Abstract
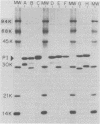
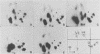
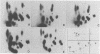
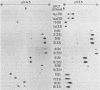
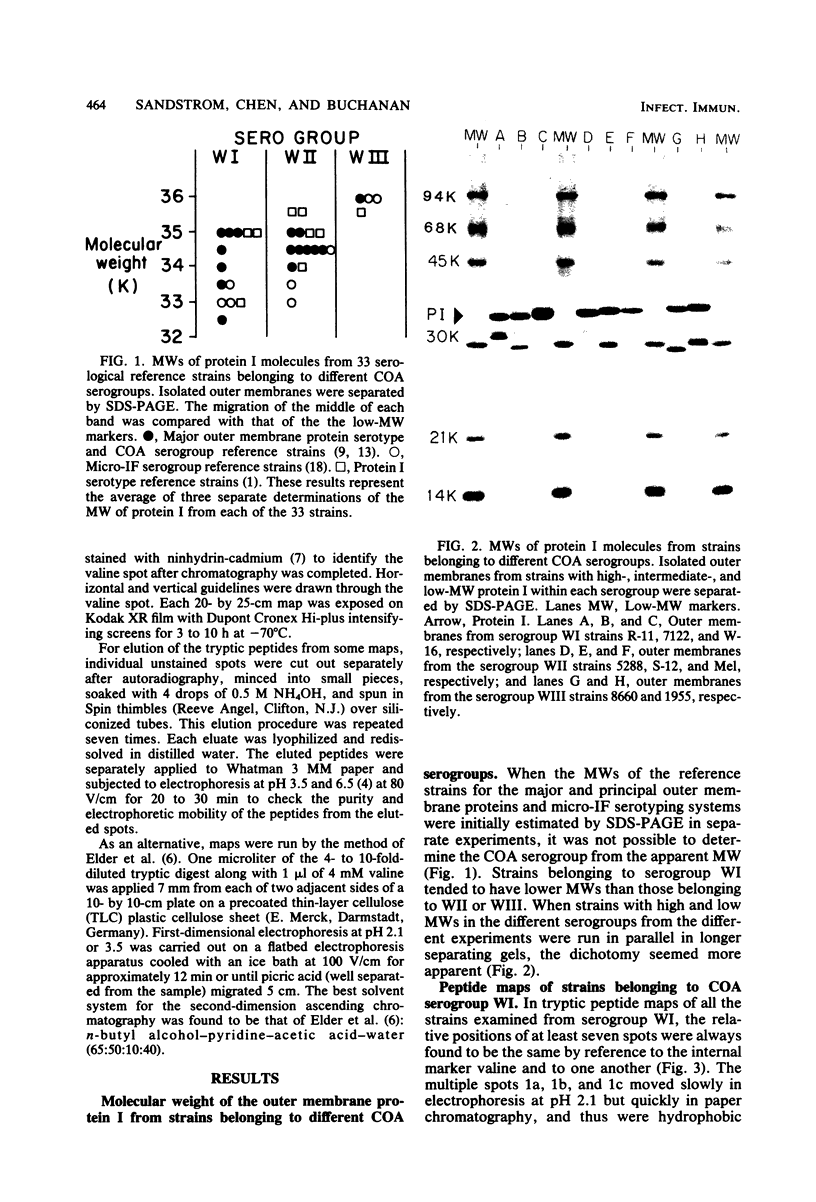
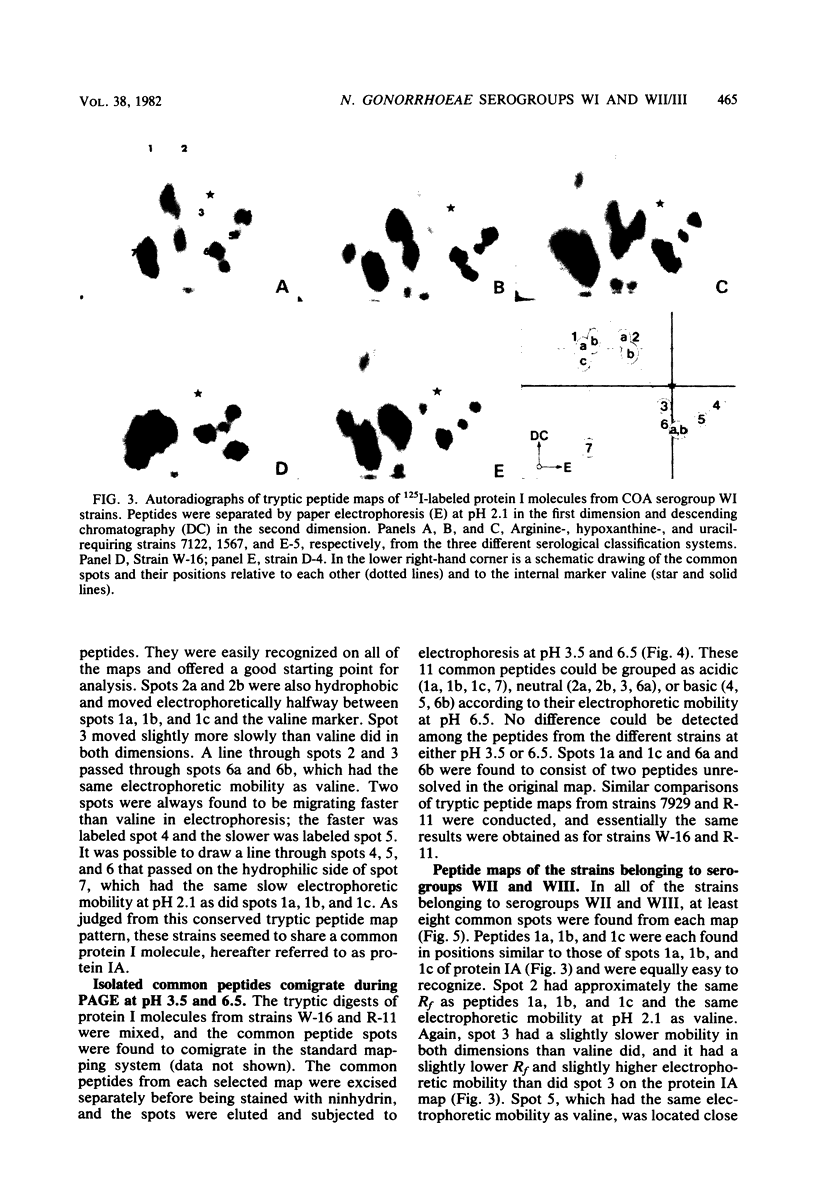
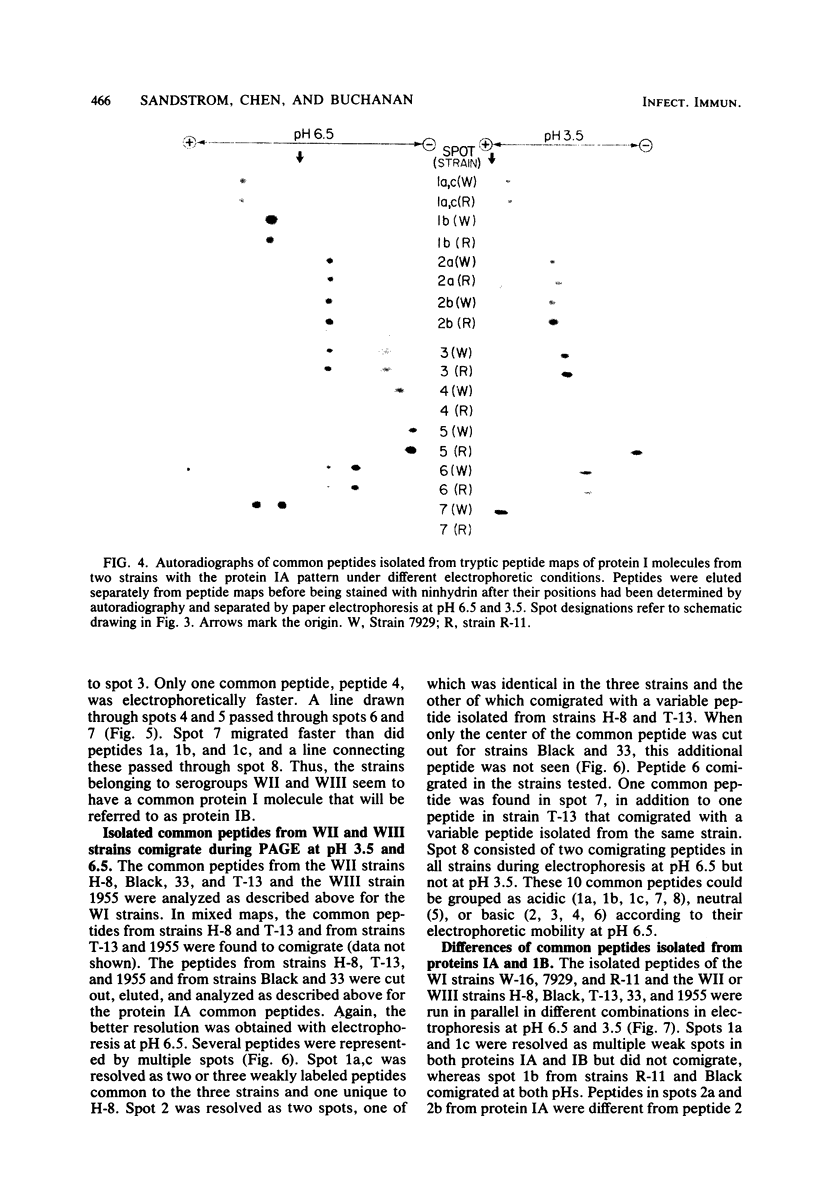
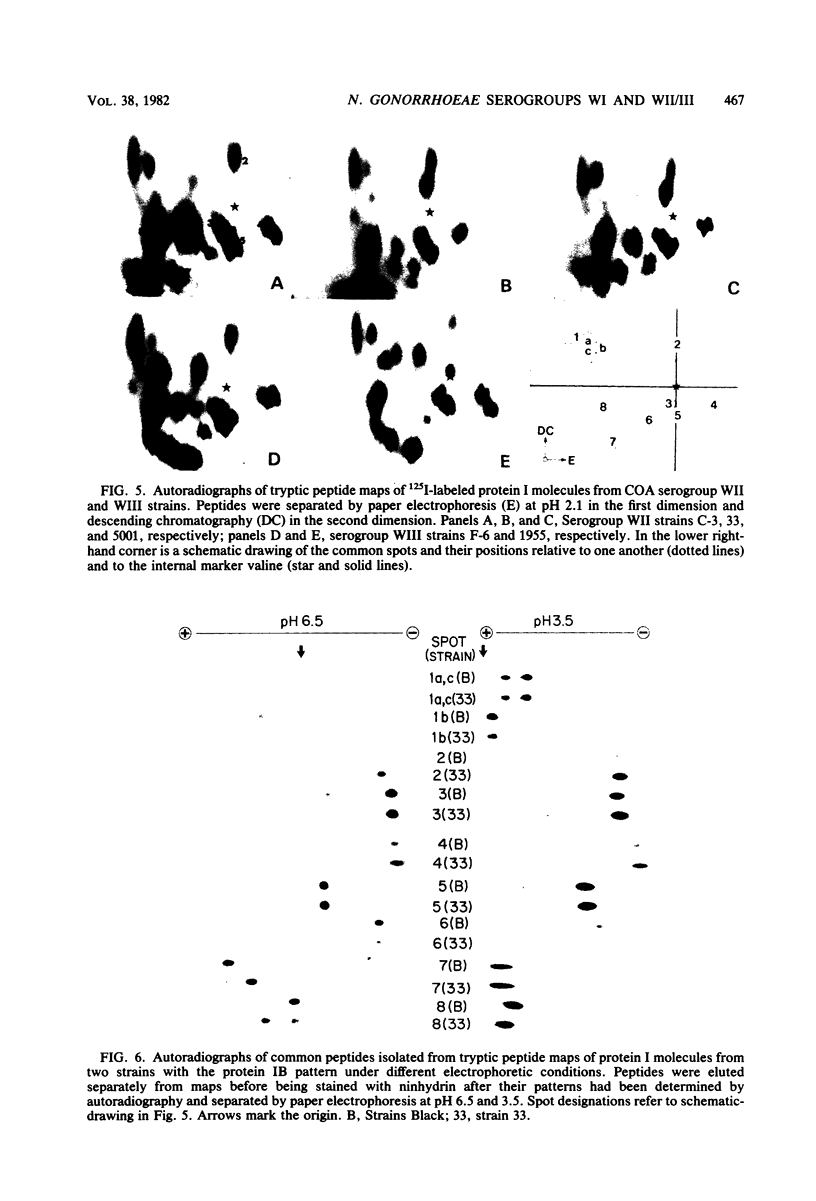
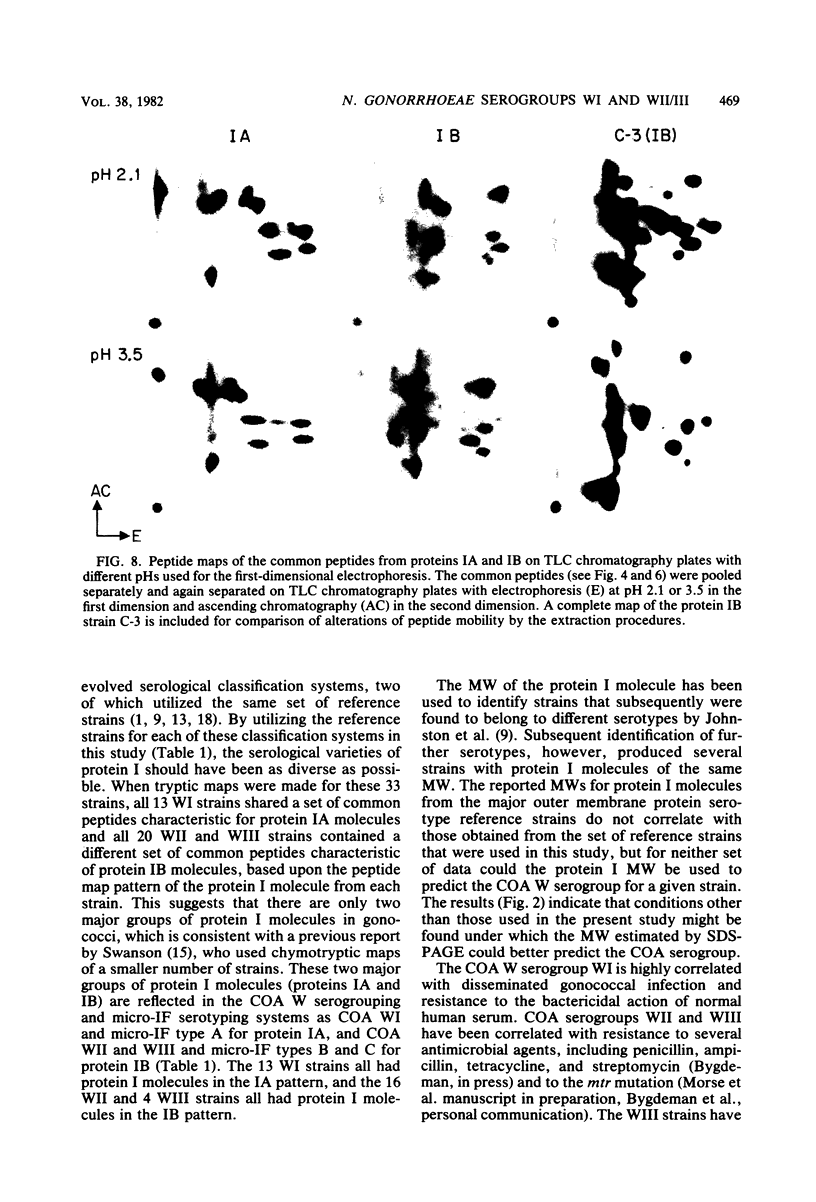
The 125I-labeled tryptic peptides of the outer membrane protein I of 33 previously characterized serological reference strains of Neisseria gonorrhoeae were investigated by peptide maps in relation to their coagglutination W serogroup. Serogroup WI strains tended to have lower-molecular-weight protein I molecules than did WII strains, and WIII strains had the highest-molecular-weight