Abstract
The alphaherpesvirus US3 kinase is a conserved multifunctional serine/threonine kinase that plays a role in several processes, including modulation of the actin cytoskeleton, egress of virus particles from the nucleus and inhibition of apoptosis. However, the mechanisms used by the US3 protein to exert its functions remain poorly understood. Recently, we identified the group A p21-activated kinases PAK1 and PAK2 as important effectors in the US3-mediated cytoskeletal rearrangements. Here, we investigated if group A PAKs are also involved in the anti-apoptotic properties of US3. Infection experiments using a group A PAK inhibitor pointed at a moderate role for group A PAKs in the anti-apoptotic properties of US3. Furthermore, infection assays using wild type and US3null PRV in wild type MEF, PAK1−/−MEF and PAK2−/− MEF indicated that PAK2 does not play a role in US3-mediated inhibition of apoptosis during infection, whereas PAK1 plays a significant, yet limited role. Experiments in US3-transfected MEF using staurosporine as apoptosis trigger confirmed these observations. These results show that PAK1 plays a significant, yet limited, role in the anti-apoptotic activity of US3.
Alphaherpesviruses constitute a subfamily of closely related large DNA viruses, including the human pathogens herpes simplex virus-1 and 2 (HSV-1 and HSV-2) and varicella-zoster virus (VZV), and the animal viruses pseudorabies virus (PRV) in pigs, Marek’s disease virus (MDV) in poultry and equine herpes virus-1 (EHV-1) in horses.
The US3 protein is a serine/threonine kinase that is conserved among alphaherpesviruses. It is a multifunctional protein that plays a role in nuclear egress of progeny virus particles and in cytoskeletal rearrangements, and displays anti-apoptotic properties (Deruelle et al., 2007; Favoreel et al., 2005; Geenen et al., 2005; Klupp et al., 2001; Leopardi et al., 1997; Ogg et al., 2004; Reynolds et al., 2002; Schumacher et al., 2005; Van Minnebruggen et al., 2003; Wagenaar et al., 1995). The kinase activity of US3 appears to be critical for most of these functions (Brzozowska et al., 2010; Deruelle et al., 2007; Finnen et al., 2009; Ogg et al., 2004; Ryckman and Roller, 2004; Van den Broeke et al., 2009a). However, the exact mechanisms by which US3 exerts its diversity of functions remain poorly understood. Recently, we have shown that group A p21-activated kinases (PAKs), central regulators of Cdc42 and Rac GTPase signaling, are involved in PRV US3-induced cytoskeletal rearrangements. PAK1 and PAK2 are the most abundant group A PAKs and US3 can phosphorylate both PAK1 and PAK2 directly (Van den Broeke et al., 2009b). Interestingly, PAK1 and PAK2 have separable contributions to US3-mediated cytoskeleton rearrangements: PAK1 is involved in US3-mediated formation of actin- and microtubule-containing cell projections whereas PAK2 is required for US3-mediated disassembly of actin stress fibers (Van den Broeke et al., 2009b). These data added to the growing body of evidence that the closely related PAK1 and PAK2 display different functions on the actin cytoskeleton (Bright et al., 2009; Coniglio et al., 2008).
Regarding its role in apoptosis, US3 protects cells from apoptosis induced upon infection or other stress agents, at least in part by inducing phosphorylation and inactivation of the pro-apoptotic Bad protein (Cartier et al., 2003; Deruelle et al., 2007; Kato et al., 2005). Interestingly, group A PAKs are known to also play a role in regulating apoptotic processes. PAK1 is activated by different stimuli that promote cell survival signaling and phosphorylates the pro-apoptotic Bad protein which leads to inactivation of Bad (Schurmann et al., 2000). PAK2 on the other hand, has been implicated in both cell survival and cell death pathways. Caspase-mediated proteolytic cleavage of PAK2 plays a role in cell death, while activation of full-length PAK2 was shown to regulate the activity of Bad in a similar way as PAK1 does (Jakobi et al., 2001; Rudel and Bokoch, 1997).
Given the involvement of group A PAKs in apoptosis, the aim of the current study was to analyze the contribution of group A PAKs in the anti-apoptotic activity of US3.
