Abstract
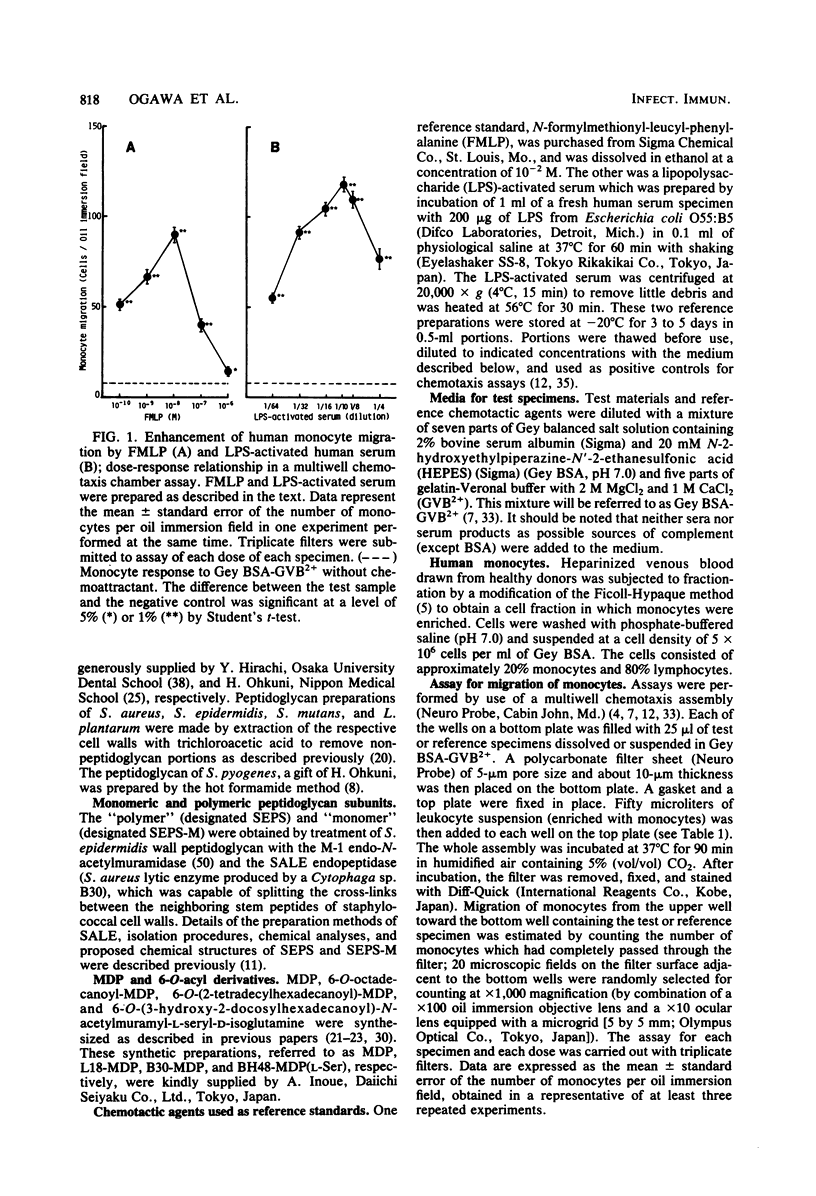
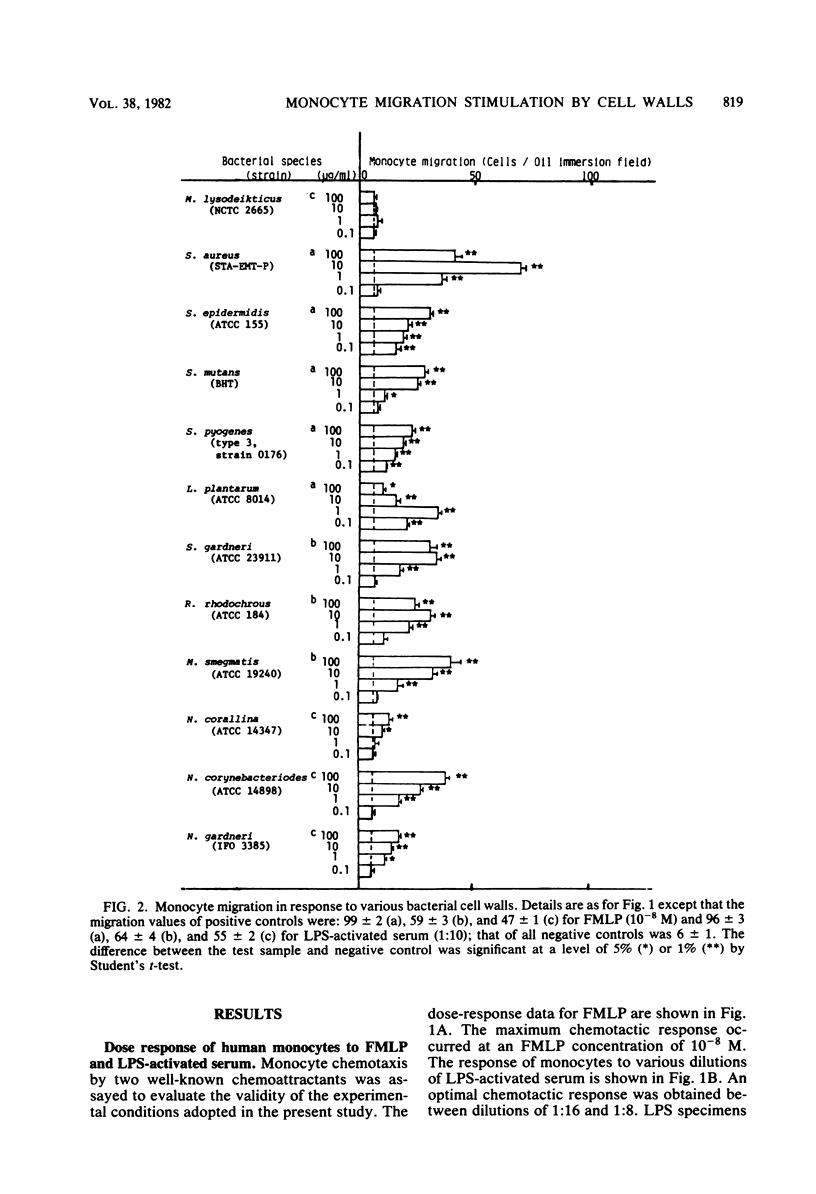
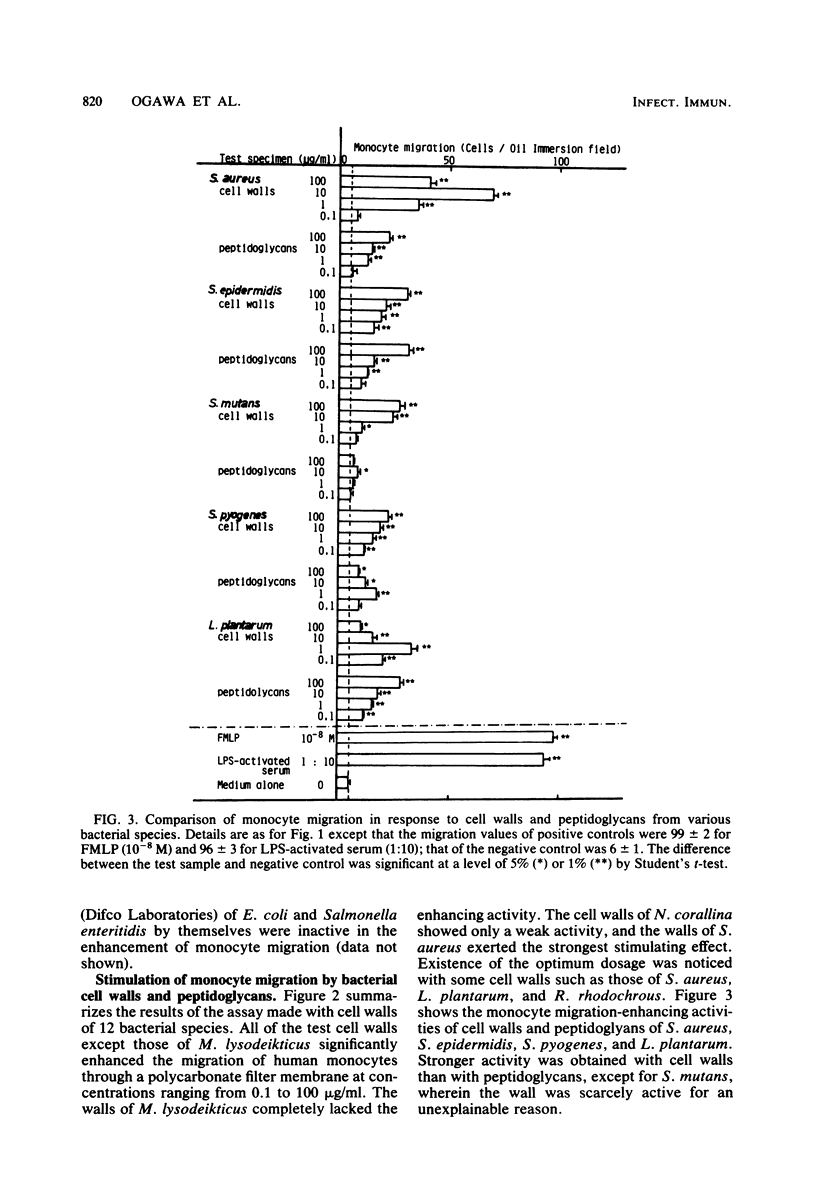
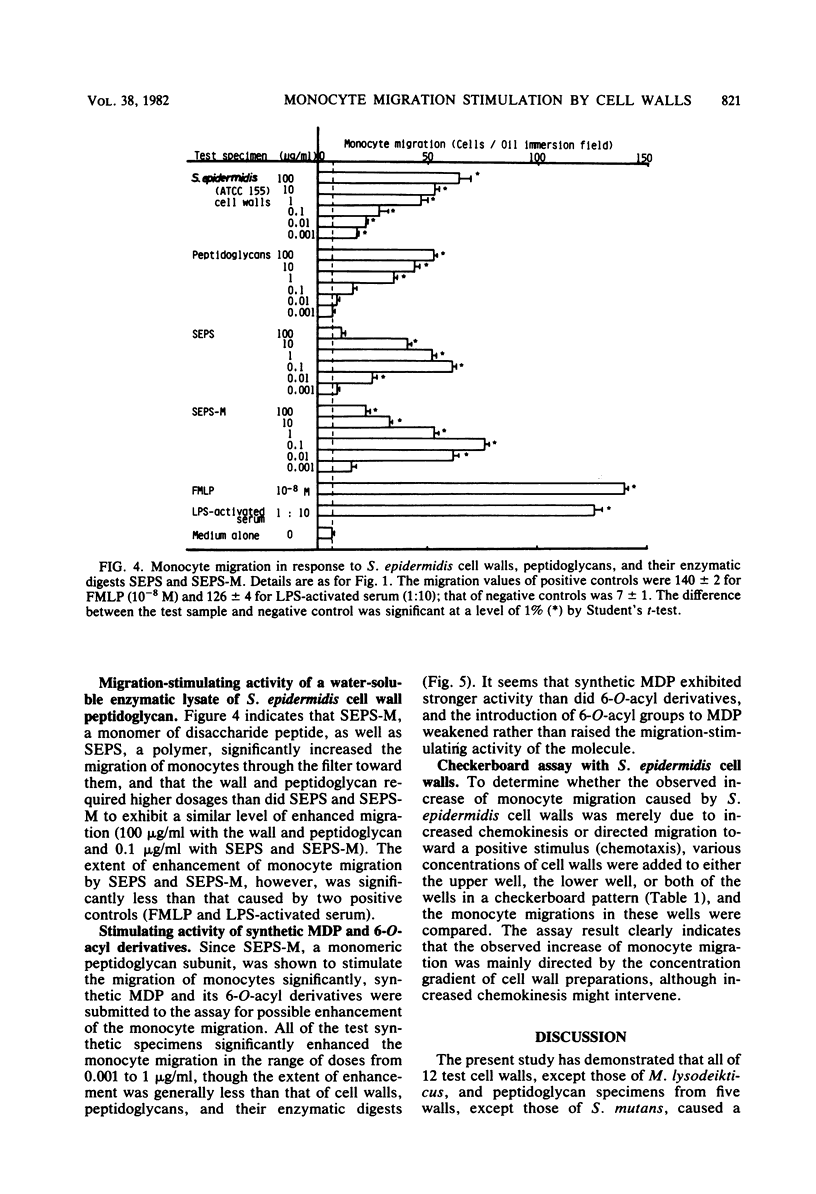
Bacterial cell walls, water-soluble fragments of the wall peptidoglycan, N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP), and 6-O-acyl derivatives of MDP were examined for migration-stimulating activity on human peripheral blood monocytes by using a multiwell chemotaxis assembly. Cell walls isolated from 11 bacterial species caused a definite increase in monocyte migration, but the walls of Micrococcus lysodeikticus were scarely active. The migration-enhancing activity of Staphylococcus epidermidis cell walls was retained by a monomer as well as a polymer of disaccharide peptides which were prepared by digestion of the peptidoglycan with enzymes. It was finally revealed that the migration of monocytes was enhanced by MDP. 6-O-Octadecanoyl-MDP, 6-O-(2-tetradecylhexadecanoyl)-MDP, and 6-O-(3-hydroxy-2-docosylhexacosanoyl)-N-acetylmuramyl-L-seryl-D-isoglutamine were active, but to a lesser extent. A checkerboard assay demonstrated that the increased monocyte migration caused by S. epidermidis cell walls was directed toward a positive stimulus (chemotaxis).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Souvannavong V., Lederer E. Non-specific MIF-like activity induced by the synthetic immunoadjuvant: N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). Biochem Biophys Res Commun. 1978 Nov 29;85(2):684–690. doi: 10.1016/0006-291x(78)91216-0. [DOI] [PubMed] [Google Scholar]
