Abstract
Background and Aims
Trifolium repens (white clover) is a valuable component of pastures due to its ability to fix nitrogen. Productivity of T. repens is sometimes threatened by insect pests, and it has been suggested that phenylpropanoid-derived isoflavonoids such as formononetin can protect white clover from insect damage. The aim of this study was to isolate and functionally characterize an isoflavone synthase (IFS2_12) from T. repens by expressing it in Nicotiana tabacum (tobacco), a plant which does not naturally produce isoflavonoids.
Methods
To induce anthocyanin production and increase isoflavonoid precursors in tobacco, the tomato R2R3 MYB transcription factor ANT1 was expressed in tobacco (Nt-ANT1 plants). IFS2_12 was heterologously expressed in tobacco both transiently and stably, and isoflavonoids in leaf extracts were analysed by liquid chromatography (LC) coupled to mass spectrometry (MSn). As a positive control, a double construct of soybean IFS and alfalfa chalcone isomerase (IFS/CHI), which had been previously shown to induce isoflavonoid production in tobacco, was also expressed. Stable transformants expressing IFS2_12, soybean/alfalfa IFS/CHI and ANT1 were crossed and the resulting plants were analysed for isoflavonoid production.
Key results
Leaves of tobacco plants expressing ANT1 had a range of phenotypes from mainly green to uniformly bronze coloured. Both transient and stable expression of the IFS2_12 or IFS/CHI constructs resulted in the production of the isoflavonoid genistein and its conjugates. The highest levels (up to 19·2 mg g−1 d. wt) accumulated in a progeny of a cross between a purple ANT1 and a IFS/ CHI transformant, while the second highest concentration was found in a plant derived from a selfed IFS2-12 transformant.
Conclusions
It is concluded that the gene IFS2_12 isolated from T. repens encodes an isoflavone synthase. This study paves the way for engineering white clover plants with higher levels of isoflavonoids than naturally found in this species for sufficient insect protection.
Keywords: Trifolium repens, Nicotiana tabacum, isoflavone synthase, isoflavonoid biosynthesis, transient gene expression, liquid chromatography–mass spectrometry, genistein
INTRODUCTION
Trifolium repens (white clover) is a valuable component of temperate pastures, providing nitrogen fixation to fertilize the soil and high quality nutrition to grazing livestock (Caradus et al., 1996). However, white clover productivity can be reduced by diseases and pests such as Sitona lepidus (clover root weevil; CRW), and, to maintain productivity, resistant or tolerant germplasm needs to be developed. Phenylpropanoids play important roles in plant defence (Dixon and Steele, 1999; Yu et al., 2000; Winkel-Shirley, 2001), and isoflavonoids, a class of phenylpropanoids which occur predominantly in legumes, have been shown to contribute to insect resistance (Lane et al., 1987; Simmonds, 2003). Feeding studies with T. pratense (red clover) suggest that the isoflavonoids formononetin and biochanin A deter both CRW larvae feeding on roots, and adults feeding on leaves (Gerard et al., 2005; Murray et al., 2007). Compared with red clover, white clover contains much lower levels of formononetin and biochanin A which are insufficient to deter CRW feeding.
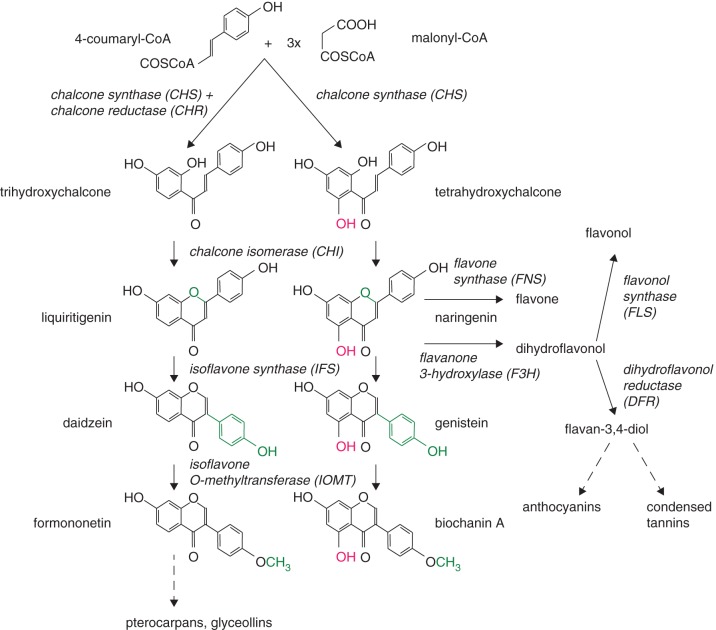
Trifolium repens germplasm shows little variation in isoflavonoid content, and attempts to increase isoflavonoids by conventional breeding strategies have not been successful so far. Traditional phenotype-based breeding is limited to the use of available germplasm, and it takes many years to produce a new white clover cultivar with a different phenotype. Marker-assisted selection can speed up this process but is still limited to the available germplasm. Metabolic engineering allows the incorporation and controlled expression of structural or regulatory genes to produce a new biochemical phenotype that may not exist within any of the current genetic diversity of the species of interest, and can thus potentially achieve greater enhancements than conventional breeding (Dixon and Steele, 1999). Isoflavonoids, flavonols, anthocyanins and condensed tannins are derived from phenylpropanoids and share common precursors (Winkel-Shirley, 2001; Fig. 1). The first committed step to flavonoid biosynthesis is catalysed by chalcone synthase (CHS) which condenses three malonyl-CoA and p-coumaroyl-CoA to tetrahydroxychalcone (Austin and Noel, 2003). In addition, legumes also possess a chalcone reductase (CHR) which, together with CHS, can form a trihydroxychalcone (Bomati et al., 2005). Tri- and tetrahydroxychalcones are converted by chalcone isomerases (CHIs) to the flavanones liquiritigenin and naringenin, respectively (Dixon et al., 1988; Jez and Noel, 2002). Isoflavone synthase (IFS) subsequently converts liquiritigenin to daidzein, and naringenin to genistein (Hashim et al., 1999; Sawada et al., 2002). Methylation of daidzein and genistein by isoflavone-O-methyltransferases (IOMTs) leads to the formation of formononetin and biochanin A (He et al., 1998; Liu and Dixon, 2001).
Fig. 1.
Isoflavonoid biosynthetic pathway in legumes showing parallel processing of 5-reduced chalcones (left side) and 5-hydroxylated (right side) chalcones and isoflavones. Naringenin is the key branch point for producing isoflavonoids or other flavonoids such as flavonols, anthocyanins and condensed tannins. Enzymes and their abbreviations are labelled in italics; dashed arrows indicate several enzymatic reactions.
