Abstract
The paradigm of SIRS-to-CARS transition implies that hyperinflammation triggers acute sepsis mortality, while hypoinflammation (release of anti-inflammatory cytokines) in late sepsis induces chronic deaths. However, the exact humoral inflammatory mechanisms attributable to sepsis outcomes remain elusive. In part I of the study, we characterized the systemic dynamics of the chronic inflammation in dying (DIE) and surviving (SUR) mice suffering from CLP sepsis (days 6-28). In part II, we combined the current chronic and previous acute/chronic sepsis data to compare the outcome-dependent inflammatory signatures between these two phases. To compare global inflammatory responses, a composite cytokine score (CCS) was calculated. Mice were never sacrificed but sampled daily (20μl) for blood. Part I: parameters from chronic DIE mice were clustered into the 72h, 48h and 24h prior-to-death time-points and compared to SUR of the same post-CLP day. Cytokine increases were mixed and never preceded chronic deaths earlier than 48h (3 to 180-fold increase). CCS demonstrated simultaneous and similar upregulation of pro-and anti-inflammatory compartments at 24h prior to chronic death (DIE 80 and 50-fold higher vs. SUR). Part II: cytokine ratios across sepsis phases/outcomes indicated steady pro-vs. anti-inflammatory balance. CCS showed the inflammatory response in chronic DIE was 5-fold lower versus acute DIE mice, yet identical to acute SUR. Concluding, the systemic MARS-like pattern (concurrent release of pro-and anti-inflammatory cytokines) occurs irrespectively of the sepsis phase, response magnitude and/or outcome. Although different in magnitude, neither acute nor chronic septic mortality is associated with a predominating pro-and/or anti-inflammatory signature in the blood.
Keywords: Chronic sepsis, SIRS, CARS, Cytokines, Cytokine receptors, Inflammation
Introduction
Despite improvements in supportive care, sepsis continues as a life-threatening condition in patients of all ages (1,2). In sepsis, infection leads to a systemic immune reaction termed the systemic inflammatory response syndrome (SIRS). The historical consensus characterized the early phase of sepsis by a prompt rise of circulating pro-inflammatory cytokines, such as IL-1β, TNFα and IL-6 (3). Since this strong pro-inflammatory response was believed to be responsible for early septic deaths (3), inactivation/removal of cytokines during raging SIRS was the focus of experimental and clinical intensive care research over the last decades. Yet, dismal failure of numerous large-scale anti-inflammatory treatment trials (4) was recently followed by the failure of eritoran tetrasodium (a TLR-4 antagonist) (5) and withdrawal of drotrecogin alpha (recombinant human activated protein C) (6), the only existing drug specifically indicated for treatment of sepsis. Due to these drawbacks, the understanding of the traditional concept of the pro- versus anti-inflammatory immune response in sepsis has been rapidly evolving in the recent years. For example, both experimental (7,8) and clinical (9-11) findings demonstrated that pro- and anti-inflammatory cytokines are released in early sepsis and signs of immunosuppression are already manifested in the acute stage of sepsis. Hence, it is evident that sepsis does not progress along a preset disease pattern but needs to be perceived as a highly dynamic biological process (12,13).
Since the trajectory of the systemic immuno-inflammatory response in sepsis can alternate between hyper-activity or immunossupression, an uncorrected, escalating deviation from homeostasis in either direction may result in death. Effective corrective measures should either blunt hyperactive responses or boost the suppressed responses before the window of therapeutic opportunity closes. Given the above, the future of sepsis treatment lies in a more individual approach to septic patients (14-16): the same drug may be beneficial, non-effective or even harmful depending on the patient's immunological status. A notion of using biomarkers (such as cytokines) and their temporal response patterns for identification of homogenous cohorts with the greatest projected benefit from specifically tailored immuno-modulatory therapeutics seems especially attractive (17-20). The temporal evolution of the immuno-inflammatory response in septic patients is central to both of the above concepts. A precise characterization of these changes, relatively straightforward immediately after the onset of sepsis in non-immunocompromised patients, is much more problematic during the later phases of the disease, and remains largely unexplored. This creates a dangerous dissonance since advances in the ICU care have considerably reduced incidences of acute (early) mortality (5). Improved survival throughout the initial stages of sepsis often translates into higher mortality in the later stages of the disease (21-23). In other words, the treatment did not cure the disease, it only delayed death. In the blood, late mortality has been typically associated with increased levels of anti-inflammatory cytokines termed as compensatory anti-inflammatory response syndrome (CARS). This has been postulated to cause a protracted dampening of immune functions in chronically ill septic patients (12,24). Apart from the proposed shift in the profile of circulating cytokines, this late-occurring “immune paralysis” is also reflected by deregulation of cellular compartment, e.g., reduced macrophage antigen presentation (7,25), increased lymphocyte apoptosis (9,26,27) and altered leukocyte recruitment (28) - all of which increase patient's susceptibility to secondary complications.
Despite its relevance, the evolution of immuno-inflammatory signaling in chronic sepsis and its contribution to late mortality have not been widely investigated. In the current study, we characterized the protracted evolution of the chronic pre-lethal inflammatory response to specifically compare the outcome-based profiles of the pro-and anti-inflammatory cytokines and leukocytes in the blood. Additionally, we sought to identify whether a global outcome-dependent pattern of cytokine responses occurring in acute (early) and chronic (late) CLP sepsis defines their key similarities and differences. These findings would provide insight into whether the same basic mechanisms drive the disease process in both acute and chronic sepsis.
Materials and Methods
Animals
ICR outbred mice (Harlan-Sprague Dawley, Inc., Frederick, MD) with an average weight of 22 g were used (n=97). To eliminate gender-related variability, only female mice were included in the study. The mice were acclimated to the laboratory environment for at least 48 hrs before surgery and housed in a temperature-controlled room with a 12-hr light-12-hr dark diurnal cycle. Standard rodent chow and water were provided ad libitum. All experiments were carried out in accordance with the National Institutes of Health guidelines and the Boston University Animal Care and Use Committee.
Sepsis model
To ensure adequate reproducibility, surgeries were performed on separate groups of mice (typical n=10/experiment). The CLP model is widely accepted (29) and used by many to study the immunopathology of sepsis (30-32). We used a medium-grade CLP severity (18 gauge needle, double puncture) to emulate a typical 30% mortality rate occurring in chronic patients with abdominal sepsis (33). The original CLP protocol was followed (34) and previously described modifications were implemented (8) including broad-range antibiotic (imipenem, 25 mg/kg) therapy with 1ml/mouse fluid resuscitation (Lactated Ringers, 1ml/mouse) administered (twice daily) only during the first 5 days post-CLP. All animals were followed for 28 days or until death, whichever occurred first. Sham surgeries were not performed since we were comparing the response in dying mice with long-term survivors, rather than just cataloguing the response to sepsis.
