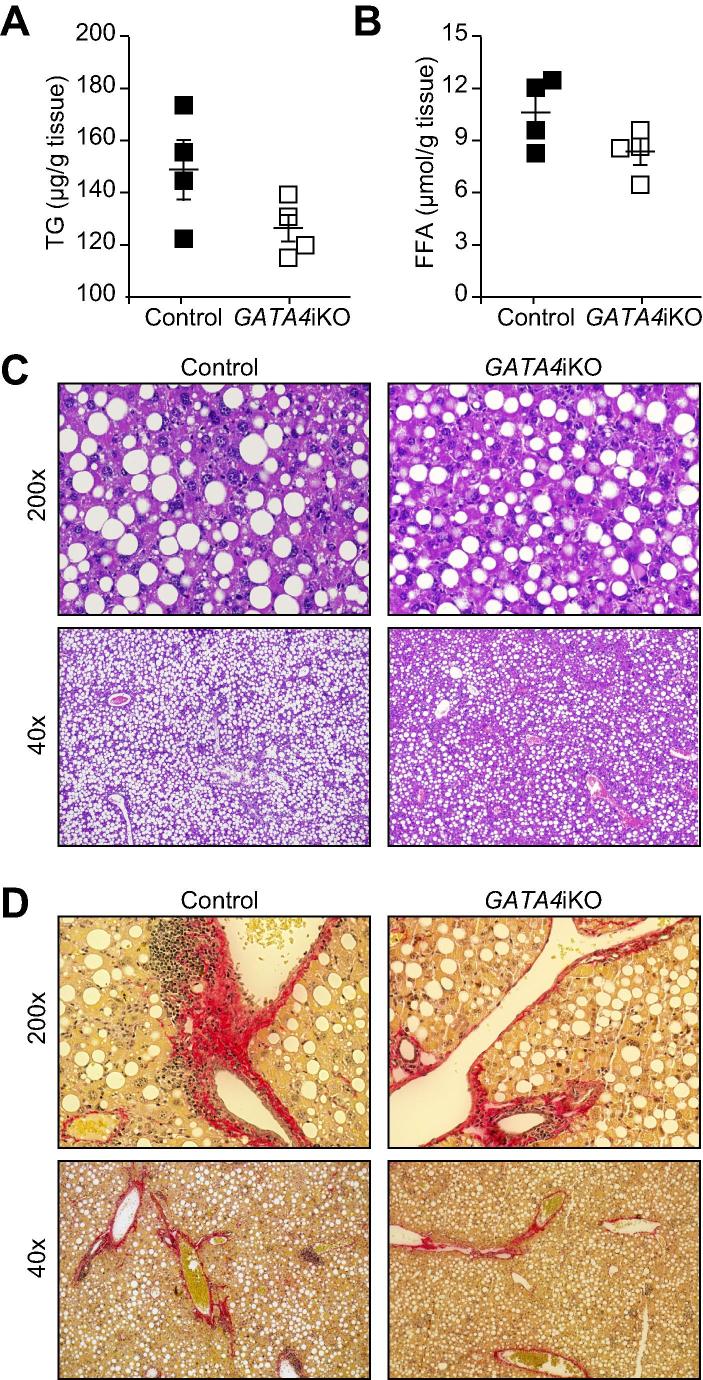
We observed that the protective effects of GATA4iKO were absent when mice were challenged with MCDD. MCDD feeding led to a similar extent of weight loss (Supplementary Fig. 5) and hepatic injury in both genotypes as indicated by comparable levels of plasma AST, ALT, and LDH (Table 3). Hepatic accumulation of TG and FFA was unaffected (Fig. 4A and B). Assessment of liver damage as determined by haematoxylin and eosin as well as Sirius Red staining also indicated significant fat accumulation and fibrosis in both genotypes (Fig. 4C and D). In accordance, mRNA expression of genes involved in lipogenesis and fibrosis was unchanged (Table 4).
Table 3.
Plasma parameters for hepatic damage in MCDD-fed GATA4iKO mice compared to controls.
 |
Values are means ± SE (n = 4/group).
Fig. 4.
Intestinal GATA4 deficiency does not protect from methionine and choline-deficient diet (MCDD)-induced hepatic steatosis. Control and GATA4iKO mice (n = 4/group) were fed MCDD for 3 weeks. (A) Hepatic TG and (B) FFA concentrations determined in hepatic lipid extracts. (C) Representative images of liver sections stained for haematoxylin and eosin. (D) Representative images of liver sections stained for Sirius Red revealing collagen fibers (red), cytoplasm (yellow) and nuclei (gray). Values represent means ± SE.
Table 4.
mRNA fold change and p value for various genes in MCDD-fed GATA4iKO mice compared to controls.
