Soluble guanylate cyclase (sGC) is a key receptor in the mammalian nitric oxide (NO) signaling pathway, which is involved in important physiological processes such as vasodilation, platelet aggregation, and neurotransmission.[1–5] NO directly activates sGC, leading to increased formation of the second messenger cyclic guanosine 3′,5′-monophosphate (cGMP) from guanosine 5′-triphosphate (GTP), which goes on to mediate the diverse physiological functions noted above.[6] sGC is a heterodimeric hemoprotein; most commonly found as the α1/β1 isoform. The N-terminus of the β1 subunit contains a Heme-Nitric oxide/OXygen binding (H-NOX) domain, a conserved gas-sensing domain found in prokaryotes and eukaryotes.[7–9] The C-termini of the α1 and β1 subunits together form the catalytic domain that is responsible for the Mg2+-dependent conversion of GTP to cGMP. NO activates sGC by binding to the ferrous heme in the H-NOX domain leading to the cleavage of the Fe2+-His bond.[9–11] Ferrous heme is essential for NO-mediated sGC function; NO is a poor ligand for ferric sGC and does not activate the enzyme.[12,13] sGC is highly resistant to oxidation by molecular oxygen (O2); the inability to form a ferrous-oxy complex certainly contributes to this stability. However, reactive oxygen species can oxidize sGC heme under conditions of oxidative stress, leading to a decreased sensitivity to NO in the diseased tissue.[14–16] This desensitization to NO, results in tolerance to treatments involving NO-donors.[17–19] While the extent of sGC oxidation in vivo is not known, oxidation of sGC under pathological conditions is thought to be an important contributor to the development of cardiovascular disorders and the decrease in effectiveness of treatments involving NO-donors.[17–19] Therefore, sGC has emerged as a promising pharmacological target for the treatment of cardiovascular and pulmonary disorders.[17,19, 20]
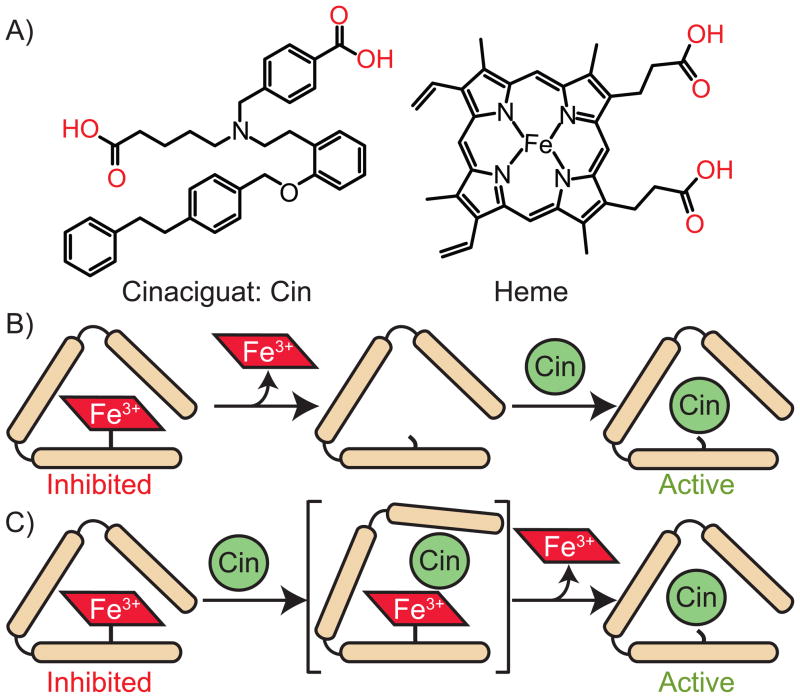
Since the emergence of sGC as a therapeutic target for cardiovascular disease, two classes of molecules have been developed: sGC stimulators and sGC activators. sGC stimulators, such as YC-1, the first sGC stimulator discovered, and BAY 41–2272, act directly on native, ferrous sGC.[21–24] In contrast, recently discovered sGC activators, such as BAY 58–2667 (cinaciguat, Figure 1A) and HMR-1766, have effects on both oxidized (ferric) and/or heme-free sGC.[25,26] Cinaciguat, one such activator, can cause a 200-fold increase in the activity of oxidized and/or apo sGC.[25] Previous studies have shown that cinaciguat binds to the ferrous, ferric and apo states of sGC, but only activates the oxidized or apo forms of the enzyme.[25] A recently reported co-crystal structure of a homologous H-NOX domain from Nostoc sp. (35% sequence identity to sGC) with cinaciguat showed the sGC activator bound in the heme pocket in place of the native heme cofactor.