protein I molecules, although the serogroup could not be predicted from the molecular weights of the protein I molecules for a given strain. All 13 strains belonging to serogroup WI were found to have 11 peptides in common, as judged by their migration with respect to one another and to the internal marker valine in the peptide maps. Common peptides isolated from a given strain were found to comigrate with the corresponding common peptides from other strains in the same serogroup under various electrophoretic conditions. The 20 strains belonging to serogroups WII and WIII were all found to have 10 common peptides by the same criteria. When common peptides from serogroup WI were compared with the common peptides of serogroups WII and WIII, only three of these peptides appeared to be similar. Thus, two different outer membrane protein I molecules seem to exist which are mutually exclusive. Protein IA molecules contain the antigens recognized as serogroup WI, and protein IB molecules contain the antigens that characterize serogroups WII and WIII.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect Immun. 1981 May;32(2):547–552. doi: 10.1128/iai.32.2.547-552.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Buchanan T. M. Hydrolases from Neisseria gonorrhoeae. The study of gonocosin, an aminopeptidase-P, a proline iminopeptidase, and an asparaginase. J Biol Chem. 1980 Feb 25;255(4):1704–1710. [PubMed] [Google Scholar]
- Chen K. C., Krause R. M. A peptide mapping technique--a three map system. Anal Biochem. 1975 Nov;69(1):180–186. doi: 10.1016/0003-2697(75)90579-5. [DOI] [PubMed] [Google Scholar]
- Danielsson D., Sandström E. Serology of Neisseria gonorrhoeae. Demonstration by co-agglutination and immunoelectrophoresis of antigenic differences associated with colour/opacity colonial variants. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):39–46. doi: 10.1111/j.1699-0463.1980.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J Bacteriol. 1980 Jan;141(1):169–176. doi: 10.1128/jb.141.1.169-176.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Holmes K. K., Knapp J. S., Ott S., Kyzer D. D. Immunologic classification of Neisseria gonorrhoeae with micro-immunofluorescence. J Immunol. 1977 Sep;119(3):795–803. [PubMed] [Google Scholar]