Study design
In the first, chronic sepsis phase, part of the study (Figures 1-5), the experiment investigated the protracted immuno-inflammatory responses in between days 6-28 post-CLP. The selection of the 5-day cut-off was justified based on dissimilar mechanism(s) of death between the acute (days 1–5 post-CLP) vs. chronic sepsis (16,35,36). Death was used as a reference time-point for all DIE mice. Consequently, data are plotted in an inverted fashion: the 24h time-point represents the last individual (and/or average) parameter value in an animal that died within the 24h of sampling (all tables and figures), while 48h and 72h time-points (additionally present in Fig. 2-4 and Supplementary Fig. 2) represent measurements taken within 48h and 72h of death, respectively. For comparisons, all chronic DIE mice (irrespective of the day of death) were pooled (based on the sequence of their prior-to-death time-points) and retrospectively matched with two (randomly selected) SUR (alive at day 28) animals of the same post-CLP day (see Supplementary Fig. 1 schematic). After selection, three consecutive (i.e. 72-24h SUR time-points), daily measurements were taken from the same SUR mice to match the pre-death measurements from a DIE mouse of each given (one-DIE/two-SUR) triplet (e.g. for a death on day 13, both SUR and DIE values from days 11, 12 and 13 were tallied).
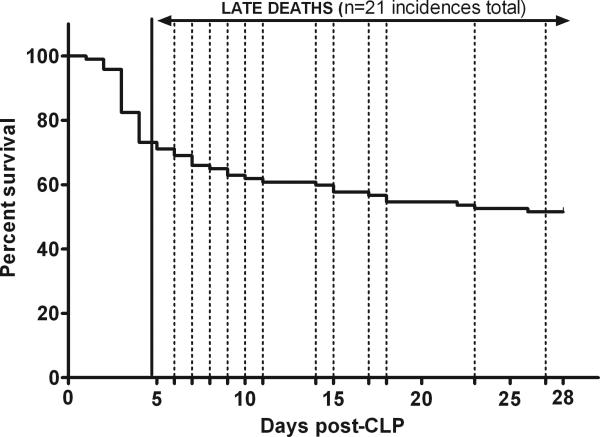
Figure 1. 28-day survival curve after CLP-induced sepsis.
CLP was performed (total n=97) with an 18-gauge needle to produce ~30% mortality during the end of chronic phase of sepsis. Using a subjective cut-off, 28-day follow up was separated into the acute (days 1-5) and chronic (days 6-28) phase. A total of 21 deaths (21 out of 71) occurred in chronic sepsis. Animals included in the analysis (15 mice) underwent daily 20μl blood sampling between days 6-28. Solid line indicates the end of the acute sepsis phase, whereas dotted lines indicate the day of death of mice included in the analysis.
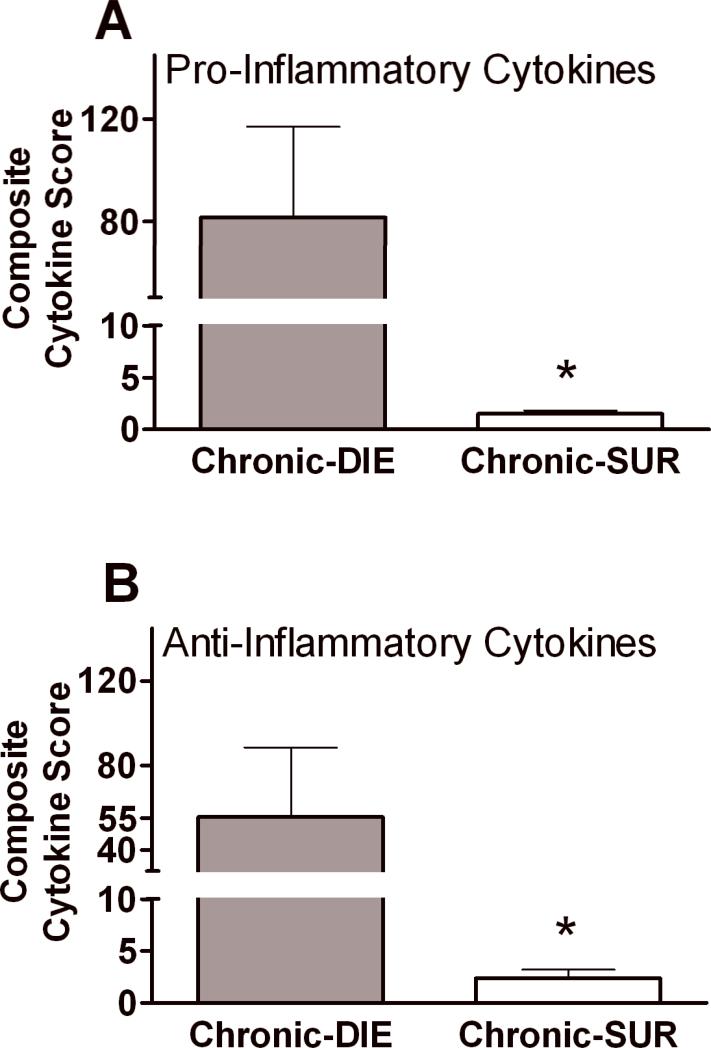
Figure 5. Composite Cytokine Scores for pro-and anti-inflammatory compartments based on outcome in chronic sepsis.
All cytokine values were normalized (each mediator individually) to the median DIE value of that specific cytokine. The individual activation scores for each tested cytokine were then combined into the pro-inflammatory (i.e. IL-1β, IL-2, IL-5, IL-6, IL-12, IL-17, TNFα, IFNγ, ICAM-1, MIP-1α, MIP-2, MCP-1, Eotaxin, Eotaxin-2) and anti-inflammatory (IL-1ra, IL-4, IL-10, IL-13, TNF srI, TNF srII) panels, and the average response scores were compared based on outcome. DIE (any 6-28 post-CLP day) n=15 SUR (alive by 28 post-CLP day) n=30. Chronic DIE bar represents pooled cytokine values collected within 24h of death. Cytokine values collected from SUR animals were sampled on the same post-CLP day as the DIE mice (see study design). Data presented as mean + SEM. * p < 0.01.
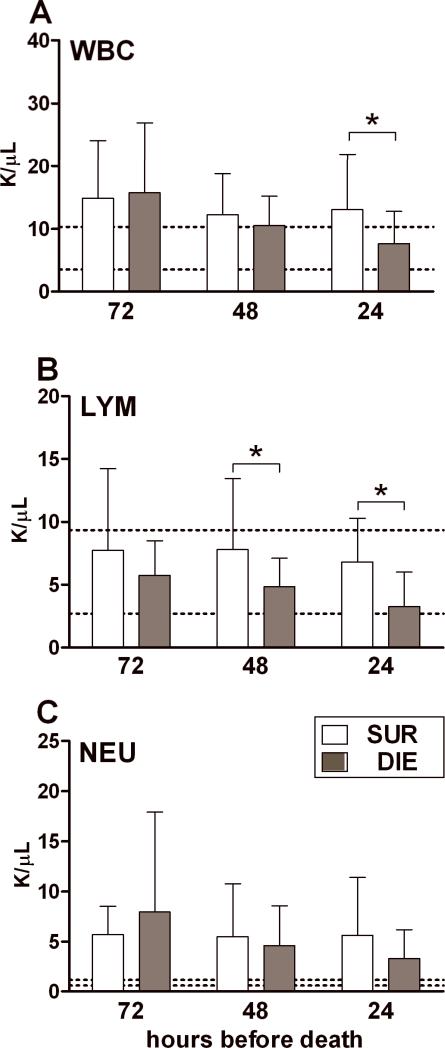
Figure 2. Protracted profiles of WBC, lymphocyte and neutrophil counts based on outcome during chronic sepsis.