[27,28] The co-crystal structure showed that the two carboxylic acid moieties of cinaciguat mimicked the interaction of the heme propionate side chains (Figure 1A) with the conserved Y-S-R motif, three conserved amino acids known to be important for heme binding in H-NOX domains.[27–31] However, the exact mechanism of cinaciguat activation of ferric sGC has so far remained unclear. Two different mechanisms are possible: Cinaciguat could bind to the vacant heme pocket of apo sGC or alternatively, cinaciguat could interact with the ferric enzyme and facilitate the direct displacement of the heme (Figure 1B and 1C). Due to the significant role of oxidized sGC in the progression of cardiovascular diseases as well as the therapeutic potential of sGC activators, it is vital to understand the underlying mechanism of action of these compounds. In this study, we demonstrate that oxidized sGC loses heme more readily than the ferrous enzyme and that the activation mechanism of cinaciguat involves facilitation of heme loss from ferric sGC and subsequent replacement of heme in the binding pocket.
Figure 1.
The structure of cinaciguat (Cin) and heme (A), possible mechanisms for the replacement of ferric heme in sGC by cinaciguat (B and C). In (B), oxidized sGC loses heme and then cinaciguat binds and activates the apo form of the enzyme. In (C), cincaciguat is shown to play a direct role in ferric heme displacement forming a cinciguat-sGC complex.
Therapeutic interest in sGC is in part based on the hypothesis that sGC oxidation and heme loss play a role in cardiovascular disorders and that sGC activators could potentially rescue oxidized or apo sGC. This hypothesis relies on the following untested assumptions: 1) oxidation of the sGC heme occurs, 2) reduced affinity of the ferric heme to the protein leads to heme dissociation and the formation of apoprotein, and 3) the pharmacological effects of sGC activators involve the rescue of ferric and/or apo-sGC. The work reported here directly tests these fundamental assumptions of sGC dysfunction and the underlying mechanism for the activation of sGC by the activator cinaciguat.
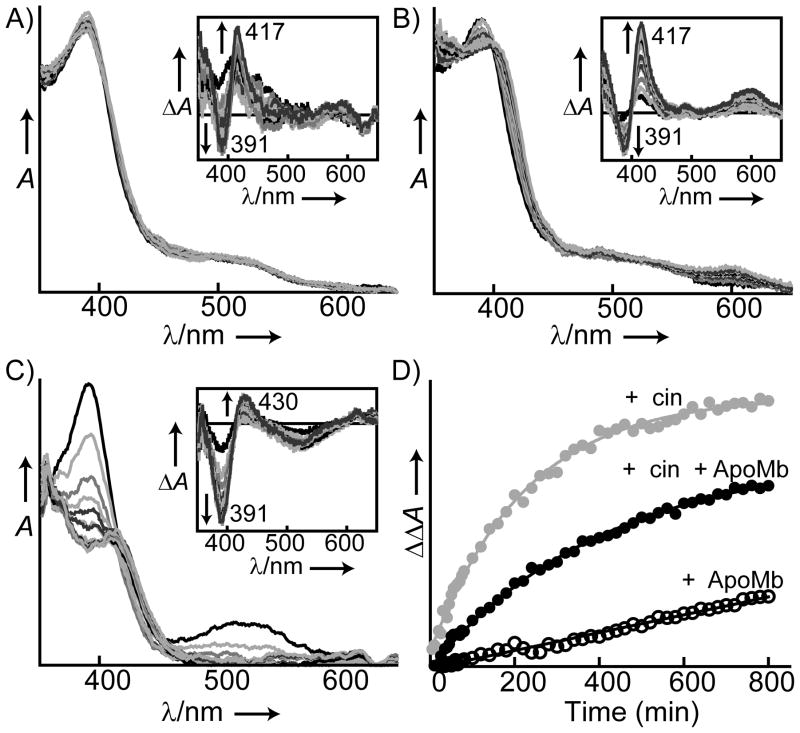
To assess the stability of oxidized sGC, the rate of heme dissociation from the ferric enzyme was investigated using both the full-length mammalian enzyme and previously reported H-NOX domain truncations of sGC.[9,33] To prevent heme rebinding, an apomyoglobin (apoMb) variant H64Y/V68F was used as a heme trap.[37] Previous studies have shown that apomyoglobin H64Y/V68F binds heme rapidly (kH ~ 1 × 108 M−1s−1) and exhibits a distinct absorbance spectrum.[38] Full length sGC (2.7 μM) was oxidized with 5 μM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), and heme oxidation was confirmed by the spectral shift in the Soret maximum from 430 to 391 nm.