Genes coding for these enzymes have been cloned and functionally characterized from a range of legumes (for a review, see Rasmussen, 2009). However, no isoflavonoid pathway genes from white clover have been functionally assessed so far. As a start to exploring whether it is possible to produce high levels of isoflavonoids in white clover, a metabolic engineering approach was initiated, which focused on testing the functionality of an IFS gene cloned from white clover. As knowledge of the mechanisms of isoflavonoid biosynthesis enzyme function is limited, cloned isoflavonoid biosynthesis genes cannot be confirmed as being functional by sequence analysis alone (Deavours et al., 2006). While Nicotiana tabacum (tobacco) is considered not to produce isoflavonoids (Yu et al., 2000; Joung et al., 2003; Liu et al., 2007), it has been shown previously to accumulate isoflavonoids when expressing soybean IFS or a soybean/alfalfa IFS/CHI fusion gene (Tian and Dixon, 2006). We have therefore used this model system to test the functionality of a putative IFS gene cloned from white clover. It had also been shown that expression of soybean IFS in anthocyanin-accumulating tobacco tissues resulted in enhanced levels of isoflavonoid production (Yu et al., 2000; Tian and Dixon, 2006). Anthocyanin biosynthesis can be induced in tobacco leaves by transformation with the R2R3 MYB transcription factor ANT1 from tomato (Mathews et al., 2003), and we tested here if the expression of white clover IFS in ANT1-expressing tobacco plants results in enhanced isoflavonoid production.
MATERIALS AND METHODS
Plant materials
Nicotiana tabacum ‘Wisconsin 38’ (‘W38’) seed was obtained from stocks held at Agresearch Grasslands (Palmerston North, New Zealand). Seeds were surface-sterilized in bleach (5 % available chlorine) for 20 min followed by five washes with sterile water. The seed was germinated and grown on MS0 nutrient agar without hornones (Murashige and Skoog, 1962) under sterile conditions in a growth room under 16 h d−1 of diffuse fluorescent lighting at 20 °C.
The white clover (Trifolium repens) cultivar ‘Sustain’ and the tomato (Solanum lycopersicum) variety ‘Grosse Lisse’ were grown outdoors and used for genomic DNA isolation.
Database query and identification
To obtain white clover IFS genes, ‘isoflavone synthase’ was used as a keyword to query the public NCBI (National Center for Biotechnology Information) nucleotide database (http://www.ncbi.nlm.nih.gov/). Fifty-one sequences from a range of species including white clover (GenBank accession nos AF195814 and AF195815; Jung et al., 2000) were returned. One of the IFS sequences (AF195814) was used to query the Pastoral Genomics in-house GeneThresher® database, and two full-length IFS open reading frames of 1575 bp were assembled and translated into a 524 amino acid protein. The genomic sequence was arranged in two exons spanning a 122 bp intron. Primers (Supplementary Data Table S1) were designed to amplify full-length IFS sequences from genomic DNA isolated from the white clover cultivar ‘Sustain’. Seven sequence variants were isolated, cloned and sequenced (data not shown). IFS2_12 (GenBank accession no. JQ756454), which had an intron of 368 bp, was used for tobacco transformation.
Isolation of genomic DNA, PCR, cloning and sequencing
Genomic DNA was isolated from fresh or frozen plant tissues using a DNeasy Plant Mini kit (Qiagen, BioLab Ltd, Auckland, New Zealand) following the manufacturer's instructions. DNA preparations were treated with RNase A (Sigma NZ Ltd., Auckland, New Zealand) to remove RNA from the samples. The concentration and purity of DNA samples were assessed by determining the ratio of absorbance at 260 and 280 nm using a NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Thermo Scientific, DE, USA).
The reaction mixture (20 µL) for PCR amplification of IFS2_12 contained 1× high fidelity PCR buffer, 2·5 U of Triple Master polymerase (Eppendorf, Hamburg, Germany), 0·2 mm of each dNTP, 0·4 µm of each primer (Supplementary Data Table S1) and 10 ng of DNA template. PCRs were performed in a thermocycler (MJResearch PTC200, ABI9700, ABI2720, or BioRad iCycler) using the following temperature profile: hot start at 94 °C for 2 min, 30 cycles of 94 °C for 20 s, 55 °C for 20 s and 72 °C for 180 s, followed by 72 °C for 7 min and a 4 °C hold. The reaction mixture (50 µL) for PCR amplification of ANT1 from tomato contained 1× Pfx50 PCR buffer, 5 U of Pfx50 Polymerase (Invitrogen), 2·5 mm MgSO4, 0·3 mm of each dNTP, 0·4 µm of each primer and 10 ng of genomic DNA template. The temperature profile was as follows: 94 °C for 4 min, 35 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 90 s, followed by 72 °C for 5 min and a 12 °C hold. PCR products were separated by agarose gel (1 % w/ v) electrophoresis and visualized by ethidium bromide staining. Bands were excised and DNA extracted from the gel slice using a MinElute Gel Extraction Kit (Qiagen) or by freezing in liquid nitrogen and subsequent centrifugation at 16 600 g. PCR products were cloned into the pENTR-D-TOPO vector (Invitrogen) and transformed into OneShot Top10 Escherichia coli cells by chemical transformation. Bacterial plasmid DNA was extracted using the QIAprep Spin Miniprep Kit (Qiagen) and sequenced using the dideoxynucleotide chain termination method (Sanger et al., 1977).
Stable and transient transformation of tobacco
The Gateway-adapted binary vector pRSh1 was used to transform genes into tobacco. This contains, within the left and right borders of the transferred DNA, the BAR gene, which provides resistance to ammonium glufosinate herbicide (Thompson et al., 1987), and a Gateway cloning site where the gene of interest is inserted. Both genes are driven by a Cauliflower mosaic virus (CaMV) 35S promoter. The white clover IFS2_12 gene, the soybean–alfalfa IFS/CHI construct (Tian and Dixon, 2006) and the tomato ANT1 gene (Mathews et al., 2003) were cloned into pRSh1 (Supplementary Data Fig. S1) and used for stable and transient transformation of tobacco. The empty vector pRSh1 contains the ccdB gene which is lethal to E. coli. As this gene may adversely affect plant cells, it was not used as a vector control. The binary vector pHZbar was used as an empty vector control instead, containing only the BAR gene within the left and right borders. The binary vector pBin61 (Bendahmane et al., 2000) contains the P19 protein gene, from Tomato bushy stunt virus, which is a suppressor of post-transcriptional gene silencing (Voinnet et al., 2003). Transformations were also performed using the pRS13 vector which contains green fluorescent protein (GFP) and β-glucuronidase (GUS) genes (Supplementary Data Fig. S2) as a visual control in the transient tobacco expression system.
To produce stable tobacco transformants, leaf discs from N. tabacum were transformed using Agrobacterium-mediated transformation and plant regeneration protocols as described in An (1985) and Horsch et al. (1985). The presence of the three transgenes was confirmed by PCR with appropriate primers (Supplementary Data Table S2, and Fig. S3).