Daily blood samples were collected between days 6-28 post-CLP. The day of death (any day between 6-28 days) served as the reference point. DIE values were retrospectively plotted in the 72h, 48h and 24h prior-to-death trajectory and were time-matched with SUR values from the same post-CLP day (see study design). 2:1 SUR to DIE ratio at each time-point for each parameter: n= 20/10 (SUR/DIE) at 72h; n=28/14at 48h and 24h. Data (box and whiskers) presented as mean + SD. Dotted lines indicate normal range.* p < 0.05.
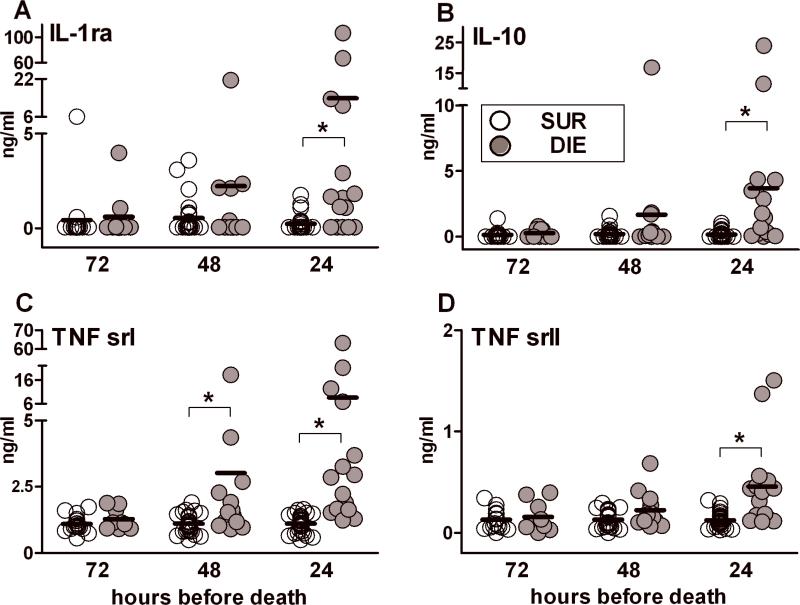
Figure 4. Protracted profiles of IL-1ra, IL-10, TNF srI and TNF srII based on outcome during chronic sepsis.
Daily blood samples were collected between days 6-28 post-CLP. The day of death (any day between 6-28 days) served as the reference point. DIE values were retrospectively plotted in the 72h, 48h and 24h prior-to-death trajectory and were time-matched with SUR values from the same post-CLP day (see study design). 2:1 SUR to DIE ratio at each time-point for each parameter: n= 18/9 (SUR/DIE) at 72h; n= 26/13 at 48h; n=29/15 at 24h. Data presented as scatter dot plot: each dot represents an individual mouse, horizontal line represents mean.* p < 0.01.
In the second part of the study, the current data from the chronic sepsis experiment were combined with the historical data recorded in the previous acute (8) and chronic (35) sepsis experiments (Figures 7-8 and Tables). Only the 24h prior-to-death time-points (regardless of sepsis phase) from all three studies were combined for comparison of inflammatory responses in acute versus chronic sepsis. Data merger was justified by identical experimental protocol in all three studies, high reproducibility of measurements and by the improved statistical power of such combined analysis.
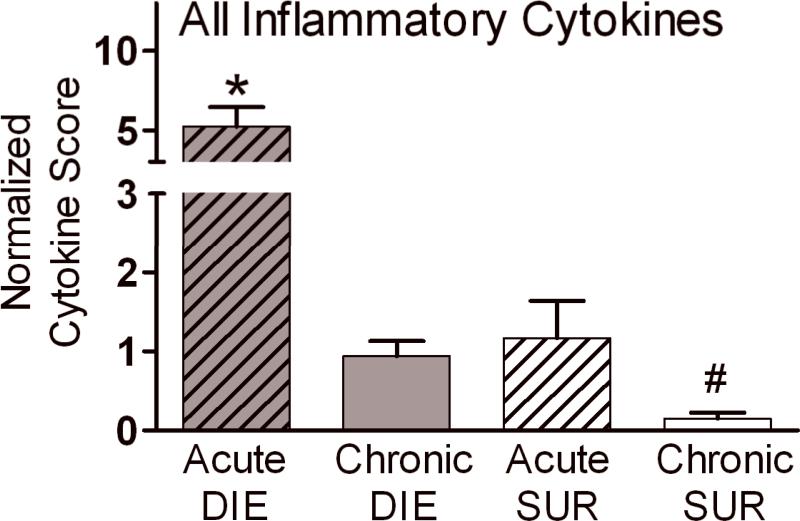
Figure 7. Normalized Cytokine Score based on outcome in the acute and chronic sepsis.
To enable comparison between septic phases, cytokine values from the current and two previous studies (25,32) were combined for this analysis. All cytokine values were normalized and cytokine scores for individual cytokines were calculated (see statistical section). Those normalized individual scores were then combined into an overall score for each group and compared between DIE and SUR and across acute and chronic sepsis. DIE bars represent pooled cytokine values collected within 24h of death in the respective phase of sepsis. Only the same cytokines that were measured in all three studies were analyzed (n = 10/each group; IL-1β, IL-6, TNFα, MIP-2, MCP-1, Eotaxin and IL-1ra, IL-10, TNF srI, TNF srII). For simplicity, pro-and anti-inflammatory cytokines were pooled in each group. Data presented as mean + SEM. * p < 0.01 and # p < 0.001 compared to all remaining groups.
Sampling
All animals that survived the period of acute sepsis (day 1-5) were sampled daily in the chronic phase of sepsis (days 6-28). Blood (20μl) was collected from all animals by facial vein (vena submandibularis) puncture (alternating cheeks daily) using a 23-gauge needle for an optimal sampling precision (37). Repetitive daily sampling over 22 days (days 6-28 post-CLP) was safe: it affected neither 28-day mortality nor any of the recorded parameters (i.e. hemoglobin, cell blood counts, circulating cytokines) (38). All samples were drawn into a pipette rinsed with EDTA and then immediately diluted 1:10 in PBS with 2% (vol/vol) EDTA. After centrifugation (1000xg, 5min, 22°C) plasma was removed and stored at –80°C until analysis. Overall, 15 chronic DIE mice (out of total 21) were included in the final analysis: 1 mouse was excluded from the study due to a sampling injury, and irregularity in the sampling schedule eliminated the remaining 5. Figure 1 shows the temporal distribution of sampled animals.
Cytokine immunoassays
In the current study, we used a validated microarray immunoassay methodology with a capacity to simultaneously measure multiple mouse cytokines (39). All targets contained on our microarray platform were shown to be in-and/or directly implicated in the septic inflammatory sequele (40,41). Other classical biomarkers such as procalcitonin (PCT) and C reactive protein were not assessed due to the sample volume constraints (20μ/mouse) and/or lack of commercial murine assays.