[14] The oxidized enzyme was diluted to final concentration of 0.6 μM in the buffer containing the apomyoglobin variant (50 μM, an 80-fold excess), and the spectral change was monitored for 13 hr at 22 °C (Figure 2A).[14,39] Heme loss from oxidized sGC and the subsequent binding to the apomyoglobin variant resulted in a decrease in the absorbance at 391 nm and a concurrent increase in the absorbance at 417 nm (Figure 2A, inset). The spectral change [ΔΔAbs(417–391)] was fit to a single exponential equation to determine the rate of heme dissociation (Figure 2D). Ferric sGC exhibited a rate of heme loss of (5.0 ±1.0) ×10−4 min−1 and a t1/2 of approximately 23 hrs. When ferrous sGC (1.9 μM) was incubated with an excess of the apomyoglobin variant (50 μM) under the same conditions, no heme loss was observed (Figure S1 in the Supporting Information). Additionally, ferrous sGC maintained both basal and fully NO-stimulated activity following 15 hours of incubation with the myoglobin trap (Figure S1, inset). Taken together, these observations demonstrate that heme dissociation from ferrous sGC is very slow and that ferrous sGC is very stable. The same would appear to be true in vivo as these data parallel previous findings regarding the stability of sGC in endothelial cells.[40] In previous studies Stasch et al. observed that cells treated with ODQ exhibited significant decreases (40%) in sGC protein levels after 8 hr; showing that oxidized sGC is prone to degradation in vivo. However, inhibiting global protein expression without ODQ resulted in no detectable loss of sGC during the same time period.[40] The apparent stability of native sGC can be explained by the intrinsic stability of ferrous sGC as seen in Figure S1. These results support the hypothesis that ferric sGC is more prone to heme loss, and therefore, is less stable than the ferrous enzyme, both in vitro and in cellular conditions.
Figure 2.
Time-dependent heme loss from oxidized sGC with and without cinaciguat. Representative changes in the UV–visible spectra of ferric sGC (0.6 μM) under various conditions over time (13 hr): A) in the presence of the apoMb H64Y/V68F trap (50 μM), B) in the presence of cinaciguat (20 μM) and the H64Y/V68F apoMb trap (50 μM) and C) in the presence of cinaciguat (20 μM) without the H64Y/V68F apoMb trap. The Soret absorbance maximum shifts from 391 nm to 408 nm in A and B, while it shifts from 391 nm to 415 nm in C. D) Timecourse of heme dissociation from oxidized sGC was obtained from the spectral changes: with the H64Y/V68F apoMb trap (ApoMb) in the absence (open circles) and presence (black) of cinaciguat (cin), and in the presence of cinaciguat without H64Y/V68F apoMb trap (grey). Data was extracted from the difference spectra and plotted with a single exponential fit.