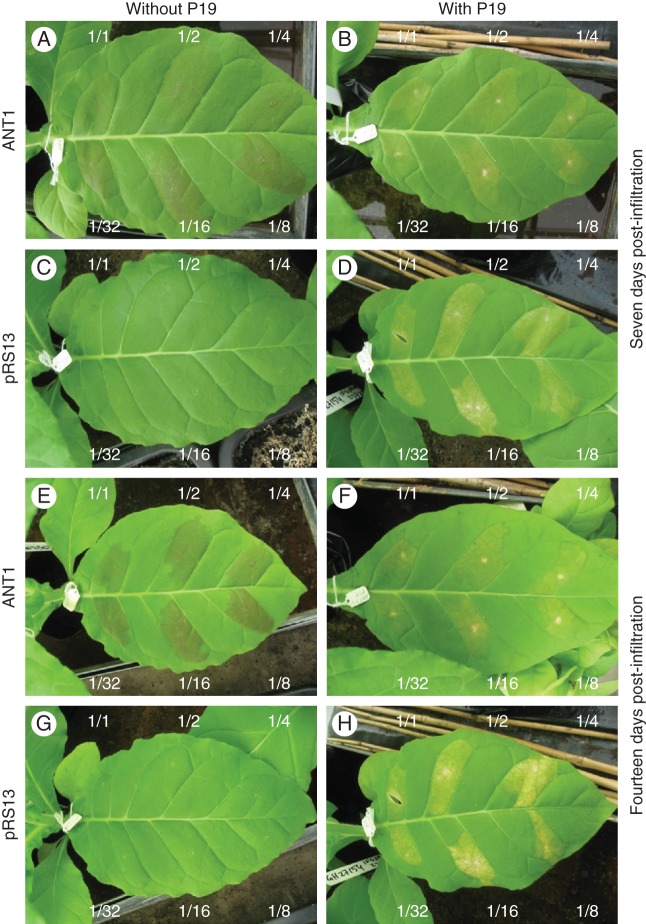
Transient tobacco transformants were produced using the leaf infiltration method (Kapila et al., 1997). An A. tumefaciens ‘GV3101’ strain carrying the desired vector was grown overnight in 20 mL of liquid MGL medium (Murashige and Skoog, 1962; without hormone supplement) containing 100 µg ml−1 spectinomycin or 50 µg ml−1 kanamycin, at 28 °C with shaking at 200 rpm. The bacteria in the broth were pelleted by centrifugation and the cells resuspended in 10 mL of sterile infiltration buffer (10 mm MgCl2, 10 µm acetosyringone). The bacterial suspension was incubated for 2–3 h at room temperature and, for co-expression experiments separate bacterial suspensions were mixed together prior to infiltration. Tobacco leaves were infiltrated by pressing the suspension with a needleless syringe into the abaxial side of the leaf through a small incision. To determine the optimal concentration of bacterial suspensions, spatially separated leaf areas were infiltrated with six serial dilutions (1/1, 1/2, 1/4, 1/8, 1/16 and 1/32) and visually examined for anthocyanin production and leaf vitality (Fig. 2). The optimal tobacco tissue to infiltrate was the tissue between veins of the first fully expanded leaves developed approx. 3 weeks after transplanting small plants from peat plugs into pots in the greenhouse. Infiltrated areas were left for between 1 and 4 weeks (7 d unless mentioned otherwise) to give time for the transgene to be expressed and for metabolites to accumulate. Infiltrated areas were then dissected from the leaves with a scalpel, freeze-dried and weighed prior to isoflavonoid extraction.
Fig. 2.
Serial dilution of A. tumefaciens tobacco leaf infiltrates of ANT1 or pRS13 (contains GFP) without (left hand panels) or with (right hand panels) P19, at 7 d (upper panels) and 14 d (lower panels) post-infiltration in wild-type tobacco plants. The dilutions of the infiltrate are labelled around each leaf.
Crossing of stable tobacco transformants
Mature tobacco plants transformed with white clover IFS2_12 (Nt-IFS2_12), soybean/ alfalfa IFS/CHI (Nt-IFS/CHI) and ANT1 (Nt-ANT1) that produced flowers at the same time were crossed in various combinations as well as self-fertilized. Crossed flowers were labelled with tags identifying the pollen donor, and mature seed pods were collected and stored at 4 °C. Seedlings were grown and leaf tissue collected from 3–6 young plants from each cross. The original Nt-ANT1 5P01963 plant and three ‘W38’ wild-type control plants were also sampled. Leaf tissue was freeze-dried, ground, and isoflavonoids extracted and analysed by liquid chromatography coupled to mass spectrometry (LCMS).
Isoflavonoid extraction and detection
Freeze dried (Flexi-Dry™ μP freeze drier) tobacco leaf samples were placed into 2 mL screw-top microtubes (Sarstedt) pre-loaded with five 3 mm steel balls or a 200 µL volume of 1 mm ceramic beads and ground in a Fast Prep FP120 mixer mill twice for 30 s at 4 m s−1. Isoflavonoids were extracted from 150 mg of dry tissue three times with 1 ml of acetone for 30 min, extracts were centrifuged at 16 600 g for 1 min, supernatants combined and the acetone evaporated under nitrogen gas. Residues were dissolved in 200 µL of 80 % (v/v) methanol containing 0·1 % (v/v) acetic acid and samples mixed in an Eppendorf Comfort Thermomixer at room temperature with shaking at 1400 rpm for 30 min. Samples were centrifuged at 16 600 g for 10 min and the supernatants decanted into new tubes, stored at –20 °C and centrifuged again before transferring approx. 700 µL into glass vials.
Extracts were separated in a high-performance liquid chromatography (HPLC) system consisting of two JASCO X-LC 3085PU high pressure pumps (JASCO International Co. Ltd, Tokyo, Japan), and a HTS PAL autosampler with a 25 µL syringe and a 15k psi injection valve (CTC Analytics AG, Zwingen, Switzerland), coupled to a Thermo LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Thermo Finnigan Xcalibur software (version 2·1) was used for data acquisition and processing. A 20 µL injection of sample was made onto a Phenomenex Luna C18(2)-HST column (100 × 2·0 mm, 2·5 µm particle size; Phenomenex, Torrance, CA, USA). The HPLC solvents used were: solvent A = 0·1 % (v/v) formic acid in MilliQ® water; solvent B = 0·1 % (v/v) formic acid in acetonitrile (LiChroSolv grade, Merck, New Zealand). The flow rate was 400 µL min−1 and the solvent gradient used was: 0 min 5 % (v/v) B, 6 min 5 % (v/v) B, 11 min 10 % (v/v) B, 26 min 17 % (v/v) B, 31 min 23 % (v/v) B, 41 min 30 % (v/v) B, 45 min 50 % (v/v) B, 52 min 97 % (v/v) B, 59 min 97 % (v/v) B, 61 min 5 % (v/v) B, and 65 min 5 % (v/v) B. The acetonitrile concentration changed linearly between time points.