Using IPA3, a specific small-molecule inhibitor of group A PAKs (Deacon et al., 2008), the involvement of group A PAKs on apoptosis of cells infected with wild type (wt) and US3null PRV was analyzed. Mouse embryonic fibroblasts (MEF) were inoculated with wt PRV or US3null PRV at a multiplicity of infection of 10 in the presence or absence of the inhibitor IPA3 (Deacon et al., 2008; Van den Broeke et al., 2009b). To determine the percentage of apoptotic cells, at 24h post inoculation (hpi), cells were stained for active caspase-3 and, to confirm infection, the PRV gB glycoprotein as described previously (Deruelle et al., 2007). In brief, cells were fixed in 3% paraformaldehyde and permeabilized in 0.1 % Triton X 100, and stained using a rabbit antibody against active caspase 3 (Sigma-Aldrich, St. Louis, Missouri, USA) and a mouse antibody against PRV gB (Nauwynck and Pensaert, 1995) followed by a FITC-labeled goat anti-rabbit and a Texas Red-labeled goat anti-mouse secundary antibody (Invitrogen, Carlsbad, California, USA). Finally, nuclei of the cells were counterstained with Hoechst (Invitrogen, Carlsbad, California, USA) and the percentage of active caspase 3 positive cells was determined by fluorescence microscopy.
In line with previous reports in ST and Hep-2 cells (Deruelle et al., 2007; Geenen et al., 2005), the number of apoptotic cells was significantly higher upon infection with US3null PRV (21.4 ± 1.9%) than upon wt PRV infection (2.8 ± 0.3%), underscoring the anti-apoptotic properties of US3. Inhibition of group A PAKs using IPA3 resulted in a modest, but significant increase in apoptosis in wt PRV infected cells (7.4 ± 2.3% versus 2.8 ± 0.3%), while this was not the case in US3null PRV infected cells (21.7 ± 3.9% versus 21.4 ± 1.9%)(Figure 1). Addition of the IPA3 inhibitor alone did not increase the percentage of apoptosis in mock-infected cells (0.1 ± 0.1% versus 0.1 ± 0.1%)(Figure 1). These results therefore point at a noticeable, yet limited role for group A PAKs in US3-mediated inhibition of apoptosis during virus infection.
Figure 1. Group A PAKs play a modest role in the ability of US3 to protect PRV-infected cells from apoptotic cell death.
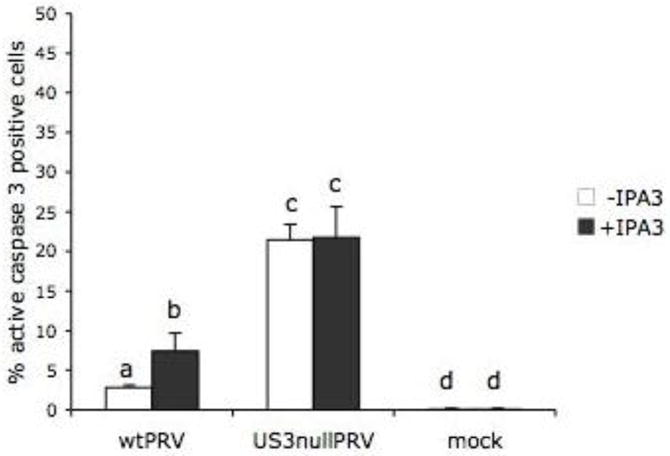
Percentage of active caspase 3 positive mock-infected MEF or MEF at 24h post inoculation with wt PRV or US3null PRV in the presence or absence of the group A PAK inhibitor IPA3 (30 μM). Three independent replicates of each experiment were performed, data shown are means and standard deviations, and statistical analysis was performed using the SPSS software. Means were compared with an analysis of variance and a least significant difference post hoc test for a multiple comparison of means (α=0.05). Different letters indicate significant differences at the 0.05 level.
To confirm these results and to determine which group A PAKs are predominantly involved in the US3-mediated effect on apoptosis, PAK knockout cells were used. Control MEF, PAK1−/− MEF, and PAK2−/− MEF were used earlier to elucidate the effect of PAKs on US3-mediated cytoskeletal rearrangements (Van den Broeke et al., 2009b). These cell lines were cultured as described before and all three cell lines were inoculated with wt PRV or US3null PRV at a multiplicity of infection of 10. To determine the percentage of apoptotic cells, at 24hpi, cells were again stained for active caspase-3 (Deruelle et al., 2007).