The most remarkable property of sGC is perhaps the aerobic stability of the ferrous heme cofactor, a property attributable, in part, to the fact that the enzyme does not form a stable ferrous-oxy complex.[12,41] Although prone to oxidation, studies with various heme domain truncations have been helpful in characterization, since the amounts available of the full-length enzyme often preclude certain types of experiments. Indeed, a recent study with a truncated insect sGC from Manduca sexta (Ms) reported on the heme dissociation from the oxidized protein.[15] These results from Montfort and colleagues are in agreement with the findings reported here, showing that oxidized sGC is more prone to heme dissociation than reduced sGC.[15] However, the rate of heme dissociation for truncated Ms sGC, lacking the catalytic domain [α(49–450)β(1–380)], was found to be 18 times faster (9 × 10−3 min−1) than that of the full-length mammalian enzyme. To assess the influence of the other sGC domains on heme stability, rates of heme loss were measured for shorter sGC H-NOX domain truncations.[9,33] The rate of heme dissociation from sGC constructs β1(1–385), β1(1–194) and β2(1–217) were investigated using the same apomyoglobin variant as a heme trap. As with the shorter Ms construct, these sGC truncations were found to have significantly faster heme dissociation rates (0.9 – 10 × 10 −2 min−1) compared to the full-length enzyme (5 × 10−4 min−1) (Figure S2). These results provide quantitative evidence that the heme affinity of sGC is influenced by domain architecture remote from the immediate heme environment. The increase in rate of heme dissociation in H-NOX domains and truncations of sGC may be due to a greater degree of flexibility in these truncated constructs thereby facilitating heme dissociation.
Cinaciguat was shown to activate both the oxidized and apo states of sGC through a mechanism that likely involves binding in place of the heme cofactor.[25] However, it was not clear whether the effects of cinaciguat are due to binding to the apo protein or if cinaciguat actively displaces the ferric heme (Figure 1B and 1C).[25,42] There are two most likely possibilities. One, cinaciguat acts on apo sGC and action is delayed until heme dissociates from ferric sGC (t1/2 ~ 23 hrs, vide supra). Alternatively, cinaciguat may interact with ferric sGC directly, inducing and accelerating heme loss. To probe the mechanism of action, the effect of cinaciguat on heme dissociation was determined using the heme trap method as described above. sGC (2.7 μM) oxidized with 5 μM ODQ was diluted to 0.6 μM in buffer with cinaciguat (20 μM) in the presence or absence of the apomyoglobin trap (50 μM), and UV-vis spectral changes were monitored (Figure 2B). The rate of heme dissociation from ferric sGC was determined by fit of the data to a single exponential (Figure 2D). In the presence of cinaciguat, the rate of heme dissociation from ferric sGC was (2.3 ± 0.15) × 10−3 min−1, five times faster than heme dissociation from ferric sGC without cinaciquat (5 × 10−4 min−1). A rapid decrease in the Soret absorbance of sGC at 391 nm was also observed, when sGC (0.6 μM) was incubated with cinaciguat (20 μM) in the absence of apomyoglobin trap (Figure 2C). The rate of heme dissociation in the absence of apomyoglobin was faster (4.5 ± 1.4 × 10−3 min−1). The decrease in rate of heme dissociation in the presence of apomyoglobin was due to a weak binding between apomyoglobin and cinaciguat as verified by equilibrium dialysis (results not shown). In addition, cinaciguat had no effect on heme dissociation from the reduced enzyme (Figure S3). The effect of cinaciguat on heme dissociation cannot be explained by binding to apo-sGC and shifting the equilibrium (Figure 1B), because apomyoglobin, which has a very high affinity for heme (KD ~10−14 M), is in excess (80 equiv); thus heme re-binding to sGC never takes place.[37] In addition, since the binding of heme to apomyoglobin is rapid the only rate-determining step in this process is the dissociation of heme from sGC. Taken together, cinaciguat acts to increase the rate of heme dissociation through direct interaction with ferric sGC. These results confirm that cinaciguat can selectively activate oxidized sGC by preferentially binding to ferric state and further suggest that cinaciguat actively displaces the ferric heme cofactor (Figure 1C).