Mass spectra were acquired using electrospray ionization (ESI) in positive mode with a spray voltage of +4·5 kV, an ion source capillary temperature of 275 °C and the nitrogen sheath, auxiliary and sweep gas flow rates set to 20, 5 and 0 (arbitrary units), respectively. The first 3 min of flow from the HPLC were diverted to waste. The 61 min run protocol was used and the LCMS was set to scan for MS1 ions of m/z 271 (for genistein), 433 (for genistein glycoside = genistin) and 519 (for genistin malonate), MS2 fragments of m/z 271 and MS3 fragments of m/z 153. LCMS peaks were identified and quantified by comparison with a genistein standard (Sigma) for elution time, mass spectrum and Retro-Diels-Alder (RDA) fragment masses of collision energy fragmentations performed in the trap with helium.
Quantification was carried out by creating a calibration curve of the chemical standard of known concentration, using MS ion count peak areas. Quantification of the peaks was based on ion counts of fragmented m/z 271 ions, and the concentrations and weights of genistein and its conjugates were measured in ‘genistein aglycone only’ units. In other words, the weights do not include the added weights of the glucoside or glucoside-malonate molecules. The true weight of genistin, including the glucose, in the sample can be calculated by multiplying the measured weight by 433/271 = 1·60 times. Likewise the genistin malonate results can be multiplied by 519/271 = 1·92 times to obtain the true weight. The MS2 ion count peak area was compared with that of known aglycone quantity standards to quantify the isoflavonoid content in the sample.
RESULTS
Optimization of tobacco leaf infiltration
Mature young wild-type tobacco leaves were infiltrated with A. tumefaciens transformed with the R2R3 MYB transcription factor ANT1 from tomato (Mathews et al., 2003) which induces anthocyanin biosynthesis resulting in red tissue coloration. To optimize the infiltration method, bacterial suspensions were serially diluted and infiltrated into spatially separated leaf areas (Fig. 2). Anthocyanins were clearly visible 7 d post-infiltration as a speckled red tinge in the ANT1 infiltrations of wild-type tobacco plants, with 1/8 and 1/16 dilutions giving the darkest colour change compared with the other dilutions (Fig. 2A, E). As an additional control, wild-type tobacco leaves were also infiltrated with A. tumefaciens transformed with GFP and GUS genes. Leaves infiltrated with GFP were microscopically analysed for the presence of GFP which accumulated in cells within the infiltrated areas but not in non-infiltrated areas (Supplementary Data Fig. S4).
The binary vector pBin61 contains the P19 protein gene, from Tomato bushy stunt virus, which is a suppressor of post-transcriptional gene silencing (Bendahmane et al., 2000). Voinnet et al. (2003) had shown previously that co-infiltration of P19 with GFP into N. benthamiana leaves resulted in an increase in GFP expression compared with infiltration of GFP alone. We therefore also tested the effects of combinations of ANT1 + P19 and GFP + P19. Comparison of the four different infiltrates showed the effect of P19 on tobacco leaves (Fig. 2). The ANT1- and GFP-infiltrated areas without P19 remained healthy throughout the life of the leaf (Fig. 2, left hand panels), while ANT1 + P19- and GFP + P19-infiltrated areas both turned yellow at 7 d post-infiltration at all dilutions (Fig. 2, right hand panels). The GFP + P19 infiltrations even became necrotic in some areas, and at 14 d post-infiltration this effect was more pronounced (Fig. 2H). Clearly the presence of A. tumefaciens carrying the P19 plasmid was detrimental to the infiltrated leaf areas.
Stable transformation of tobacco with ANT1
The isoflavonoid and anthocyanin pathways share common precursors such as naringenin. To increase precursor supply, we stably transformed tobacco plants with the R2R3 MYB transcription factor ANT1 from tomato (Mathews et al., 2003), thus increasing flux through the phenylpropanoid pathway. Ten tobacco plants transformed with ANT1 were regenerated and named Nt-ANT1 in the following text, and the presence of the transgene was confirmed using PCR (Supplementary Data Fig. S3). The Nt-ANT1 plants had a range of phenotypes from mainly green to uniformly bronze or darker purple coloured (Fig. 3). Many plants had intermediate colour levels with red vasculature (Fig. 4A). Root phenotypes of young regenerants growing on agar plates developed from colourless young roots to dark red root tips and eventually turning over to completely red roots (Fig. 4B). Some regenerants also had leaves with both green and purple areas simultaneously, even though PCR analysis demonstrated the presence of the transgene in both types (Supplementary Data Fig. S3C). Nt-ANT1 plants had a growth habit, form and vigour similar to those of wild-type plants, but the most intensely coloured plants were smaller compared with the wild type. Flowers of Nt-ANT1 plants were more intensely pink and purple coloured than the light pink wild type, and seeds were darkly coloured compared with the light brown colour of wild-type seed (data not shown).
Fig. 3.

Nt-ANT1 regenerants showing the diversity of colour phenotypes.
Fig. 4.

Nt-ANT1 regenerants showing (A) red vasculature and (B) roots.
Isoflavonoid production in infiltration experiments
Stable Nt-ANT1 tobacco transformants were used for infiltration experiments to determine the functionality of the white clover IFS2_12 gene. We also used a soybean/alfalfa IFS/CHI construct, which had been shown previously to result in isoflavonoid production in tobacco (Tian and Dixon, 2006; Nt-IFS/CHI), as a positive control with or without ANT1 infiltration. In a preliminary study, we infiltrated Nt-ANT1 plants with the IFS/CHI construct and co-infiltrated the precursor naringin (naringenin rhamnoglucoside). Interestingly, Nt-ANT1 leaf areas infiltrated with IFS/CHI and naringin showed noticeably reduced pigment levels and were greener than the bronze red colour of the surrounding leaf (Fig. 5), indicating reduced production of anthocyanins probably due to competition for substrates between flavanone 3-hydroxylase (F3H) and the IFS/CHI enzymes. However, no peaks corresponding to isoflavonoids were detected by HPLC-photodiode array (PDA) which might have been due to the relatively low sensitivity of PDA detection. We therefore developed an LCMSn-based method allowing for detection of much lower concentrations of isoflavonoids.
Fig. 5.
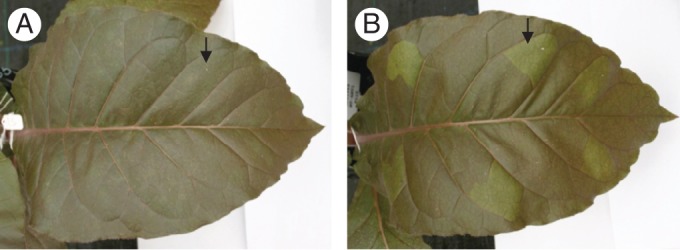
Nt-ANT1 leaves infiltrated with infiltration solution only (A) or A. tumefaciens carrying an IFS/CHI fusion construct (B) showing the loss of colour in the IFS/CHI infiltration area.