In all three cell types, mock-infected cells showed only very few apoptotic cells without significant differences between the three cell types: 0.6 ± 0.3% in MEF, 1.6 ± 0.6% in PAK1−/− MEF and 1.7 ± 0.8% in PAK2−/− MEF. In infected cells, percentages of apoptosis in PAK2−/− MEF were not significantly different from those in control MEF: 5.0 ± 1.5% versus 5.0 ± 2.8% for cells infected with wt PRV and 25.6 ± 8.1% versus 34.5 ± 7.6% for cells infected with US3null virus. The percentage of apoptosis observed in PAK1−/− MEF inoculated with US3null virus (26.2 ± 6.6%) was also not significantly different from corresponding percentages observed for control or PAK2−/− MEF. However, a limited yet significant increase in apoptotic cells was observed in PAK1−/− MEF inoculated wt PRV (11.5 ± 2.4%), compared to both other cell lines inoculated with wt PRV (Figure 2A and B). These data suggest that the limited role of group A PAKs in the ability of US3 to protect infected cells from apoptotic cell death can be attributed to PAK1, but not PAK2. We reported earlier that viral replication is similar in the three cell types (Van den Broeke et al., 2009c), excluding the possibility that the difference in apoptosis observed in PAK1−/− MEF is due to differences in viral replication. As a consequence, this indicates that the involvement of PAK1 in US3-mediated protection from apoptosis does not directly contribute to the replication efficiency of the virus. This result is in line with the recent finding that US3-mediated inhibition of apoptosis does not directly contribute to virus replication since a broad-spectrum caspase-inhibitor had no direct effect on virus production (Deruelle et al., 2010).
Figure 2. PAK1, but not PAK2, is involved to some extent in the ability of US3 to protect PRV-infected cells from apoptotic cell death.
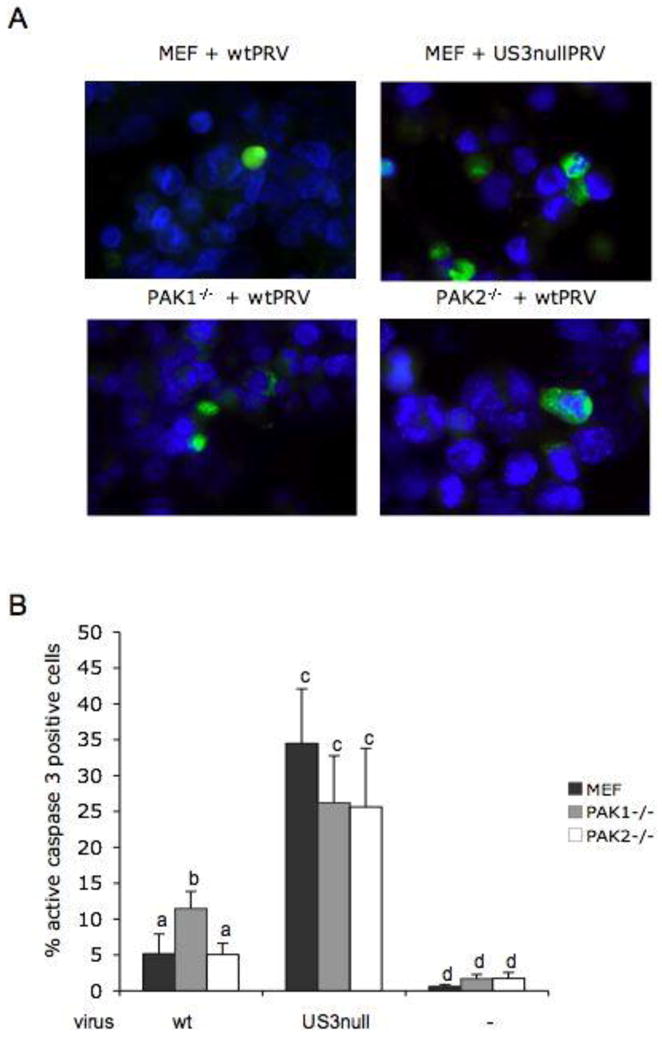
A. MEF, PAK1−/− MEF and PAK2−/− MEF at 24hpi with wtPRV or US3null PRV, stained for active caspase-3 (green) and nuclei (blue) B. Percentage of active caspase-3 positive MEF, PAK1−/− MEF and PAK2−/− MEF that were either mock infected or infected with wt PRV or US3nullPRV (24 hpi). Three independent replicates of each experiment were performed, data shown are means and standard deviations, and statistical analysis was performed using the SPSS software. Means were compared with an analysis of variance and a least significant difference post hoc test for a multiple comparison of means (α=0.05). Different letters indicate significant differences at the 0.05 level.