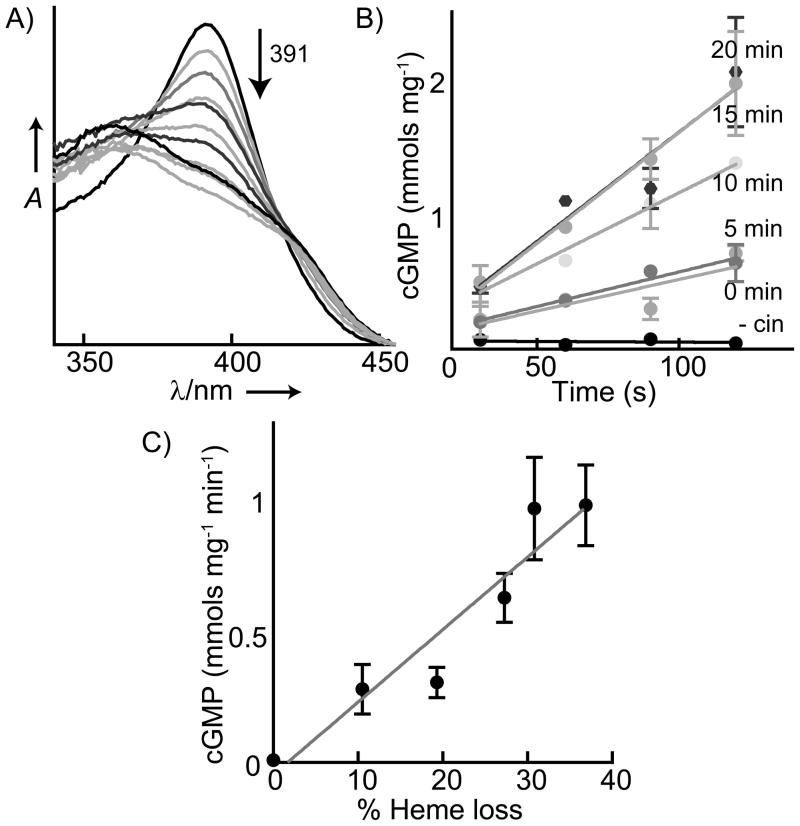
Although cinaciguat accelerated heme loss from sGC, it was not clear if the displacement of the heme leads to increased activity levels. To establish whether the binding of cinaciguat to the sGC heme pocket is the basis of activation, heme loss and activity were measured concurrently. sGC (1.8 μM) was oxidized with ODQ (7.5 μM) and incubated with cinaciguat (20 μM). Throughout the time-course of heme loss (Figure 3A) aliquots of sGC were withdrawn and frozen for subsequent activity measurements. The percent of heme loss was monitored from UV–vis spectra by following the decrease in the ferric sGC Soret maximum (391 nm). The specific activity for each time-point was determined by measuring the initial rate of cGMP formation as quantified by an ELISA assay (Figure 3B). Initially, as cinaciguat replaced oxidized heme, there was an increase in the activity of oxidized sGC, and the increase in activity correlated with the percent of heme displaced (Figure 3C). This result shows that the binding of cinaciguat at the heme pocket directly leads to enzyme activation. The trend toward increased sGC activity ceased after 30 minutes (approximately 60% oxidized heme remaining) despite ongoing heme displacement for the duration of the assay (Figure S4). The activity of the oxidized enzyme alone did not change during this time period (Figure S5). These data suggest that the decrease in the potency of cinaciguat at longer time periods is most likely linked to the long term stability of cinaciguat-bound sGC. Nevertheless, in the initial phase of the incubation, it is clear that rescued sGC activity correlates well with cinaciguat displacement of the oxidized heme.
Figure 3.
Correlation of ferric sGC heme loss with activity in the presence of cinaciguat. A) Heme dissociation from ferric sGC (1.8 μM) in the presence of cinaciguat (20 μM) was observed via the decrease in the sGC Soret maximum (391 nm) with time. B) The initial rate of sGC activity is shown from aliquots withdrawn at the same time as spectra were taken in (A) (without cinaciguat: - cin). C) Correlation of the increase in ferric sGC activity with heme replaced by cinaciguat.