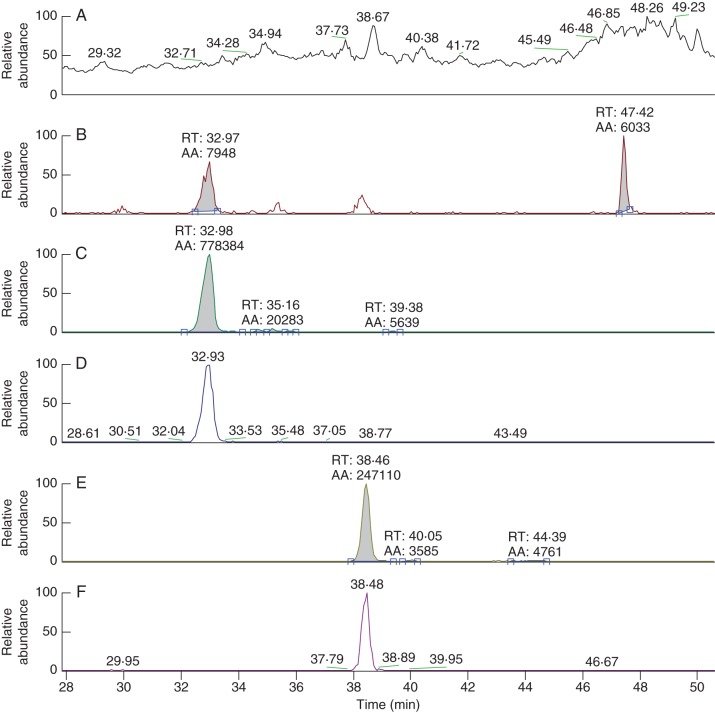
Tobacco plants transformed with the soybean/alfalfa IFS/CHI construct had been shown previously to accumulate the isoflavonoids genistein, its glycosylated conjugate genistin, as well as genistin malonate (Tian and Dixon, 2006). To detect these compounds, the LCMS was set to scan for MS1 ions and for MS2 fragments specific for genistein, genistein glucoside and genistin malonate. When leaves of Nt-ANT1 plants were infiltrated with IFS2_12 or IFS/CHI, or Nt-IFS/CHI plants were infiltrated with or without ANT1, all of the samples which had an IFS gene present (as either a stable or a transient transgene) produced MS2 peaks of m/z 271 and MS3 peaks of m/z 153 (Fig. 6), confirming that genistein and its conjugates were produced in these tissues. No genistein or genistein conjugates were detected in tobacco plants that were not transformed with an IFS gene, confirming that tobacco does not produce isoflavonoids, as has been shown in other studies (Tian and Dixon, 2006; Liu et al., 2007).
Fig. 6.
LCMS spectra of leaf tissue extracts from the Nt-IFS/CHI transformant 5P02034 infiltrated with ANT1. (A) MS1 total ion count of all masses over retention times of 28–50 min. (B) Genistein and genistein conjugates – MS2 fragments of m/z153 from MS1 ions of m/z271. (C) Genistin – MS2 fragments of m/z271 from MS1 ions of m/z433. (D) Genistin – MS3 fragments of m/z153 derived from the MS2 fragments in (C). (E) Genistin malonate – MS2 fragments of m/z271 from MS1 ions of m/z519. (F) Genistin malonate – MS3 fragments of m/z153 derived from the MS2 fragments in (E).
Genistin was the major genistein component, being present at 2–9 times the concentration of genistin malonate. The genistein aglycone was present in concentrations too low to be quantifiable (data not shown). No genistein or genistein conjugates were detected in a no-sample extraction or in any samples that did not contain an IFS gene, i.e. the NT-ANT1 plants without infiltration or infiltrated with the pHZbar empty vector control. The levels of genistin and genistin malonate in Nt-ANT1 leaf tissues infiltrated with white clover IFS2_12 or the soybean/alfalfa IFS/CHI construct ranged from 0·07 to 1·26 µg g−1 d. wt (Fig. 7). The two stably transformed Nt-IFS/CHI plants produced genistein without any infiltrate at lower levels of 0·04–0·15 µg g−1 d. wt. When infiltrated with ANT1, the amount of genistein conjugates increased, reaching very high levels in plant 5P02034 of 4·52 µg g−1 d wt. Concentrations of genistein conjugates were below the detection level in Nt-IFS/CHI leaves infiltrated with the pHZbar empty vector control.
Fig. 7.
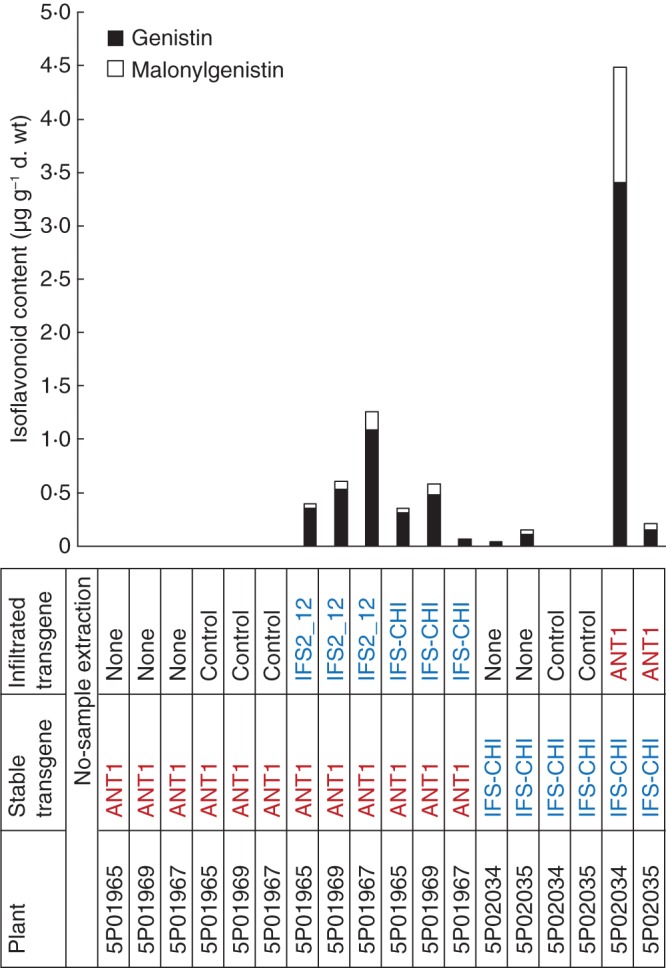
Genistin and genistin malonate content of tobacco plants with combinations of stable and infiltrated IFS, ANT1 and negative control (pHZbar) transgenes. The aglycone genistein is not shown because its concentrations were too low.