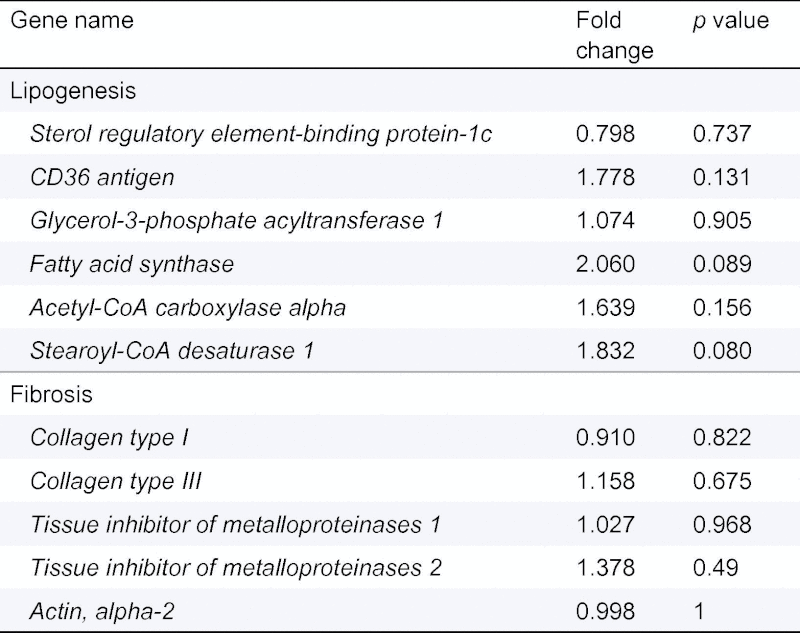 |
Values are means ± SE (n = 4/group).
Discussion
In this report, we show that WTD but not MCDD-fed GATA4iKO mice accumulate less hepatic TG and have reduced fibrosis than control mice. These findings suggest that intestinal deficiency of GATA4 has a role on hepatic metabolism and prevents diet-induced liver damage only in a setting that mimics metabolic disease. We had previously reported that GATA4iKO mice were resistant to diet-induced obesity on long-term WTD feeding [17]. A reduction in intestinal lipid absorption leads to a compensatory increase in hepatic DNL. Although GATA4iKO mice showed reduced intestinal TG absorption, hepatic DNL was suppressed. We speculate that GLP-1 leads to hepatic protection in GATA4iKO mice since TG-induced GLP-1 release is increased [17]. Hepatic protection by GLP-1 has also been demonstrated in genetic or dietary models that resemble obesity and metabolic syndrome but not under MCDD conditions. Therefore, GLP-1 release could be one of the endocrine mechanisms involved in the observed suppression of hepatic DNL in GATA4iKO mice. It has been reported that exendin-4, a GLP-1 receptor agonist, significantly reduces hepatic steatosis in genetically obese ob/ob mice and exerts a direct effect on the reduction of hepatic steatosis [23,24]. We found more GLP-1 positive cells and increased GLP-1 mRNA expression in GATA4iKO jejuna. These findings are in good correlation with data obtained in rats that have undergone IIS [14]. GLP-1 is known to suppress hepatic lipogenesis via activation of the AMPK pathway [21]. In livers from GATA4iKO mice, increased intestinal GLP-1 secretion resulted in AMPK phosphorylation, leading to phosphorylation and inhibition of its target gene ACC, a key lipogenic enzyme. A recent study has shown that AMPK activation phosphorylates SREBP-1c at Ser372 and thus inhibits the cleavage and subsequent nuclear translocation of this lipogenic transcription factor [25]. Our results indicate AMPK-mediated inhibition of SREBP-1c maturation and subsequent downregulation of DNL being operative in livers of GATA4iKO mice. We conclude that apart from reduced intestinal absorption [17], GLP-1-mediated suppression of hepatic DNL is an important contributor to the observed hepatic protection in GATA4iKO mice.
The major cellular event in the development of liver fibrosis is the triggering of quiescent HSC into an active state [26]. Pparg is a known suppressor of HSC activation. Increased serum leptin levels have a net pro-fibrotic effect on the liver since leptin inhibits Pparg gene expression in HSC [27]. In contrast, adiponectin inhibits HSC proliferation and suppresses DNL by inhibiting SREBP1c maturation. Therefore, adiponectin attenuates progression of both alcoholic and NAFLD [28,29]. We also found decreased transcript levels of fibrotic and inflammatory marker genes and an increase of Pparg mRNA in the liver of GATA4iKO mice, indicating reduced HSC activation. GATA4iKO mice have decreased serum leptin (even on normal chow) and increased adiponectin concentrations [17,30]. We speculate that the leptin and adiponectin status in GATA4iKO mice leads to the observed upregulation in Pparg transcript levels in a HSC and not hepatocyte-specific manner. The effect of these adipokines on the activity and expression of hepatocyte Pparg is so far unknown. Expression of α-SMA, a marker of activated HSC, was reduced in GATA4iKO livers supporting our hypothesis that a favorable adipokine status in GATA4iKO mice may operate as an additional protective mechanism against fibrosis.
In summary, our results provide evidence that GATA4iKO in mice reduces intestinal TG uptake and lowers hepatic DNL through increased GLP-1 production. Our findings imply beneficial effects of GATA4iKO protecting against NAFLD and hepatic steatosis under conditions of metabolic syndrome.