Cytokine data from two previous studies that were used for comparisons between acute and chronic sepsis (Figures 7-8 and all Tables), were obtained by either the same microarray immunoassay (previous chronic sepsis study; 35) or a sequential ELISA (previous acute sepsis study; 8) method described elsewhere (42). In all three studies (regardless of the employed assay), the same standard ELISA-based and previously optimized antibody pairs were used (43). Reliability of the presented comparisons was assured, as the employed microarray and classical ELISA immunoassays display a virtually perfect correlation and detect the same relative levels of cytokines (with the same degree of variability) in the blood plasma samples (39).
In brief, the primary (capture) antibodies were spotted on the bottoms of 96-well microtiter plates (the current study and 8) or nitrocellulose pads (35). Next, samples were incubated with a biotinylated secondary antibody, then streptavidin conjugated to HRP or an infrared (IR) fluorophore, and plates were read with the ELISA plate reader (Bio-Tek Instruments, Winooski, VT) or the Odyssey IR imaging system (LI-COR Biosciences, Lincoln, NE), respectively.
Hematology
Following blood collection, the cell pellet was immediately re-suspended in 480μl of Hemavet solution (CDC Technologies, Oxford, CT). A complete blood count including differential was performed with a Hemavet 1500 (CDC Technologies, Oxford, CT).
Statistical analysis
28 day-survival (Fig. 1) was plotted using the Kaplan-Meier curve. Figures 1-5 (and in Supplementary Fig. 2) were based on the data generated in this study only. For data presented in Table I and Figures 6-7 (and in supplementary Tables I-II), cytokine values (i.e. IL-1β, IL-6, TNFα, MIP-2, MCP-1, Eotaxin and IL-1ra, IL-10, TNF srI, TNF srII) obtained in this and two previous (25,32) studies were combined for analysis. Specifically, the current dataset (n=97) was supplemented by additional measurements from the previous chronic (42 values total) and acute (70 values total) sepsis datasets. In all three studies, the CLP protocols were identical (i.e. severity, antibiotic and fluid resuscitation treatment). Detailed n distribution and the source of data plotted in all individual Tables and Figures are present in their respective legends.
Table I.
Average activation score for individual pro-and anti-inflammatory cytokines separated based on the outcome and phase of CLP sepsis.
| Cytokinea | Acute-DIEb | Chronic-DIEb | Acute-SURc | Chronic-SURc |
|---|---|---|---|---|
| Interleukin (IL)-1β | 4.78 | 0.65 | 2.22 | 0.11 |
| IL-6 (Pro-I) | 11.55 | 0.93 | 0.47 | 0.01 |
| TNFα (Pro-I) | 4.96 | 0.33 | 0.88 | 0.02 |
| MIP-2 (Pro-I) | 8.72 | 1.26 | 0.92 | 0.03 |
| MCP-1 (Pro-I) | 1.11 | 0.96 | 0.58 | 0.14 |
| Eotaxin (Pro-I) | 3.76 | 2.09 | 0.57 | 0.82 |
| IL-10 (Anti-I) | 1.74 | 0.74 | 0.53 | 0.04 |
| TNF srI (Anti-I) | 1.88 | 1.70 | 0.30 | 0.27 |
| TNF srII (Anti-I) | 11.33 | 0.15 | 5.09 | 0.03 |
| IL-lra (Anti-I) | 2.27 | 0.59 | 0.09 | 0.02 |
All cytokine values were normalized (see statistical section) to the median DIE value of that specific cytokine. Acute-DIE/SUR n=35/group, Chronic-DIE n=29, Chronic-SUR n=58. Listed response scores were computed from combined cytokine values measured in this and two previous studies (25,32). Pro-I, pro-inflammatory; Anti-I, anti-inflammatory.
Pre-lethal cytokine values were collected within 24h of death within the respective phase.
Cytokine values collected from SUR animals (alive at day 28 post-CLP) were sampled for comparison on the same post-CLP day as the DIE mice.
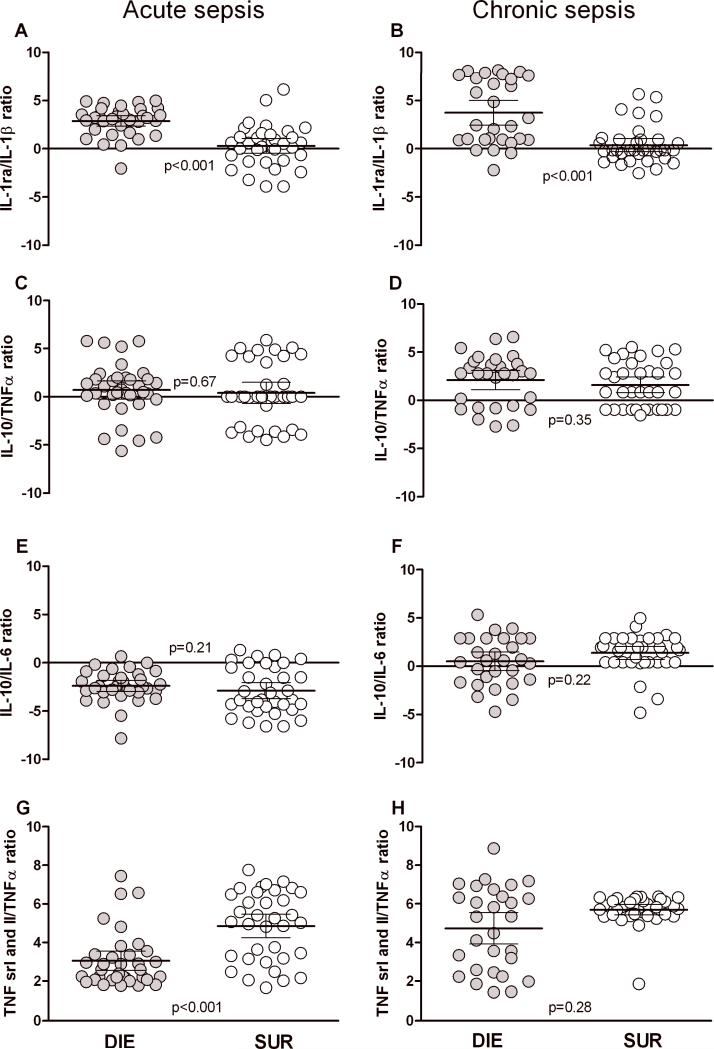
Figure 6. Comparison of pro/anti-inflammatory ratios in acute and chronic sepsis.
To enable comparison between septic phases, cytokine values from the current and two previous studies (25,32) were combined for this analysis. Each dot represents a ratio for a given cytokine set in an individual mouse. DIE group (left) represents ratios calculated from cytokine values collected within 24h of death. SUR group (right) represents ratios calculated from cytokine values collected on the same post-CLP day as the DIE mice (see study design). n=35 in acute DIE, n=29 in chronic DIE; n=30 in both SUR groups. Data presented as scatter plot on log N scale, horizontal bars represent mean ± 95% confidence intervals.
Data in Figures 2-6 were analyzed by either Student's t test (Gaussian distribution) or Mann Whitney test (skewed distribution). Cytokine ratios (Fig. 6) were computed for each individual animal, averaged across respective groups and log transformed (Y=Ln(Y)) for comparison. In Figures 2-4, each time-point was analyzed separately since the differences between SUR vs. DIE groups were the primary endpoints.