Isoflavonoid content in progeny of stable tobacco transformant crosses
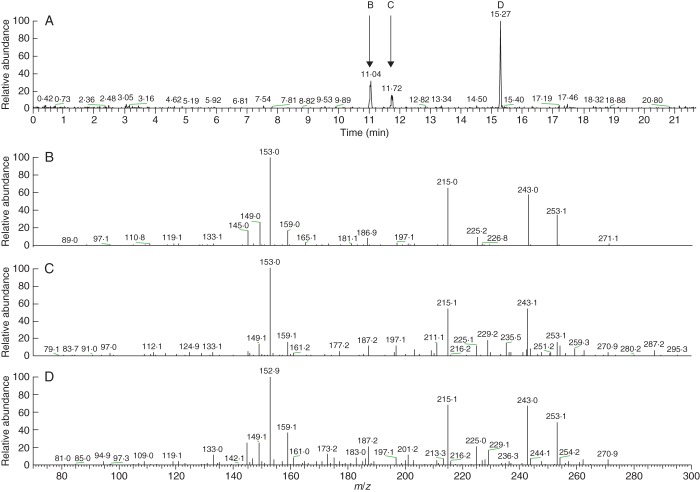
Once mature, tobacco plants transformed with white clover IFS2_12 (Nt-IFS2_12), soybean/alfalfa IFS/CHI (Nt-IFS/CHI) and ANT1 (Nt-ANT1) were either self-fertilized or crossed in various combinations. Seedlings were grown from each cross. Isoflavonoids were extracted from leaves of young plants and analysed by LCMS. MSn fragmentation analysis confirmed the presence of genistein in the forms of genistein, genistin and genistin malonate in a number of transgenic plants (Fig. 8).
Fig. 8.
Mass spectra of three peaks containing MS2 fragments of m/z271 in a leaf tissue extract from a selfed offspring of tobacco plant 23722 expressing the white clover IFS2_12 transgene. (A) MS3 fragments of m/z153 derived from MS2 fragments of m/z271 over the retention times of 0–21 min. (B) Genistin: peak in (A) and MS3 mass spectrum of that peak. (C) Genistin malonate: peak in (A) and MS3 mass spectrum of that peak. (D) Genistein: – peak in (A) and MS3 mass spectrum of that peak.
Quantification of the three compounds again found genistin to be the most common form (95–100 % of the total genistein conjugate content), while genistein was the second most prevalent at concentrations of 1/20th to 1/200th of the amount of genistin. Unlike in the infiltration experiment, genistin malonate was present in very low concentrations, at approximately only a quarter of that observed for genistein (data not shown).
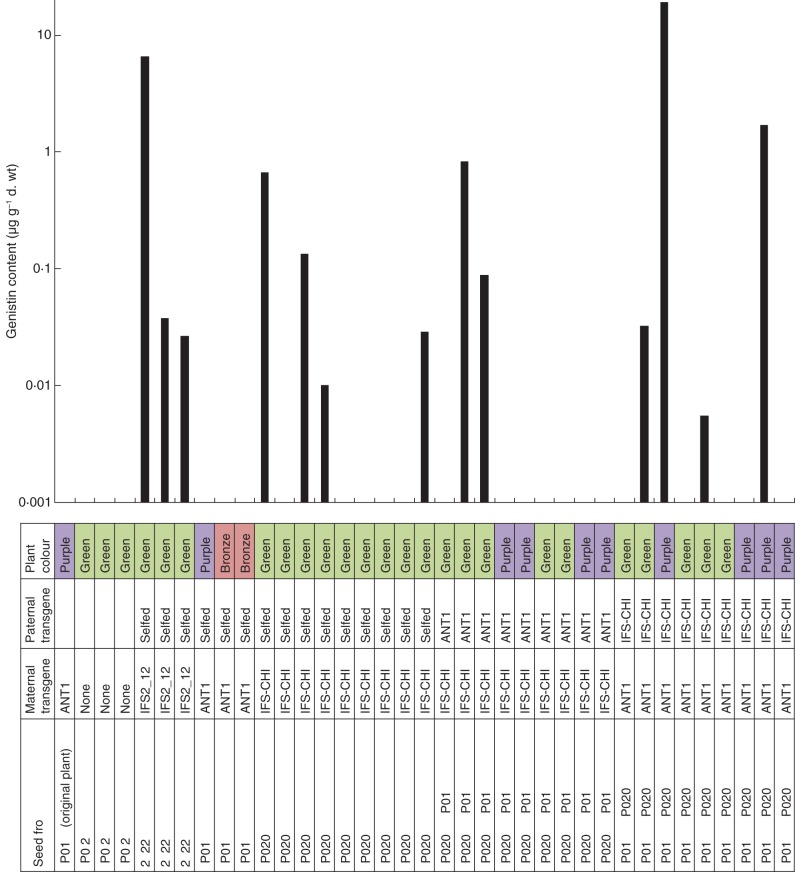
All of the plants that contained no transgene or only ANT1 produced no detectable genistein or genistein conjugates. Of the 30 plants that were progeny of IFS2_12 or IFS/CHI transformants, 13 produced a detectable level of genistin ranging from 0·006 to 19·2 µg g−1 d. wt (Fig. 9). The highest level was detected in a purple plant arising from a cross between Nt-ANT1 and Nt-IFS/CHI, while the second highest concentration was found in a plant derived from a selfed Nt-IFS2_12 tobacco plant (Fig. 9). There was no clear correlation between genistin levels and the colour phenotype.
Fig. 9.
Genistin content of seedlings from crossings of Nt-ANT1, Nt-IFS2_12 and Nt-IFS/CHI plants. A logarithmic scale was used to visualize the four orders of magnitude of genistin content. For the sake of clarity, the minor components, genistein and genistin malonate, are not shown.
DISCUSSION
Selection of a suitable model plant to assess functionality of cloned genes requires a plant amenable to transformation, and one that does not normally produce the key metabolites. To characterize functionally a white clover gene putatively coding for an IFS isolated in this study, we expressed IFS2_12 in tobacco, and analysed transformed plants for isoflavonoid production by LCMSn. Tobacco was chosen as it is readily transformed and generally accepted as a model species which does not produce isoflavonoids naturally (Joung et al., 2003; Liu et al., 2007). It had previously been shown to produce the isoflavonoid genistein and its conjugates following transformation with an IFS/CHI gene fusion construct, while untransformed plants did not accumulate any detectable levels of the isoflavonoids (Yu et al., 2000; Tian and Dixon, 2006). A major finding of our study was that both transient and stable expression of white clover IFS2_12 results in the production of genistein conjugates in tobacco leaves (Figs 6–8), confirming the functionality of the IFS2_12 gene and showing that its expression is necessary and sufficient for producing genistein in a naïve model plant. Analysis of progeny from crosses of Nt-ANT1 and Nt-IFS/CHI or selfed Nt-IFS2_12 plants also showed that the transgenes were inherited and isoflavonoids accumulated in progeny (Fig. 9).