- Adamu S. A., Sperry J. F. Polymorphonuclear neutrophil chemotaxis induced and inhibited by Bacteroides spp. Infect Immun. 1981 Sep;33(3):806–810. doi: 10.1128/iai.33.3.806-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagawa K. S., Tokunaga T. Effect of synthetic muramyl dipeptide (MDP) on differentition of myeloid leukemic cells. Microbiol Immunol. 1980;24(10):1005–1011. doi: 10.1111/j.1348-0421.1980.tb02906.x. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W. Effects of isoprinosine, levamisole, muramyl dipeptide, and SM1213 on lymphocyte and macrophage function in vitro. Cancer Treat Rep. 1978 Nov;62(11):1981–1985. [PubMed] [Google Scholar]
- Harada K., Kotani S., Takada H., Tsujimoto M., Hirachi Y., Kusumoto S., Shiba T., Kawata S., Yokogawa K., Nishimura H. Liberation of serotonin from rabbit blood platelets by bacterial cell walls and related compounds. Infect Immun. 1982 Sep;37(3):1181–1190. doi: 10.1128/iai.37.3.1181-1190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Imai K., Tanaka A. Effect of muramyldipeptide, a synthetic bacterial adjuvant, on enzyme release from cultured mouse macrophages. Microbiol Immunol. 1981;25(1):51–62. doi: 10.1111/j.1348-0421.1981.