To generate the average inflammatory scores (Fig. 5 and 7), all cytokine values were normalized (each mediator individually) to the median DIE value of that specific cytokine. The scores for the same chronic-DIE and SUR groups are different in Fig. 5 and 7 since an effective graphical depiction required two different median DIE values to compute them: median was selected from the chronic dataset in Fig. 5 (henceforth referred to as the “Composite Cytokine Score”) and from combined acute and chronic sepsis datasets in Fig. 7 (henceforth referred to as the “Normalized Cytokine Score”). The means of all normalized cytokine values (now scores) were allocated to different groups based on outcome (Fig. 5) and/or phase of sepsis (Table I, Fig. 7), averaged and presented as average response scores of each group. In Fig. 5, the outcome-dependent Composite Cytokine Scores of chronically septic mice were additionally divided into the pro-and anti-inflammatory panels. For comparison of global outcome-dependent responses between acute and chronic sepsis in Fig. 7, pro-and anti-inflammatory cytokines were pooled together in each respective group. Normalized Cytokine Scores were then log transformed and analyzed by one-way ANOVA followed by Tukey's test.
Cytokine levels below the limit of detection were assigned a value that was equal to one-half of the lower limit of detection in the standard curve. Significance was assigned where p < 0.05, and all tests were two-tailed. All statistical analyses were performed using either Prism 5 (GraphPad Software Inc., San Diego, CA) and SAS software 9.1.2 on Windows (SAS Institute Inc., Cary, NC, USA).
Results
Mortality in the chronic phase of CLP sepsis
To experimentally replicate the level of mortality typical for septic patients in the modern ICU, mice were subjected to CLP resulting in moderate severity and their survival was followed for 28 days (Fig.1). CLP-induced sepsis resulted in an acute mortality of 29% (or 26/97 of total deaths by day 5 post-CLP) followed by the additional chronic mortality of 30% (or 21/71 by day 28). Only those animals which survived/died within days 6-28 post-CLP were subjected to daily blood sampling. A total of 15 (out of 21) late death mice (designated by dotted lines) were subsequently included in the analysis.
Comparison of leukocyte trajectories in SUR vs. DIE mice in chronic sepsis
To characterize protracted changes preceding chronic mortality for all parameters included in this study, we used the day of death during the chronic phase (any day between 6-28 days) as the reference point. We then clustered all parameter values recorded at 72h, 48h and 24h prior to the death of each mouse and compared them to the matching values recorded in SUR mice (see study design and Supplementary Fig. 1 schematic). An identical analytical approach was used for data in Figures 2-4 (and Supplementary Fig. 2).
We previously reported pronounced lympho-and neutropenia in acutely ill CLP mice (44,45). To establish whether similar shifts also occur during chronic sepsis, we compared circulating WBC and their subsets in DIE and SUR mice.
Regardless of outcome, chronically septic mice displayed slight leukocytosis due primarily to pronounced neutrophilia (Fig. 2A and C). Lymphocyte counts were within the range of normal values at all time points. Compared to SUR, total WBC counts in DIE mice were similar at 72h and 48h but a 42% reduction was observed at 24h prior to death (Figure 2A). A similar decrease for DIE mice was observed in LYM counts: a 30% drop at 48h and 50% at 24h compared to the respective SUR groups (Figure 2B). Conversely, no significant differences in the numbers of NEU were observed between the SUR and DIE groups at any time point (Figure 2C). These results indicate that the decrease in WBC counts recorded immediately prior to death was due to the reduction in LYM but not NEU counts. Additionally, this effect was accompanied by a pronounced pre-lethal thrombocytopenia without overt signs of anemia (Supplementary Fig.1).
Comparison of pro-inflammatory cytokine trajectories in SUR vs. DIE mice in chronic sepsis
We have previously demonstrated that acute septic deaths after CLP were preceded by a robust rise of several inflammatory cytokines (8). To characterize the protracted evolution of inflammatory responses preceding late deaths in individual mice, their cytokine values were clustered to display profiles for the last three days before death (i.e. 72h, 48h and 24h). These values were then compared with time-matched SUR values.
A strikingly consistent pattern emerged when the data are analyzed relative to the day of death. This analysis is possible because the study design sampled all 15 mice on a daily basis. Compared to SUR, mean plasma levels of TNFα (Fig. 3A), IL-6 (Fig. 3B), MIP-2 (Fig. 3C) and MCP-1 (Fig. 3D) recorded over the three days prior to death were significantly increased at 24h.
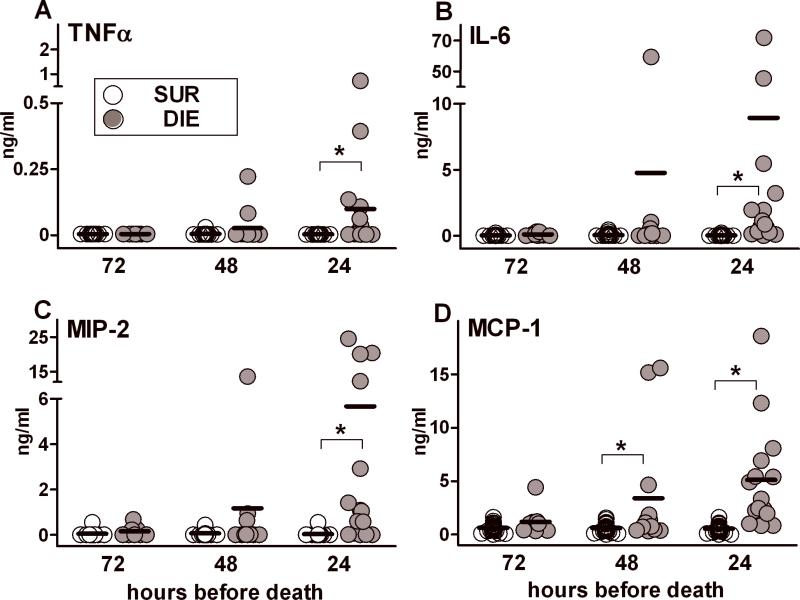
Figure 3. Protracted profiles of TNFα, IL-6, MIP-2 and MCP-1 based on outcome during chronic sepsis.
Daily blood samples were collected between days 6-28 post-CLP. The day of death (any day between 6-28 days) served as the reference point. DIE values were retrospectively plotted in the 72h, 48h and 24h prior-to-death trajectory and were time-matched with SUR values from the same post-CLP day (see study design). 2:1 SUR to DIE ratio at each time-point for each parameter: n= 18/9 (SUR/DIE) at 72h; n= 26/13 at 48h; n=30/15 at 24h. Data presented as scatter dot plot: each dot represents an individual mouse, horizontal line represents mean.* p < 0.01.