US3 not only protects cells from apoptosis during PRV infection, but also from apoptosis induced by exogenous stimuli such as staurosporine (Geenen et al., 2005). In order to find out whether group A PAKs may play a role in the US3-mediated suppression of apoptosis induced by staurosporine, cells of all three cell lines were transfected using lipofectamine (Invitrogen, Carlsbad, California, USA), with a plasmid encoding PRV US3 (Geenen et al., 2005). At 12h post transfection, cells were treated with 1.5 μM staurosporine (Sigma Chemical Company, St. Louis, Missouri, USA) in culture medium with 1% FBS for 12 hours, and fixed and stained for active caspase 3 and US3 as described earlier. In line with earlier findings in ST cells (Deruelle et al., 2007; Geenen et al., 2005), expression of the US3 protein protected cells from staurosporine-induced apoptosis in the control MEF cell line (0.6 ± 0.6% apoptotic cells in cells transfected with US3 compared to 24.9 ± 5.7% apoptotic cells in mock-transfected cells). Lack of PAK2 did not significantly affect the percentages of apoptosis observed (0.6 ± 0.6% apoptotic cells in US3-transfected cells versus 35.2 ± 4.0% apoptotic cells in mock-transfected cells). Lack of PAK1 also did not affect the percentage of apoptotic cells in mock-transfected cells (32.6 ± 4.8%). However, lack of PAK1 modestly, but significantly, reduced the ability of US3 to protect cells from staurosporine-induced apoptosis (3.3 ± 1.5% apoptotic cells in US3-transfected PAK1−/− MEF versus 0.6 ± 0.6% in US3-transfected control MEF) (Figure 3).
Figure 3. PAK1, but not PAK2, is involved in the ability of US3 to protect cells from apoptosis induced by staurosporine.
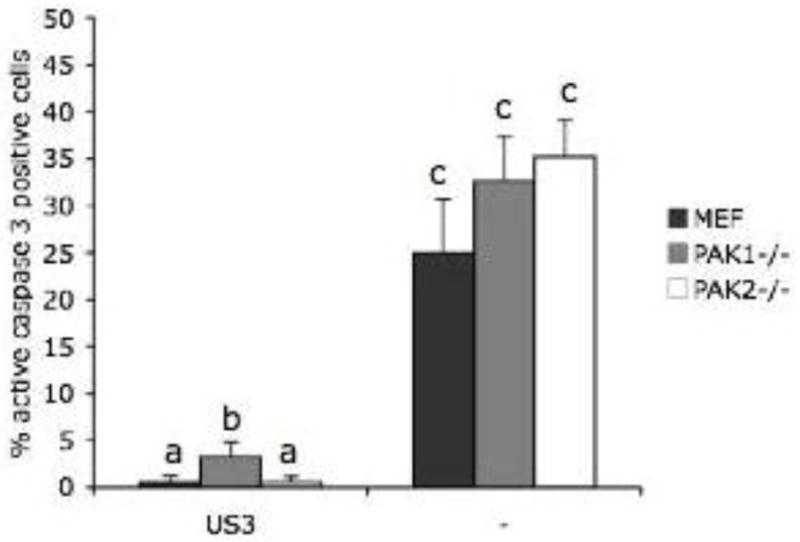
Percentage of active caspase-3 positive, staurosporine-treated mock-transfected or US3-transfected MEF, PAK1−/− MEF and PAK2−/− MEF (24h post transfection). Three independent replicates of each experiment were performed, data shown are means and standard deviations, and statistical analysis was performed using the SPSS software. Means were compared with an analysis of variance and a least significant difference post hoc test for a multiple comparison of means (α=0.05). Different letters indicate significant differences at the 0.05 level. Figure 1
In conclusion, PAK1 not only contributes to some extent to the ability of US3 to protect cells from apoptosis during PRV infection, but also plays a noticeable role in US3-mediated protection from staurosporine-induced apoptosis, whereas PAK2 is again not involved.
Based on the current data, we conclude that PAK1 plays a limited, yet significant role in the anti-apoptotic function of US3, while PAK2 does not appear to be involved in this activity of US3.