Financial support
This work was supported by the PhD Program “Molecular Medicine” of the Medical University of Graz, the Austrian Science Fund (P22832, SFB LIPOTOX F30, DK-MCD W1226 and P19186), the Austrian Federal Ministry of Science and Research (GEN-AU project Genomics of Lipid-associated Disorders-GOLD) and the Austrian National Bank (12929). Jay Patankar and Prakash Doddapattar were funded by and Sascha Obrowsky is a fellow of the PhD program “Molecular Medicine”.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Acknowledgements
The authors thank S. Povoden and A. Ibovnik for technical help and S. Schauer for assistance with histochemistry. We also thank I. Hindler for support with animal maintenance and care.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2012.06.028.
Appendix Supplementary. data
This document file contains Supplementary Figs. 1–5.
References
- 1.Muoio D.M., Newgard C.B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Postic C., Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 3.Savage D.B., Semple R.K. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol. 2010;21:329–336. doi: 10.1097/MOL.0b013e32833b7782. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve L.D., Wang X., Kanel G.C., Atkinson R.D., McCuskey R.S. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brackmann C., Gabrielsson B., Svedberg F., Holmaang A., Sandberg A.S., Enejder A. Nonlinear microscopy of lipid storage and fibrosis in muscle and liver tissues of mice fed high-fat diets. J Biomed Opt. 2010;15:066008. doi: 10.1117/1.3505024. [DOI] [PubMed] [Google Scholar]
- 6.Choi D.H., Choi J.H., Whang S.K., Kim Y.S. Regulation of acetyl CoA carboxylase mRNA in rat liver by high carbohydrate diet and insulin. Yonsei Med J. 1989;30:235–245. doi: 10.3349/ymj.1989.30.3.235. [DOI] [PubMed] [Google Scholar]
- 7.Anstee Q.M., Goldin R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polak J.M., Bloom S.R., Pearse A.G. Gut endocrinology: correlation of physiology and morphology. Clin Endocrinol (Oxf) 1976;(5 Suppl.):205S–216S. doi: 10.1111/j.1365-2265.1976.tb03829.x. [DOI] [PubMed] [Google Scholar]
- 9.Bloom S.R., Polak J.M. Gut hormones. Adv Clin Chem. 1980;21:177–244. doi: 10.1016/s0065-2423(08)60089-x. [DOI] [PubMed] [Google Scholar]
- 10.Mummadi R.R., Kasturi K.S., Chennareddygari S., Sood G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Culnan D.M., Albaugh V., Sun M., Lynch C.J., Lang C.H., Cooney R.N. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. Am J Physiol Gastrointest Liver Physiol. 2010;299:G751–G760. doi: 10.1152/ajpgi.00525.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strader A.D., Vahl T.P., Jandacek R.J., Woods S.C., D’Alessio D.A., Seeley R.J. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kindel T.L., Yoder S.M., Seeley R.J., D’Alessio D.A., Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 14.Kohli R., Kirby M., Setchell K.D., Jha P., Klustaitis K., Woollett L.A. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–G660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battle M.A., Bondow B.J., Iverson M.A., Adams S.J., Jandacek R.J., Tso P. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686. doi: 10.1053/j.gastro.2008.07.074. e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosse T., Fialkovich J.J., Piaseckyj C.M., Beuling E., Broekman H., Grand R.J. Gata4 and Hnf1alpha are partially required for the expression of specific intestinal genes during development. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1302–1314. doi: 10.1152/ajpgi.00418.2006. [DOI] [PubMed] [Google Scholar]
- 17.Patankar J.V., Chandak P.G., Obrowsky S., Pfeifer T., Diwoky C., Uellen A. Loss of intestinal GATA4 prevents diet-induced obesity and promotes insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2011;300:E478–E488. doi: 10.1152/ajpendo.00457.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy G.K., Enwemeka C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosse T., Piaseckyj C.M., Burghard E., Fialkovich J.J., Rajagopal S., Pu W.T. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Shlomo S., Zvibel I., Shnell M., Shlomai A., Chepurko E., Halpern Z. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 23.Ding X., Saxena N.K., Lin S., Gupta N.A., Anania F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta N.A., Mells J., Dunham R.M., Grakoui A., Handy J., Saxena N.K. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safadi R., Friedman S.L. Hepatic fibrosis – role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] [Google Scholar]
- 27.Sun K., Wang Q., Huang X.H. PPAR gamma inhibits growth of rat hepatic stellate cells and TGF beta-induced connective tissue growth factor expression. Acta Pharmacol Sin. 2006;27:715–723. doi: 10.1111/j.1745-7254.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu A., Wang Y., Keshaw H., Xu L.Y., Lam K.S., Cooper G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi M., Brenner D.A. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677–685. doi: 10.1002/hep.21991. [DOI] [PubMed] [Google Scholar]
- 30.Shulzhenko N., Morgun A., Hsiao W., Battle M., Yao M., Gavrilova O. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document file contains Supplementary Figs. 1–5.