A similar outcome-dependent difference (i.e. at 24h) was also observed in virtually all other cytokines/chemokines we measured (Supplementary Table I). Conversely, the mean DIE versus SUR difference was not significant at 48h and 72h (Fig. 3 and data not shown) in most of the recorded pro-inflammatory biomarkers, with the exception of MCP-1 (Fig. 3D, approx. 6-fold difference at 48h). SUR values of TNFα, IL-6, MIP-2 and MCP-1 (Fig. 3A-D) and remaining pro-inflammatory biomarkers (data not shown) were either below detection level or marginally low at the 48 and 72 hour pre-death time-points.
Comparison of anti-inflammatory cytokine trajectories in SUR vs. DIE mice in chronic sepsis
It has been suggested that CARS predominates in the chronic phase of sepsis, and that late septic mortality is due to immuno-suppression associated with a strong release of anti-inflammatory mediators (46). Using the analytical approach described above, we analyzed fluctuations of key anti-inflammatory cytokines known to contribute to suppressed immune responses.
Compared to the late pro-inflammatory response, the pre-lethal trajectory of anti-inflammatory biomarkers in chronic sepsis was virtually identical. At 24h, mean DIE values of circulating IL-1ra (Fig. 4A), IL-10 (Fig. 4B),TNF srI (Fig. 4C) and TNF srII (Fig. 4D) were 60-fold, 24-fold, 8-fold and 4-fold higher compared to SUR mice, respectively. A similar outcome-dependent effect was also observed in 5 (out of 6) other anti-inflammatory cytokines recorded at 24h prior to death (Supplementary Table II). In contrast, in none of the anti-inflammatory cytokines except TNF srI (Fig. 4C, approx. 3-fold difference at 48h) was the mean DIE concentration elevated (vs. SUR) at 48h and 72h (Fig. 4, and data not shown). Combined data from Figures 3 and 4 show that the pre-lethal rise of both pro-inflammatory and anti-inflammatory cytokines during chronic sepsis is very transient and typically occurs only immediately before death.
Comparison of Composite Cytokine Scores in chronic sepsis
Lethal outcome in sepsis is not caused by a single key mediator but is likely driven by concurrent deregulation of numerous immuno-inflammatory pathways. To examine general and outcome-dependent inflammatory responses in chronic sepsis, we combined all cytokines into the two panels (pro-inflammatory Fig 5A and anti-inflammatory 5B) and generated outcome-dependent, Composite Cytokine Scores (CCS; by normalizing all individual cytokine values - see statistical section). The Composite Cytokine Scores recorded at 24h prior to death in DIE mice were higher from the ones calculated in SUR mice (Fig. 5): the difference reached approximately 80-fold in the pro-inflammatory and 50-fold in the anti-inflammatory panel (p>0.1 between pro-inflammatory and anti-inflammatory CCS). These data demonstrate that lethality in chronic sepsis is preceded not only by a simultaneous but also a similar-grade release of pro-and anti-inflammatory cytokines.
Comparison of cytokine ratios based on outcome and phase of sepsis
The data in Fig. 3-5 (and Supplementary Tables I-II) demonstrated that during chronic sepsis, both non-and lethal responses were associated with a simultaneous and similar release of both pro-and anti-inflammatory cytokines. To further examine the systemic, outcome-dependent balance between pro-and anti-inflammatory responses across acute and chronic sepsis, ratios for key pro- and anti-inflammatory mediators were computed.
Despite occasional statistical inter-group differences, cytokine ratios were similar both across phases and outcomes. The most consistent difference was noted in the IL-1ra/IL-1β ratio, which showed an identical increase in DIE versus SUR mice in both acute and chronic phase of sepsis (Fig. 6A-B). The opposite finding was true for the TNF srI + II/TNFα ratio: SUR was slightly higher compared to DIE but only in the acute sepsis (Fig. 6G).
Additionally, both acute sepsis IL-10/IL-6 ratios (also TNF srI + II/TNFα ratio in acute DIE vs. chronic DIE; Fig. G-H) were statistically lower compared to the corresponding ratios in chronic mice (Fig. 6E-F), whereas all remaining ratios were virtually identical. These data underline that regardless of the phase and/or outcome, a typical humoral response in sepsis always features pro-and anti-inflammatory components.
Comparison of Normalized Cytokine Scores in acute and chronic sepsis
The above data are indicative of the MARS-like release pattern, but do not reveal differences in the magnitude of septic responses between acute and chronic sepsis. In the next analysis, we aimed to compare the relative inflammatory response levels across the acute and chronic sepsis. To accomplish this, we combined cytokine data from the current study and two previous studies investigating both acute (8) and chronic (35) sepsis. Similarly to Figure 5, cytokine scores were generated (see statistical section). Scores were computed only for cytokines that overlapped in all three studies, and the scores were divided into four groups depending on outcome (i.e. SUR vs. DIE) and the phase of sepsis (i.e. acute vs. chronic). For simplicity, no additional separation into the pro- and anti-inflammatory panels was performed. The group scores for each individual cytokine are listed in Table I.
The highest Normalized Cytokine Score (NCS) was recorded in mice that died during the acute phase of sepsis: NCS was approximately 5-fold higher than in acute SUR mice and chronic DIE animals (Fig. 7). The difference was even more pronounced (approx. 35-fold) when compared to NCS in chronic SUR mice. Additionally, the chronic SUR group NCS was on average 5-fold lower compared to both chronic DIE and acute SUR animals (all p<0.01).
Discussion
Measurement of circulating biomarkers constitutes the most practical method to rapidly describe the immuno-inflammatory status of a septic ER/ICU patient. To characterize the chronic pre-lethal inflammatory response in the first part of the study (Figs. 1-5), we incorporated three vital experimental elements: 1) a model of moderate severity sepsis (displaying frequent late mortality) by polymicrobial, CLP-induced peritonitis, 2) a study design where mice were never sacrificed to allow a natural progression of the disease to survival/death, and 3) small-volume daily sampling during the chronic phase of sepsis (days 6-28). The above design was dictated by the clinical reality, where a large fraction of septic patients die in the chronic disease stage, the onset of sepsis is typically unknown (compared to pre-clinical studies), and patients are monitored frequently to provide optimal management and avoid another and ultimate reference point - death.