Here, we also used leaf infiltration to express transiently clover IFS2_12, IFS/CHI fusion (Tian and Dixon, 2006) and the R2R3 MYB transcription factor ANT1 from tomato (Mathews et al., 2003). This method is very rapid, with anthocyanin and isoflavonoid synthesis being observable after only 7 d (Figs 3 and 4). Quantities of genistein conjugates accumulating in tobacco plants transiently expressing IFS genes were similar to those in stably transformed plants, making this a viable functional testing technique that is faster than producing stable transformants (Figs 5 and 6). The P19 protein had previously been shown to suppress post-transcriptional gene silencing and thus increase the production of transiently expressed transgene products and also maintain this production until senescence of the leaf in N. benthamiana (Voinnet et al. 2003). However, N. tabacum leaves used in this study infiltrated with P19 turned yellow, while those without P19 remained green and healthy, showing that the P19 protein itself or some other factor of the P19 vector was toxic (Fig. 2).
Tobacco plants expressing ANT1 showed variable coloured phenotypes ranging from mainly green to dark purple (Fig. 3). It had been shown previously that ANT1 transcript levels correlated well with the concentrations of anthocyanins in both tomato and tobacco overexpressing ANT1 (Mathews et al., 2003), and it is possible that the phenotypic variation seen here is due to variable transcript levels. In our study, ANT1 expression was under the control of the cauliflower mosaic virus 35S promoter which is expressed in all tissues. Anthocyanin accumulation patterns derive from ANT1 expression levels as well as the presence of repressors and interactions of the ANT1 MYB with compatible, endogenous, differentially expressed, basic helix–loop–helix (bHLH) factors and WD-repeat proteins to form a transcriptional activation complex (Albert et al., 2011). Our findings of dark red vasculature tissues and root tips may reflect this expression of endogenous factors (Fig. 4). It had been suggested previously that plant growth and flavonoid production might be inversely correlated (Hofmann et al., 2003), and we also saw reduced growth of plants accumulating very high levels of anthocyanins (Fig. 3). Some flavonoids have been shown to regulate auxin transport, a phytohormone involved in plant growth regulation (Taylor and Grotewold, 2005), and a direct link between high levels of flavonoids and reduced growth due to inhibition of auxin transport has been shown in A. thaliana plants suppressed in lignin biosynthesis (Besseau et al., 2007).
The tobacco plants were either infiltrated or transformed with the R2R3 transcription factor ANT1 to create plants that were likely to accumulate higher levels of a precursor common to the biosynthetic pathways leading to isoflavonoids and anthocyanins, namely naringenin. Naringenin had been shown previously by Liu et al. (2002) to be a limiting factor in genistein production in Arabidopsis thaliana plants transformed with a soybean IFS. When IFS was introduced into an A. thaliana tt6/ tt3 (transparent testa) double mutant, which has structural defects in both flavanone 3-hydroxylase (F3H) and dihydroflavonol reductase (DFR), and produces much lower concentrations of flavonoids and anthocyanins, genistein levels were much higher than those produced in the non-mutant transformed plants (Liu et al., 2002). This demonstrated that a limiting factor in genistein production was competition for naringenin between IFS and F3H. Consistent with those results, we found here that tobacco plants expressing IFS/CHI and ANT1 simultaneously accumulated higher levels of genistein conjugates compared with plants transformed with the fusion construct only (Fig. 7).
The fact that the compounds detected in the tobacco plants transformed with IFS were almost entirely genistin and genistin malonate shows that endogenous glucosyl and malonyl transferases are active in tobacco and can add glucose and malonate groups to the aglycone genistein. Glycosylated isoflavonoids are more water soluble than the aglycone, and glycosylation is required for their transport into the vacuole (Jones and Vogt, 2001). We did not see production of any other isoflavonoids such as daidzein, formononetin or biochanin A, indicating that tobacco lacks genes coding for CHR and IOMT.
When bronze-coloured ANT1 leaves were infiltrated with IFS/CHI, the infiltrated areas were less red-coloured after 7 d compared with the surrounding uninfiltrated areas or the areas infiltrated with buffer only (Fig. 5). This indicated that a constituent of the infiltrate was reducing the anthocyanin concentration. It is possible that the presence of A. tumefaciens had a general effect on the tissues with a side effect of reducing anthocyanin content. Alternatively, the introduction of IFS/CHI into these cells may have resulted in a depletion of naringenin, the common precursor to isoflavonoids and anthocyanins, and thus caused a reduced production and accumulation of anthocyanins which may, as a result of turnover, actually decrease in concentration. Xie et al. (2006) produced transgenic tobacco plants expressing simultaneously the MYB transcription factor PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1) and anthocyanidin reductase (ANR) to produce condensed tannins. ANR reduces anthocyanins to cis-flavan-3-ols, monomeric precursors of condensed tannins (proanthocyanidins), and competes with anthocyanidin glycosyltransferases which produce glycosylated anthocyanins. Plants expressing PAP1 and ANR transgenes contained lower levels of anthocyanins than those expressing only PAP1 in that study.
White clover plants accumulating high levels of isoflavonoids would be useful in pastoral animal production systems as these compounds can protect clover plants from insect attack. Conventional breeding of white clover with high levels of isoflavonoids has not been successful in the past, and metabolic engineering with known pathway genes might be an alternative strategy. The functional characterization of a white clover IFS gene confirms that its constitutive expression results in increased accumulation of isoflavonoids in a heterologous system, and these findings pave the way for the production of white clover cultivars with modified isoflavonoid levels. However, formononetin is demethylated and metabolized to equol by micro-organisms in the rumen which acts as a phyto-oestrogen in grazing mammals and can reduce fertility in sheep if consumed in large quantities (Beck, 1964). It might therefore be necessary to develop a strategy for white clover which simultaneously upregulates IFS while downregulating CHR to increase flux into the biosynthesis of genistein and biochanin A, which, in contrast to formononetin, are only mildly oestrogenic. Our study shows that the tobacco model system is suitable for functional characterization of white clover biosynthetic genes and paves the way for analysis of additional isoflavonoid genes such as CHR and IOMT from white clover.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by a FRST NZ (Foundation for Research, Science and Technology) Enterprise Scholarship UOCX04009 to B.K.F. and by the Pastoral Genomics Research Consortium (VLAC0201). Pastoral Genomics is funded by DairyNZ, Beef + Lamb New Zealand, Fonterra, DEEResearch, AgResearch and the Ministry of Science and Innovation. We thank Dr R.A. Dixon (Noble Foundation, OK, USA) for providing us with the IFS/CHI fusion construct.