Although detection of heme-free sGC is the subject of active investigation,[43] thus far direct detection of oxidized sGC in vivo is unprecedented. Cinaciguat-induced activation of sGC is selective for the oxidized enzyme; cinaciguat only marginally activates the reduced or NO-bound state of sGC.[25] It is conceivable that this selectivity could be leveraged as a probe for the oxidation state of sGC in cells. Because ferric heme dissociates slowly from sGC, oxidized, dysfunctional sGC is predicted to persist in vivo. Therefore, the increase in sGC activity induced by the addition of cinaciguat could be diagnostic of the extent of sGC oxidation. Indeed, a similar approach has been used to determine the oxidative burden in coronary artery disease patients.[44] The ability to detect the redox state of sGC under normal and diseased conditions has important implications for assessing the relationship between sGC dysfunction and disease progression.
Diseased cardiovascular tissue is often less sensitive to the effects of NO.[16] Oxidation of sGC could play a central role in this desensitization to NO. The recent focus on the discovery of small molecule effectors of sGC was spurred on by the hypothesis that oxidized sGC, known to be insensitive to NO, could be targeted for treatment. The two classes of sGC effectors developed thus far can be divided into heme-dependent stimulators and heme-independent activators. sGC stimulators act to increase the activity of ferrous sGC. These types of molecules will have limited clinical utility under conditions of oxidative stress because sGC appears to become oxidized. Because of this limitation, attention has shifted towards targeting oxidized sGC. Understanding the mechanism of action of heme-independent sGC activators is vital to the development of therapeutics. The results reported here establish that the sGC activator cinaciguat actively displaces the ferric heme of oxidized sGC. Furthermore, cinaciguat-induced displacement of the sGC heme is directly associated with increased enzyme activity. Future experiments will be aimed at delineating the molecular details underlying the cinaciguat-induced displacement of the oxidized sGC heme.
Experimental Section
Materials
sGC from Rattus novegicus was obtained via a baculovirus/Sf9 expression system and purified as previously described.[32] sGC heme domain constructs β1(1–385) and β2(1–217) were expressed in E. coli and purified as previously described.[33] The mouse myoglobin mutant H64Y/V68F was obtained using site-directed mutagenesis following the Quikchange protocol (Stratagene). All DNA primers were obtained from Integrated DNA Technologies, Inc. and sequencing was performed by Quintara Biosciences. H64Y/V68F mouse myoglobin was expressed with an N-terminal hexahistidine tag from plasmid pCW. Similarly, sGC heme domain β1(1–194) was expressed with a C-terminal His tag from plasmid pCW. These proteins were expressed and purified from RP523 E. coli cells as previously described (see Supporting Information).[34,35] 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA/NONOate) were obtained from Cayman chemicals. Cinaciguat (Bay 58–2667) was provided by Bayer HealthCare (Germany). All other reagents were analytical grade.
Heme-dissociation assay
The rates of heme dissociation were determined from the decrease in Soret absorbance of sGC or sGC heme domains, and the concurrent increase in myoglobin H64Y/V68F Soret absorbance. Spectra from 350–750 nm were recorded for 13 hours on a Cary UV-vis spectrometer at 22°C using a 1-cm path length septum-sealed cuvette. The absorbance changes were plotted versus time and fit to a single exponential equation (supplemental Eq. 1). To monitor heme dissociation under various conditions, sGC (2.7 μM) was oxidized with ODQ (5 μM) in spectral buffer (40 mM HEPES, pH 7.4, 120 mM NaCl, 10 % v/v glycerol) at 4 °C under anaerobic conditions. Then, the oxidized sGC sample was diluted to a final concentration of 0.6 μM in three different samples in spectral buffer containing: a) apomyoglobin variant (50 μM), b) apomyoglobin variant (50 μM) and cinaciguat (20 μM), and c) cinaciguat (20 μM). The samples were mixed thoroughly, and the changes in UV-vis spectra were monitored for 13 hrs, spectra taken at 1hr intervals are shown in Figure 2. In Figure 2C, noise in UV spectra was reduced by 5 point adjacent averaging for clarity. Heme dissociation from sGC heme domains is described in the Supporting Information.