US3 protects cells from apoptosis at least in part by promoting phosphorylation of the pro-apoptotic Bad protein, which leads to its inactivation (Cartier et al., 2003; Deruelle et al., 2007; Kato et al., 2005). PAK1 has been shown to be able to phosphorylate the Bad protein, in vivo and in vitro (Jakobi et al., 2001; Schurmann et al., 2000), which may explain the involvement of PAK1 in US3-mediated inhibition of apoptosis. The observation that PAK2 is not involved in the anti-apoptotic activity of US3 is in line with the known opposing roles of PAK2 in apoptotic pathways. Like PAK1, PAK2 is able to phosphorylate Bad, leading to activation of the survival pathway (Jakobi et al., 2001). On the other hand, PAK2 is unique among the PAKs since it contains a cleavage site for caspase-3 or a caspase-3-like protease within the regulatory domain. Catalytic cleavage by caspase-3 results in a N-terminal 28 kDa and a C-terminal 34 kDa fragment (Rudel and Bokoch, 1997). Caspase cleavage of PAK2 is correlated with Fas- and ceramide-induced cell death of Jurkat cells, TNF-alpha-induced cell death of MCF-7 cells, heat shock-induced cell death of BALB3T3 and Hep 3B cells, and UVC light-induced cell death of A431 cells (Chan et al., 1998; Rudel and Bokoch, 1997; Tang et al., 1998). With this background in mind, our results pointing at a modest role for PAK1, but not for PAK2 in the anti-apoptotic activity of US3 are in line with the idea that PAK1 plays a preferentially anti-apoptotic role compared to PAK2, which has both pro- and anti-apoptotic activities. Our results contribute to the increasing evidence that p21-activated kinases play diverse roles in viral infections (Van den Broeke et al., 2010; Pacheco and Chernoff, 2010). Interestingly, like the alphaherpesvirus US3, the Nef protein of HIV has also been reported to activate PAK, with anti-apoptotic consequences (Wolf et al., 2001). Hence, it appears that evolutionary distinct viruses have evolved similar strategies to overcome some of the host barriers to infection.
Several cellular targets of US3 have been reported to contribute to its potent anti-apoptotic activity. US3 is able to directly target, phosphorylate and inactivate different pro-apoptotic proteins such as Bad and Bid (Kato et al., 2005; Cartier et al., 2003; Munger and Roizman, 2001). The kinase is also able to block proteolytic cleavage of procaspase-3 to active caspase-3 (Benetti and Roizman, 2007). In this context, it may not come as a surprize that our current data only point to a minor role of another US3 target, PAK1, in its anti-apoptotic activity. Apparently, US3 has developed multiple, perhaps partly redundant, mechanisms to interfere with apoptotic cell death. Our data are not the first to show the involvement of a cellular kinase in the activities of this conserved alphaherpesvirus kinase. Protein kinase A (PKA) inhibits apoptosis by phosphorylation and inactivation of Bad (Harada et al., 1999). US3 of HSV activates PKA and at the same time functionally overlaps PKA by targeting the same phosphorylation substrates, thereby interfering with apoptosis (Benetti and Roizman, 2004). Hence, at least some of the anti-apoptotic properties of US3 involve cellular kinases.
All these data point at a wide variety of US3 targets involved in its protection against apoptosis induced upon different stimuli, possibly allowing the virus to interfere with different signaling pathways to prolong the life span of the infected cell.
In conclusion, we show that, although p21-activated kinases PAK1 and PAK2 are crucial in US3-mediated actin rearrangements, they are less pivotal in the anti-apoptotic activity of this protein. PAK1 was found to play a modest yet significant role in US3-mediated apoptosis, whereas PAK2 is not involved.