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Iribe H., Koga T., Onoue K., Kotani S., Kusumoto S., Shiba T. Macrophage-stimulating effect of a synthetic muramyl dipeptide and its adjuvant-active and -inactive analogs for the production of T-cell activating monokines. Cell Immunol. 1981 Oct;64(1):73–83. doi: 10.1016/0008-8749(81)90459-7. [DOI] [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki A. [Activation of human complement system by bacterial cell walls, their water-soluble enzymatic extracts and related synthetic compounds]. Osaka Daigaku Shigaku Zasshi. 1982 Jun;27(1):46–61. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Nozawa R. T., Sekiguchi R., Yokota T. Stimulation by conditioned medium of L-929 fibroblasts, E. coli lipopolysaccharide, and muramyl dipeptide of candidacidal activity of mouse macrophages. Cell Immunol. 1980 Jul 15;53(1):116–124. doi: 10.1016/0008-8749(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Ohkuni H., Kimura Y. Increased capillary permeability in guinea pigs and rats by peptidoglycan fraction extracted from Group A streptococcal cell walls. Exp Cell Biol. 1976;44(2):83–94. doi: 10.1159/000163102. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Cummings N. P., Shiba T., Kusumoto S., Kotani S. Lipophilic derivative of muramyl dipeptide is more active than muramyl dipeptide in priming macrophages to release superoxide anion. Infect Immun. 