Correlation of sGC activity with heme dissociation
sGC (1.8 μM) was oxidized with ODQ (7.5 μM) and incubated alone or with cinaciguat (20 μM) in spectral buffer. Throughout the time-course of heme loss, aliquots of sGC (0.5 μL) were withdrawn, diluted in HEPES buffer (9.5 μL, 50 mM at pH 7.4) and immediately frozen on dry ice in the glove bag. The percent heme loss was obtained from UV-vis spectra by following the decrease in the ferric sGC Soret maximum. The final heme content of sGC was determined from the Soret peak after the removal of the labile heme by gel filtration. Frozen aliquots of sGC were thawed at 37 °C and added to HEPES activity buffer (90 μL, 50 mM at pH 7.4) containing GTP (1 mM) and MgCl2 (2.5 mM). Activity assays were carried out at 25 °C. At 30, 60, 90 and 120 seconds, the reaction mixture (20 μL) was withdrawn and the reaction quenched by the addition of Zn(CH3CO2)2 (80 μL, 125 mM) and Na2CO3 (100 μL, 125 mM). The cGMP formed was quantified using a cGMP ELISA Kit (Enzo Life Sciences) following the manufacturer’s instructions.
Activity of ferrous sGC
Activity of ferrous sGC was determined as previously described.[36] Briefly, samples contained sGC (0.2 μg) and DTT (1 mM) in HEPES buffer (50 mM at pH 7.4). Where necessary, DEA/NONOate (0.3 μL, 38 mM stock dissolved in 10mM NaOH) was added to the enzyme. The assays were initiated by the addition of MgCl2 (2.5 mM) and GTP (1 mM). The final volume of each sample was 100 μL. Reactions were quenched after 3 min by the addition of Zn(CH3CO2)2 (400 μL, 125 mM) and Na2CO3 (500 μL, 125 mM). The cGMP formed was quantified using the cGMP ELISA Kit.
Each assay point was determined in duplicate. All experiments were repeated at least two times, and representative results are shown.
Supplementary Material
Acknowledgments
We thank Eric S. Underbakke and Michael B. Winter for helpful discussions and critical reading of the manuscript. We thank Bayer HealthCare (Germany) for providing cinaciguat. This work was supported by the American Heart Association (11POST7150020) and the NIH (GM077365).
References
- 1.Derbyshire ER, Fernhoff NB, Deng S, Marletta MA. Biochemistry. 2009;48:7519. doi: 10.1021/bi900696x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Circulation. 2003;108:2172. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 3.Warner TD, Mitchell JA, Sheng H, Murad F. Adv Pharmacol. 1994;26:171. doi: 10.1016/s1054-3589(08)60054-x. [DOI] [PubMed] [Google Scholar]
- 4.Sanders KM, Ward SM. Am J Physiol Gastrointest Liver Physiol. 1992;262:G379. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- 5.Francis SH, Busch JL, Corbin JD. Pharmacol Rev. 62:525. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Physiol Rev. 2006;86:1. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 7.Wedel B, Humbert P, Harteneck C, Foerster J, Malkewitz J, Bohme E, Schultz G, Koesling D. Proc Natl Acad Sci U S A. 1994;91:2592. doi: 10.1073/pnas.91.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedel B, Harteneck C, Foerster J, Friebe A, Schultz G, Koesling D. J Biol Chem. 1995;270:24871. doi: 10.1074/jbc.270.42.24871. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Marletta MA. Biochemistry. 1997;36:15959. doi: 10.1021/bi971825x. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, Wolin MS. Biochim Biophys Acta. 1982;718:49. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- 11.Stone JR, Marletta MA. Biochemistry. 1996;35:1093. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 12.Stone JR, Marletta MA. Biochemistry. 1994;33:5636. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 13.Stone JR, Sands RH, Dunham WR, Marletta MA. Biochemistry. 1996;35:3258. doi: 10.1021/bi952386+. [DOI] [PubMed] [Google Scholar]