Acknowledgments
This research was supported by grants from the F.W.O.-Vlaanderen (grant number G.0835.09) and the special research fund of Ghent University. C.V.D.B. is supported by a post-doctoral grant from the F.W.O.-Vlaanderen. J.C. was supported by National Institutes of Health Grants R01-CA117884 and R01-CA142928.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benetti L, Roizman B. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A toblock apoptosis. Proc Natl Acad Sci U S A. 2004;101(25):9411–6. doi: 10.1073/pnas.0403160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B. In transduced cells, the US3 protein kinase of herpes simplex 1 precludes activation and induction of apoptosis by transfected procaspase 3. J Virol. 2007;81(19):10242–8. doi: 10.1128/JVI.00820-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cell Signal. 2009;21(12):1738–47. doi: 10.1016/j.cellsig.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Brzozowska A, Rychlowski M, Lipinska AD, Bienkowska-Szewczyk K. Point mutations in BHV-1 Us3 gene abolish its ability to induce cytoskeletal changes in various cell types. Vet Microbiol. 2010;143(1):8–13. doi: 10.1016/j.vetmic.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Cartier A, Komai T, Masucci MG. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosporylation of the Bcl-2 family member Bad. Exp Cell Res. 2003;291(1):242–50. doi: 10.1016/s0014-4827(03)00375-6. [DOI] [PubMed] [Google Scholar]
- Cartier A, Broberg E, Komai T, Henriksson M, Masucci MG. The herpes simplex virus-1 US3 protein kinase blocks CD8T cell lysis by preventing teh cleavage of Bid by granzyme B. Cell Death Differ. 2003;10(12):1320–8. doi: 10.1038/sj.cdd.4401308. [DOI] [PubMed] [Google Scholar]
- Chan WH, Yu JS, Yang SD. Heat shock stress induces cleavage and activation of PAK2 in apoptotic cells. J Protein Chem. 1998;17(5):485–94. doi: 10.1023/a:1022578820147. [DOI] [PubMed] [Google Scholar]
- Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28 (12):4162–72. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15(4):322–31. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res. 2007;128(1–2):65–70. doi: 10.1016/j.virusres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Deruelle MJ, De Corte N, Englebienne J, Nauwynck HJ, Favoreel HW. Pseudorabies virus US3-mediated inhibition of apoptosis does not affect infectious virus production. J Gen Virol. 2010;91:1127–32. doi: 10.1099/vir.0.015297-0. [DOI] [PubMed] [Google Scholar]
- Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci U S A. 2005;102(25):8990–5. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnen RL, Roy BB, Zhang H, Banfield BW. Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology. 2009 doi: 10.1016/j.virol.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen K, Favoreel HW, Olsen L, Enquist LW, Nauwynck HJ. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology. 2005;331(1):144–50. doi: 10.1016/j.virol.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3(4):413–22. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem. 2001;276(20):16624–34. doi: 10.1074/jbc.M007753200. [DOI] [PubMed] [Google Scholar]
- Kato A, Yamamoto M, Ohno T, Kodaira H, Nishiyama Y, Kawaguchi Y. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J Virol. 2005;79(14):9325–31. doi: 10.1128/JVI.79.14.9325-9331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Mettenleiter TC. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J Gen Virol. 2001;82:2363–71. doi: 10.1099/0022-1317-82-10-2363. [DOI] [PubMed] [Google Scholar]
- Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci U S A. 1997;94(15):7891–6. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J, Roizman B. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci U S A. 2001;98(18):10410–5. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwynck HJ, Pensaert MB. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch Virol. 1995;140(6):1137–46. doi: 10.1007/BF01315422. [DOI] [PubMed] [Google Scholar]
- Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology. 2004;319(2):212–24. doi: 10.1016/j.virol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Pacheco A, Chernoff J. Group I p21-activated kinases: emerging roles in immune function and viral pathogenesis. Int J Biochem Cell Biol. 2010;42(1):13–6. doi: 10.1016/j.biocel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76(17):8939–52. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science. 1997;276(5318):1571–4. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78(1):399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Tischer BK, Trapp S, Osterrieder N. The protein encoded by the US3 orthologue of Marek’s disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J Virol. 2005;79(7):3987–97. doi: 10.1128/JVI.79.7.3987-3997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20(2):453–61. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TK, Chang WC, Chan WH, Yang SD, Ni MH, Yu JS. Proteolytic cleavage and activation of PAK2 during UV irradiation-induced apoptosis in A431 cells. J Cell Biochem. 1998;70(4):442–54. [PubMed] [Google Scholar]
- Van den Broeke C, Deruelle M, Nauwynck HJ, Coller KE, Smith GA, Van Doorsselaere J, Favoreel HW. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology. 2009a;385(1):155–160. doi: 10.1016/j.virol.2008.11.050. [DOI] [PubMed] [Google Scholar]
- Van den Broeke C, Radu M, Chernoff J, Favoreel HW. An emerging role for p21-activated kinases (Paks) in viral infections. Trends Cell Biol. 2010;20 (3):160–9. doi: 10.1016/j.tcb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci U S A. 2009c;106(21):8707–12. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J Virol. 2003;77(16):9074–80. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar F, Pol JM, Peeters B, Gielkens AL, de Wind N, Kimman TG. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen Virol. 1995;76:1851–9. doi: 10.1099/0022-1317-76-7-1851. [DOI] [PubMed] [Google Scholar]
- Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d’Aloja P, Schürmann A, Baur AS. HIV-Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7(11):1217–24. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]