1980 Aug;29(2):617–622. doi: 10.1128/iai.29.2.617-622.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Stewart-Tull D. E. The dissociation of adjuvant properties of mycobacterial components from mitogenicity, and from the ability to induce the release of mediators from macrophages. Immunology. 1976 Sep;31(3):389–396. [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Inhibition of macrophage phagocytic activity by group A streptococcal cell walls. Infect Immun. 1978 Apr;20(1):258–261. doi: 10.1128/iai.20.1.258-261.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Phillips J. K., Gewurz H., Mergenhagen S. E. A neutrophil chemotatic factor derived from C'5 upon interaction of guinea pig serum with endotoxin. J Immunol. 1969 Sep;103(3):413–422. [PubMed] [Google Scholar]
- Sone S., Fidler I. J. Synergistic activation by lymphokines and muramyl dipeptide of tumoricidal properties in rat alveolar macrophages. J Immunol. 1980 Dec;125(6):2454–2460. [PubMed] [Google Scholar]
- Takada H., Hirachi Y., Hashizume H., Kotani S. Mitogenic activity of cytoplasmic membranes isolated from L-forms of Staphylococcus aureus. Microbiol Immunol. 1980;24(11):1079–1090. doi: 10.1111/j.1348-0421.1980.tb02913.x. [DOI] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Imai K., Mori R. Macrophage activation by muramyl dipeptide as measured by macrophage spreading and attachment. Microbiol Immunol. 