- 14.Zhao YD, Brandish PE, DiValentin M, Schelvis JPM, Babcock GT, Marletta MA. Biochemistry. 2000;39:10848. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- 15.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, Montfort WR. Biochemistry. 50:5813. doi: 10.1021/bi200794c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirkov YY, Horowitz JD. Pharmacol Ther. 2007;116:287. doi: 10.1016/j.pharmthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Stasch JP, Pacher P, Evgenov OV. Circulation. 123:2263. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladwin MT. J Clin Invest. 2006;116:2330. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch JP. Nat Rev Drug Discovery. 2006;5:755. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priviero FBM, Webb RC. J Cardiovasc Pharmacol. 2010;56:229. doi: 10.1097/FJC.0b013e3181eb4e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CC, Ko FN, Kuo SC, Lee FY, Teng CM. Br J Pharmacol. 1995;116:1973. doi: 10.1111/j.1476-5381.1995.tb16400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoenicka M, Becker EM, Apeler H, Sirichoke T, Schroder H, Gerzer R, Stasch JP. J Mol Med (Berl) 1999;77:14. doi: 10.1007/s001090050292. [DOI] [PubMed] [Google Scholar]
- 23.Stone JR, Marletta MA. Chem Biol. 1998;5:255. doi: 10.1016/s1074-5521(98)90618-4. [DOI] [PubMed] [Google Scholar]
- 24.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, Furstner C. Bioorg Med Chem Lett. 2001;11:781. doi: 10.1016/s0960-894x(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 25.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schroder H, Stahl E, Steinke W, Wunder F. Br J Pharmacol. 2002;136:773. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindler U, Strobel H, Schonafinger K, Linz W, Lohn M, Martorana PA, Rutten H, Schindler PW, Busch AE, Sohn M, Topfer A, Pistorius A, Jannek C, Mulsch A. Mol Pharmacol. 2006;69:1260. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Sayed N, Beuve A, van den Akker F. EMBO J. 2007;26:578. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Akker F, Martin F, Baskaran P, Ma XL, Dunten PW, Schaefer M, Stasch JP, Beuve A. J Biol Chem. 2010;285:22651. doi: 10.1074/jbc.M110.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch JP. J Biol Chem. 2004;279:3025. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt PM, Rothkegel C, Wunder F, Schroder H, Stasch JP. Eur J Pharmacol. 2005;513:67. doi: 10.1016/j.ejphar.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Boon EM, Marletta MA. J Inorg Biochem. 2005;99:892. doi: 10.1016/j.jinorgbio.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Winger JA, Derbyshire ER, Marletta MA. J Biol Chem. 2007;282:897. doi: 10.1074/jbc.M606327200. [DOI] [PubMed] [Google Scholar]
- 33.Karow DS, Pan D, Davis JH, Behrends S, Mathies RA, Marletta MA. Biochemistry. 2005;44:16266. doi: 10.1021/bi051601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward JJ, Martin NI, Marletta MA. Nat Methods. 2007;4:43. doi: 10.1038/nmeth984. [DOI] [PubMed] [Google Scholar]
- 35.Winter MB, McLaurin EJ, Reece SY, Olea C, Jr, Nocera DG, Marletta MA. J Am Chem Soc. 2010;132:5582. doi: 10.1021/ja101527r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derbyshire ER, Marletta MA. J Biol Chem. 2007;282:35741. doi: 10.1074/jbc.M705557200. [DOI] [PubMed] [Google Scholar]
- 37.Hargrove MS, Barrick D, Olson JS. Biochemistry. 1996;35:11293. doi: 10.1021/bi960371l. [DOI] [PubMed] [Google Scholar]
- 38.Hargrove MS, Singleton EW, Quillin ML, Ortiz LA, Phillips GN, Olson JS, Mathews AJ. J Biol Chem. 1994;269:4207. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 39.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Mol Pharmacol. 1995;48:184. [PubMed] [Google Scholar]
- 40.Schmidt HHHW, Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Kumar A, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W. J Clin Invest. 2006;116:2552. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boon EM, Marletta MA. Curr Opin Chem Biol. 2005;9:441. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Roy B, Mo E, Vernon J, Garthwaite J. Br J Pharmacol. 2008;153:1495. doi: 10.1038/sj.bjp.0707687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman LS, Schmidt PM, Keim Y, Hoffmann C, Schmidt HHHW, Stasch JP. PLoS One. 2011;6:e23596. doi: 10.1371/journal.pone.0023596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahrens I, Habersberger J, Qian H, Stasch JP, Bode C, Schmidt H, Peter K. Atheroscler Suppl. 2010;11:190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.