LITERATURE CITED
- Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. The Plant Journal. 2011;65:771–784. doi: 10.1111/j.1365-313X.2010.04465.x. [DOI] [PubMed] [Google Scholar]
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiology. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MB, Noel JP. The chalcone synthase superfamily of type III polyketide synthases. Natural Product Report. 2003;20:79–110. doi: 10.1039/b100917f. [DOI] [PubMed] [Google Scholar]
- Beck AB. The oestrogenic isoflavones of subterranean clover. Australian Journal of Agricultural Research. 1964;15:223–230. [Google Scholar]
- Bendahmane A, Querci M, Kanyuka K, Baulcombe DC. A. tumefaciens transient expression system as a tool for isolation of disease resistance genes: application to the Rx2 locus in potato. The Plant Journal. 2000;21:73–81. doi: 10.1046/j.1365-313x.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- Besseau S, Hoffmann L, Geoffrey P, Lapierre C, Pollet B, Legrand M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. The Plant Cell. 2007;19:148–162. doi: 10.1105/tpc.106.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomati EK, Austin MB, Bowman ME, Dixon RA, Noel JP. Structural elucidation of chalcone reductase and implications for deoxychalcone biosynthesis. Journal of Biological Chemistry. 2005;280:30496–30503. doi: 10.1074/jbc.M502239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradus JR, Woodfield DR, Stewart AV. Overview and vision for White Clover. In: Woodfield DR, editor. White clover: New Zealand's competitive edge. 1996. Proceedings of a joint symposium between the Agronomy Society of NZ and NZ Grassland Association, Lincoln University NZ, 21–22 November 1995. [Google Scholar]
- Deavours BE, Liu C-J, Naoumkina MA, et al. Functional analysis of members of the isoflavone and isoflavanone O-methyltransferase enzyme families from the model legume Medicago truncatula. Plant Molecular Biology. 2006;62:715–733. doi: 10.1007/s11103-006-9050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Steele CL. Flavonoids and isoflavonoids – a gold mine for metabolic engineering. Trends in Plant Science. 1999;4:394–400. doi: 10.1016/s1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Blyden ER, Robbins MP, van Tunen AJ, Mol JN. Comparative biochemistry of chalcone isomerases. Phytochemistry. 1988;27:2801–2808. [Google Scholar]
- Gerard PJ, Crush JR, Hackell D. Interaction between Sitona lepidus and red clover lines selected for formononetin content. Annals of Applied Biology. 2005;147:173–181. [Google Scholar]
- Hashim MF, Hakamatsuka T, Ebizuka Y, Sankawa U. Reaction mechanism of oxidative rearrangement of flavanone in isoflavone biosynthesis. FEBS Letters. 1999;271:219–222. doi: 10.1016/0014-5793(90)80410-k. [DOI] [PubMed] [Google Scholar]
- He X-Z, Reddy JT, Dixon RA. Stress responses in alfalfa (Medicago sativa L). XXII. cDNA cloning and characterization of an elicitor-inducible isoflavone 7-O-methyltransferase. Plant Molecular Biology. 1998;36:43–54. doi: 10.1023/a:1005938121453. [DOI] [PubMed] [Google Scholar]
- Hofmann RW, Campbell BD, Bloor SJ, et al. Responses to UV-B radiation in Trifolium repens L. – physiological links to plant productivity and water availability. Plant, Cell and Environment. 2003;26:603–612. [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jez JM, Noel JP. Reaction mechanism of chalcone isomerase: pH dependence, diffusion control, and product binding differences. Journal of Biological Chemistry. 2002;277:1361–1369. doi: 10.1074/jbc.M109224200. [DOI] [PubMed] [Google Scholar]
- Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- Joung J-y, Kasthuri GM, Park J-y, et al. An overexpression of chalcone reductase of Pueraria montana var. lobata alters biosynthesis of anthocyanin and 5′-deoxyflavonoids in transgenic tobacco. Biochemical and Biophysical Research Communications. 2003;303:326–331. doi: 10.1016/s0006-291x(03)00344-9. [DOI] [PubMed] [Google Scholar]
- Jung W, Yu O, Lau SC, et al. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nature Biotechnology. 2000;18:208–212. doi: 10.1038/72671. [DOI] [PubMed] [Google Scholar]
- Kapila J, de Rycke R, van Montagu M, Angenon G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Science. 1997;122:101–108. [Google Scholar]
- Lane GA, Sutherland ORW, Skipp RA. Isoflavonoids as insect feeding deterrents and antifungal components from roots of Lupinus angustifolius. Journal of Chemical Ecology. 1987;13:771–783. doi: 10.1007/BF01020159. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Dixon RA. Elicitor-induced association of isoflavone O-methyltransferase with endomembranes prevents formation and 7-O-methylation of daidzein during isoflavonoid phytoalexin biosynthesis. The Plant Cell. 2001;13:2643–2658. doi: 10.1105/tpc.010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-J, Blount JW, Steele CL, Dixon RA. Bottlenecks for metabolic engineering of isoflavone glycoconjugates in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2002;99:14578–14583. doi: 10.1073/pnas.212522099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Hu Y, Li J, Lin Z. Production of soybean isoflavone genistein in non-legume plants via genetically modified secondary metabolism pathway. Metabolic Engineering. 2007;9:1–7. doi: 10.1016/j.ymben.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mathews H, Clendennen SK, Caldwell CG, et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. The Plant Cell. 2003;15:1689–1703. doi: 10.1105/tpc.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Murray PJ, Cheung AKM, Abberton MT. Intraspecific variation in Trifolium pratense: impact on feeding on host location by Sitona lepidus (Coleoptera, Curculionidae) Journal of Pest Science. 2007;80:51–57. [Google Scholar]
- Rasmussen S. Biosynthesis and manipulation of flavonoids in forage legumes. In: Gould K, Davies K, Winefield C, editors. Anthocyanins: biosynthesis, functions, and applications. New York: Springer; 2009. pp. 257–281. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences, USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Kinoshita K, Akashi T, Aoki T, Ayabe S. Key amino acid residues required for aryl migration catalysed by the cytochrome P450 2-hydroxyisoflavanone synthase. The Plant Journal. 2002;31:555–564. doi: 10.1046/j.1365-313x.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Simmonds MSJ. Flavonoid–insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/s0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Grotewold E. Flavonoids as developmental regulators. Current Opinion in Plant Biology. 2005;8:317–323. doi: 10.1016/j.pbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Movva NR, Tizard R, et al. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO Journal. 1987;6:2519–2523. doi: 10.1002/j.1460-2075.1987.tb02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Dixon RA. Engineering isoflavone metabolism with an artificial bifunctional enzyme. Planta. 2006;224:496–507. doi: 10.1007/s00425-006-0233-0. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the pp19 protein of tomato bushy stunt virus. The Plant Journal. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D-Y, Sharma S, Wright E, Wang Z-Y, Dixon RA. Metabolic engineering of proanthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. The Plant Journal. 2006;45:895–907. doi: 10.1111/j.1365-313X.2006.02655.x. [DOI] [PubMed] [Google Scholar]
- Yu O, Jung W, Shi J, et al. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant Physiology. 2000;124:781–793. doi: 10.1104/pp.124.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.