1980;24(6):547–557. doi: 10.1111/j.1348-0421.1980.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Taniyama T., Holden H. T. Direct augmentation of cytolytic activity of tumor-derived macrophages and macrophage cell lines by muramyl dipeptide. Cell Immunol. 1979 Dec;48(2):369–374. doi: 10.1016/0008-8749(79)90131-x. [DOI] [PubMed] [Google Scholar]
- Tauber J. W., Polley M. J., Zabriskie J. B. Nonspecific complement activation by streptococcal structures. II. Properdin-independent initiation of the alternate pathway. J Exp Med. 1976 Jun 1;143(6):1352–1366. doi: 10.1084/jem.143.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenu J. P., Lederer E., Petit J. F. Stimulation of thymocyte mitogenic protein secretion and of cytostatic activity of mouse peritoneal macrophages by trehalose dimycolate and muramyldipeptide. Eur J Immunol. 1980 Aug;10(8):647–653. doi: 10.1002/eji.1830100813. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative pathway by pneumococcal cell walls. J Immunol. 1977 Feb;118(2):451–454. [PubMed] [Google Scholar]
- Yamamoto Y., Nagao S., Tanaka A., Koga T., Onoue K. Inhibition of macrophage migration by synthetic muramyl dipeptide. Biochem Biophys Res Commun. 1978 Feb 28;80(4):923–928. doi: 10.1016/0006-291x(78)91333-5. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