Given that plethora of pro- and anti-inflammatory cytokines were shown to correlate, both clinically (47-49) and experimentally (8,50) with sepsis severity/poor prognosis, a clinical focus has been put on selecting subjects with the highest risk of death to produce relatively homogenous cohorts more suitable for personalized treatments. Comparison of the Composite Cytokine Scores in the SUR and DIE groups at 24h prior to death (Fig. 5) shows that similar to acute sepsis (8) late deaths can also be preceded by a marked release of circulating cytokines. In line with our initial findings (35), the current data re-confirm that individual cytokine/subject responses in chronic sepsis are highly mixed, regarding both the magnitude of the increase and the selection of released mediators. By longitudinally plotting the data from the chronic phase of sepsis in the current study, we demonstrate an aspect of the chronic inflammatory response never shown before. Specifically, virtually no cytokine increases were observed earlier than 48h pre-death, regardless of whether the mediator was in the pro-or anti-inflammatory panel. This closely mirrors the cytokine response in acute CLP (8) where (SUR vs. DIE) differences did not appear earlier than the 48h prior-to-death. In the CLP setting, therefore, septic deaths (regardless whether acute or chronic) cannot be predicted earlier than approx. 48h prior to the event. In the current chronic sepsis study, none of the biomarkers exceeded AUC > 0.7 at 48h before late deaths (except AUC=0.77 for TNF srI, data not shown). Although many, seemingly promising, clinical studies investigated the viability of cytokine-prediction for long-term (typically 28 days) septic outcomes (51-53), none of the biomarkers/biomarker sets has ever translated into a routine and clinically employable tool (5,41). Current observations in the chronic sepsis part of the study may, at least partially, explain this deficit. We show that compared to the relatively steady (hyperinflammatory) signature of acute sepsis (8), the feasibility of predicting outcome in chronic sepsis is much weaker. This weakness is primarily related to the transient and erratic appearance of cytokines in the bloodstream, limiting their use as predictors, regardless whether as a single marker or in combination. Another aspect is the diverse strength of the signal, since cytokine values are much higher in the acute phase. In other words, although chronic phase spikes in selected circulating cytokines (e.g. MIP-2) are predictive for late deaths, the magnitude of these increases is negligible when compared to their massive release in the acute phase. Interestingly, with the expanded range of biomarkers measured we observed that the inflammatory response in mice dying in chronic sepsis displays an “all or nothing” character: i.e. whenever an increase (or lack of response) in a given cytokine was recorded, a similar increasing (or no-responsiveness) pattern was also true for virtually all other (or majority of) remaining biomarkers.
The two most valuable findings, however, come from a combined, across-the-board comparison of outcome-dependent cytokine responses in the acute and chronic phases of sepsis. First, based on the comparison of pro/anti-inflammatory ratios from this (chronic sepsis) and our two previous (acute and chronic sepsis) experiments (8,35), we prove that the proposed “Sepsis: Always in MARS” paradigm (54) can be uniformly applied regardless of outcome and the phase of disease. Specifically, whenever sepsis provokes a given systemic inflammatory response, the associated release of anti-inflammatory cytokines closely matches the speed and/or robustness of the pro-inflammatory mediator secretion (and vice versa). Consequently, neither acute nor chronic sepsis mortality appear to be associated with a distinct predominant pro-and/or anti-inflammatory signature in the blood (despite differences in the relative response magnitude – see below). The most recent clinical studies corroborate the above observations (10,11). The clinical translation of the above argued concept is simple: in the blood, the true cytokine make-up of SIRS/CARS is a one of MARS, and these definitions should be redefined accordingly.
Second, we demonstrate that due to the 5-fold difference in the magnitude of the response between acute DIE and chronic DIE, an effective identification of late CLP mortality in the milieu of an acute cytokine response is next to impossible. In other words, a 5ng/ml of circulating IL-6 measured in a CLP mouse may herald either its impeding death (hence a need for an immediate therapeutic intervention) in the chronic phase of sepsis, or conversely, indicate that the host mounted a successful struggle against the disease in its early, acute stage (discouraging an aggressive anti-inflammatory treatment). The above can be also directly extrapolated to the hospital ICU. The cytokine response profiles represented by Normalized Cytokine Scores indicate that the current precept of selecting septic subjects with the highest risk of death (based on the secreted cytokines) is heavily biased toward the hyper-inflammatory (MARS-like) signature, i.e., it tends to include only those who display a relatively strong cytokine response, while leaving both “non- and “low-responders” behind. As a result, chronic septic subjects with a typically weaker pre-lethal cytokine response are unable to rise above a given diagnostic threshold calibrated on the magnitude of the early inflammatory response. Hence, these subjects will be neither identified nor treated (with the latter omission paradoxically beneficial in case of aggressive immunosuppressive/anti-inflammatory therapies). The above might have been partially reflected in the MONARCS trial (15) that relied on IL-6 to direct an anti-TNF treatment in septic patients: the trial improved the overall survival by approx. 6%, yet the target group of IL-6 test positive patients was relatively small (n= 998) despite the size of the entire (n=2634) enrolled population. The hyper-inflammatory bias may be further exacerbated depending on the type/source of sepsis since innate/adaptive inflammatory signature of abdominal sepsis differs from, for example, pulmonary sepsis developing in ventilated ICU patients (55). Finally, it needs to be stressed out that plasma cytokines may not mirror the functional state of the immune cells in tissues/organs. Cavaillion et al., described this disparity as “compartmentalization” of the septic inflammatory response (56,57). Indeed, a robust systemic release of inflammatory cytokines (in sepsis) was shown to coincide with evident signs of cellular immunossupression in various tissue compartments, both in clinical (25,58) and CLP studies (59-61)
The above data do not deny a role for circulating biomarkers as a valuable tool for guiding specific immunomodulatory therapies in septic patients. In contrary, such a scenario still appears feasible, at least to some degree. Given the aforementioned differences in the magnitude of humoral cytokine response, it needs to be carefully established under what circumstances and for which patient cohorts the most clinical benefit is to be produced. Perhaps, a superior solution for parallel identification of some high-risk-of-death patients (and their immuno-inflammatory status) is to combine the most accurate existing scoring systems (e.g. Pitt bacteremia score, APACHE II and MEDS scores) (62-64) with a relatively uncomplicated (and rapid) profiling of an array of suitable circulating cytokines and/or cells. As accuracy of the latter element(s) is heavily dependent on the phase of sepsis, it should not be ignored that the absence/low levels of biomarker(s) may/should be as alerting as their overwhelming presence.
Supplementary Material
Acknowledgments
We sincerely thank Dr. S. Bahrami for the critical review of our manuscript.
Abbreviations used in this paper
- SIRS
systemic inflammatory response syndrome
- MARS
mixed anti-inflammatory response syndrome
- CARS
compensatory anti-inflammatory response syndrome
- SUR
survivors
- DIE
dying
- CCS
Composite Cytokine Score
- NCS
Normalized Cytokine Score
- ROC
receiver operating characteristic curves
- AUC
area under the curve
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- MEDS
Mortality in Emergency Department Sepsis
Footnotes
This work was supported in part by NIH GM 82962.
Reference List
- 1.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le G, Jr., Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL. EPIC II: sepsis around the world. Minerva Anestesiol. 2008;74:293. [PubMed] [Google Scholar]
- 3.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA. 1992;268:3452. [PubMed] [Google Scholar]
- 4.Remick DG. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr. Pharm. Des. 2003;9:75. doi: 10.2174/1381612033392567. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 6.Marti-Carvajal AJ, Sola I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane. Database. Syst. Rev. 2011:CD004388. doi: 10.1002/14651858.CD004388.pub4. [DOI] [PubMed] [Google Scholar]
- 7.Newton S, Ding Y, Chung CS, Chen Y, Lomas-Neira JL, Ayala A. Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg. Infect. (Larchmt. ) 2004;5:375. doi: 10.1089/sur.2004.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006;177:1967. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 9.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616. doi: 10.1016/j.imbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Tamayo E, Fernandez A, Almansa R, Carrasco E, Heredia M, Lajo C, Goncalves L, Gomez-Herreras JI, de Lejarazu RO, Bermejo-Martin JF. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011;22:82. doi: 10.1684/ecn.2011.0281. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 2009;15:496. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med. 2009;37:1567. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van ML, Daum L, Lemeshow S, Hicklin G, Doig C. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg JW, van der ZM, de Bruin RW, van Holten-Neelen C, Bastiaans J, Nagtzaam NM, IJzermans JN, Benner R, Dik WA. Mild versus strong anti-inflammatory therapy during early sepsis in mice: a matter of life and death. Crit Care Med. 2011;39:1275. doi: 10.1097/CCM.0b013e31820edf75. [DOI] [PubMed] [Google Scholar]
- 17.Bahrami S, Pelinka L, Khadem A, Maitzen S, Hawa G, van GM, Redl H. Circulating NT-proCNP predicts sepsis in multiple-traumatized patients without traumatic brain injury. Crit Care Med. 2010;38:161. doi: 10.1097/CCM.0b013e3181b78a06. [DOI] [PubMed] [Google Scholar]
- 18.Opal SM. New perspectives on immunomodulatory therapy for bacteraemia and sepsis. Int. J. Antimicrob. Agents. 2010;36(Suppl 2):S70–S73. doi: 10.1016/j.ijantimicag.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Ploder M, Pelinka L, Schmuckenschlager C, Wessner B, Ankersmit HJ, Fuerst W, Redl H, Roth E, Spittler A. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25:129. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- 20.Slotman GJ. The systemic mediator-associated response test identifies patients in failed sepsis clinical trials among whom novel drugs reduce mortality. J. Trauma. 2011;71:1406. doi: 10.1097/TA.0b013e3182159c61. [DOI] [PubMed] [Google Scholar]
- 21.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J. Leukoc. Biol. 2004;75:408. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson S, Ruokonen E, Varpula T, la-Kokko TI, Pettila V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 23.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 24.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect. Immun. 2010;78:1582. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 1997;3:678. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001;166:6952. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6:813. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 28.Ellaban E, Bolgos G, Remick D. Selective macrophage suppression during sepsis. Cell Immunol. 2004;231:103. doi: 10.1016/j.cellimm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 30.Jung E, Perrone EE, Liang Z, Breed ER, Dominguez JA, Clark AT, Fox AC, Dunne WM, Burd EM, Farris AB, Hotchkiss RS, Coopersmith CM. Cecal ligation and puncture followed by methicillin-resistant Staphylococcus aureus pneumonia increases mortality in mice and blunts production of local and systemic cytokines. Shock. 2012;37:85. doi: 10.1097/SHK.0b013e3182360faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat. Med. 2008;14:551. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr., uxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 2010;16:708. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- 33.Volakli E, Spies C, Michalopoulos A, Groeneveld AB, Sakr Y, Vincent JL. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14:R32. doi: 10.1186/cc8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J. Surg. Res. 1980;29:189. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 35.Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J. Immunol. 2007;179:623. doi: 10.4049/jimmunol.179.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006;74:5227. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weixelbaumer KM, Raeven P, Redl H, van GM, Bahrami S, Osuchowski MF. Repetitive low-volume blood sampling method as a feasible monitoring tool in a mouse model of sepsis. Shock. 2010;34:420. doi: 10.1097/SHK.0b013e3181dc0918. [DOI] [PubMed] [Google Scholar]
- 38.Weixelbaumer KM, Craciun F, Bahrami S, Redl H, Remick DG, Osuchowski MF. Daily monitoring of immuno-inflammatory response – is it feasible in septic mice? Shock. 2009;31(Supplement 1):1–92. [Google Scholar]
- 39.Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, Remick DG. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21:26. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr. Opin. Infect. Dis. 2012;25:328. doi: 10.1097/QCO.0b013e3283522038. [DOI] [PubMed] [Google Scholar]
- 41.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osuchowski MF, Siddiqui J, Copeland S, Remick DG. Sequential ELISA to profile multiple cytokines from small volumes. J. Immunol. Methods. 2005;302:172. doi: 10.1016/j.jim.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J. Immunol. Methods. 2001;255:149. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 44.Drechsler S, Weixelbaumer KM, Redl H, van GM, Bahrami S, Osuchowski MF. Experimentally approaching the ICU: monitoring outcome-based responses in the two-hit mouse model of posttraumatic sepsis. J. Biomed. Biotechnol. 2011;2011:357926. doi: 10.1155/2011/357926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 46.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 47.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007;167:1655. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, Carstina D, Oltean M. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119:155. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 49.White M, Mankan A, Lawless MW, O'Dwyer MJ, McManus R, Ryan T. Mortality in humans with pneumonia and sepsis is related to an uncompensated anti-inflammatory response to infection. Arch. Intern. Med. 2008;168:1468. doi: 10.1001/archinte.168.13.1468-b. [DOI] [PubMed] [Google Scholar]
- 50.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandia F, Resino S, Tamayo E, de Lejarazu RO, Bermejo-Martin JF. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57:332. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23:488. [PubMed] [Google Scholar]
- 54.Osuchowski M, Welch K, Yang H, Siddiqui J, Remick D. Sepsis: Always in Mars. Shock. 2006:25. [Google Scholar]
- 55.Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K, Lymberopoulou K, Mainas E, Marioli A, Massouras C, Mavrou I, Mpalla M, Michalia M, Mylona H, Mytas V, Papanikolaou I, Papanikolaou K, Patrani M, Perdios I, Plachouras D, Pistiki A, Protopapas K, Rigaki K, Sakka V, Sartzi M, Skouras V, Souli M, Spyridaki A, Strouvalis I, Tsaganos T, Zografos G, Mandragos K, Klouva-Molyvdas P, Maggina N, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care. 2010;14:R96. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavaillon JM, dib-Conquy M, Cloez-Tayarani I, Fitting C. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J. Endotoxin. Res. 2001;7:85. [PubMed] [Google Scholar]
- 57.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin. Res. 2006;12:151. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 58.Tschaikowsky K, Hedwig-Geissing M, Schiele A, Bremer F, Schywalsky M, Schuttler J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med. 2002;30:1015. doi: 10.1097/00003246-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J. Surg. Res. 1994;56:579. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- 60.Ayala A, O'Neill PJ, Uebele SA, Herdon CD, Chaudry IH. Mechanism of splenic immunosuppression during sepsis: key role of Kupffer cell mediators. J. Trauma. 1997;42:882. doi: 10.1097/00005373-199705000-00019. [DOI] [PubMed] [Google Scholar]
- 61.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J. Immunol. 2011;186:2444. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowe CA, Kulstad EB, Mistry CD, Kulstad CE. Comparison of severity of illness scoring systems in the prediction of hospital mortality in severe sepsis and septic shock. J. Emerg. Trauma Shock. 2010;3:342. doi: 10.4103/0974-2700.70761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CC, Chen SY, Tsai CL, Wu SC, Chiang WC, Wang JL, Sun HY, Chen SC, Chen WJ, Hsueh PR. Prognostic value of mortality in emergency department sepsis score, procalcitonin, and C-reactive protein in patients with sepsis at the emergency department. Shock. 2008;29:322. doi: 10.1097/shk.0b013e31815077ca. [DOI] [PubMed] [Google Scholar]
- 64.Rhee JY, Kwon KT, Ki HK, Shin SY, Jung DS, Chung DR, Ha BC, Peck KR, Song JH. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31:146. doi: 10.1097/SHK.0b013e318182f98f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.