Abstract
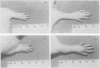
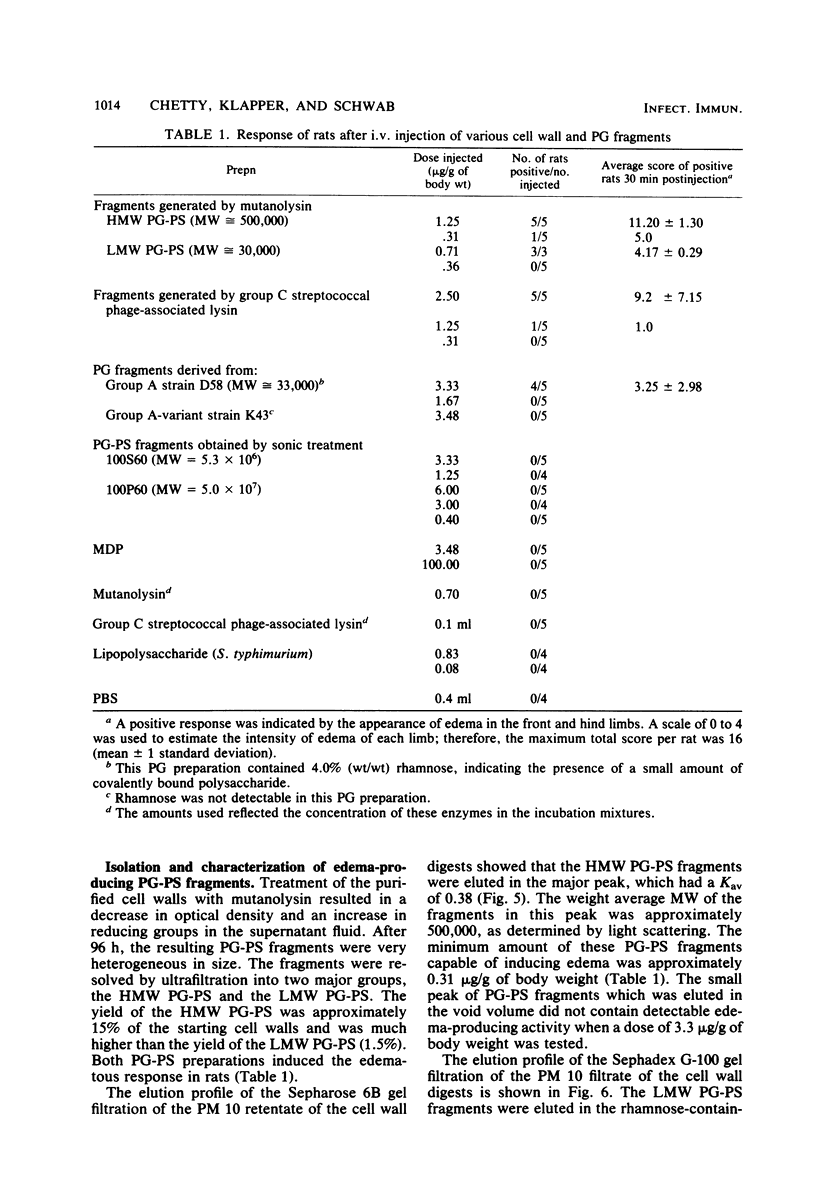
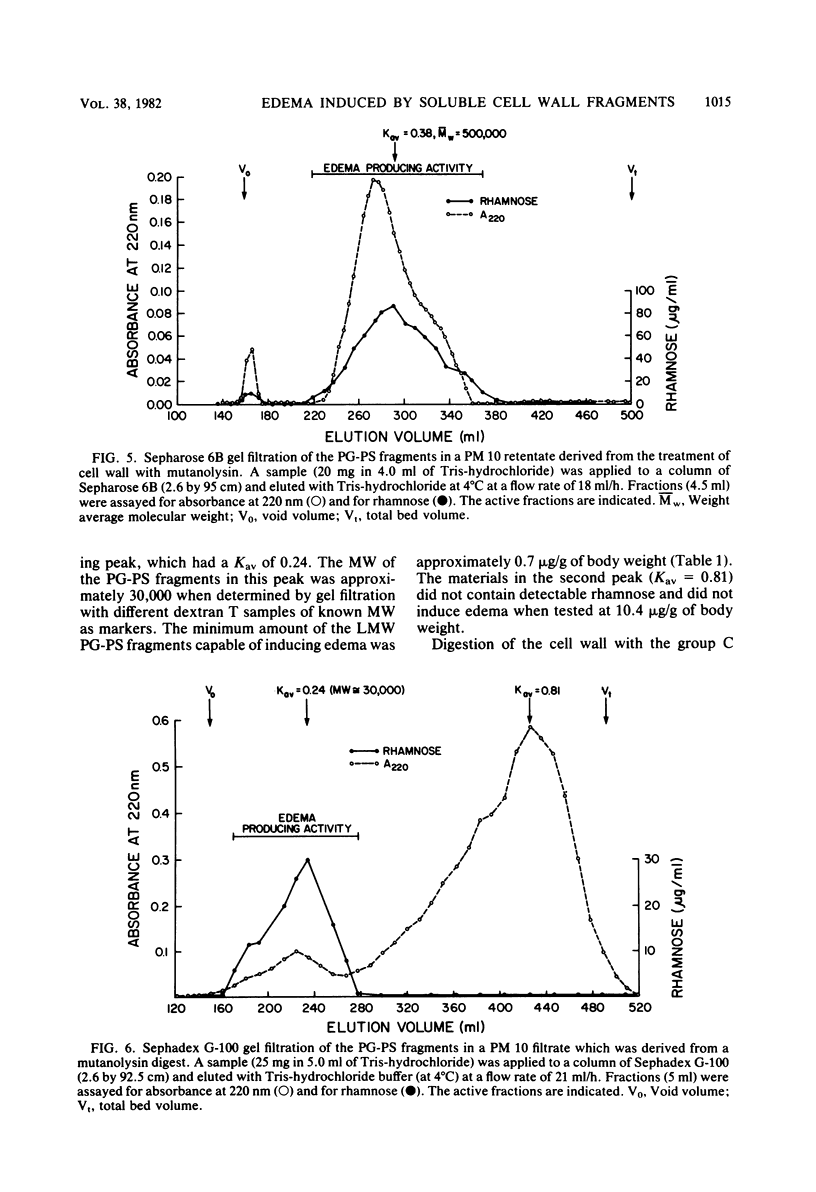
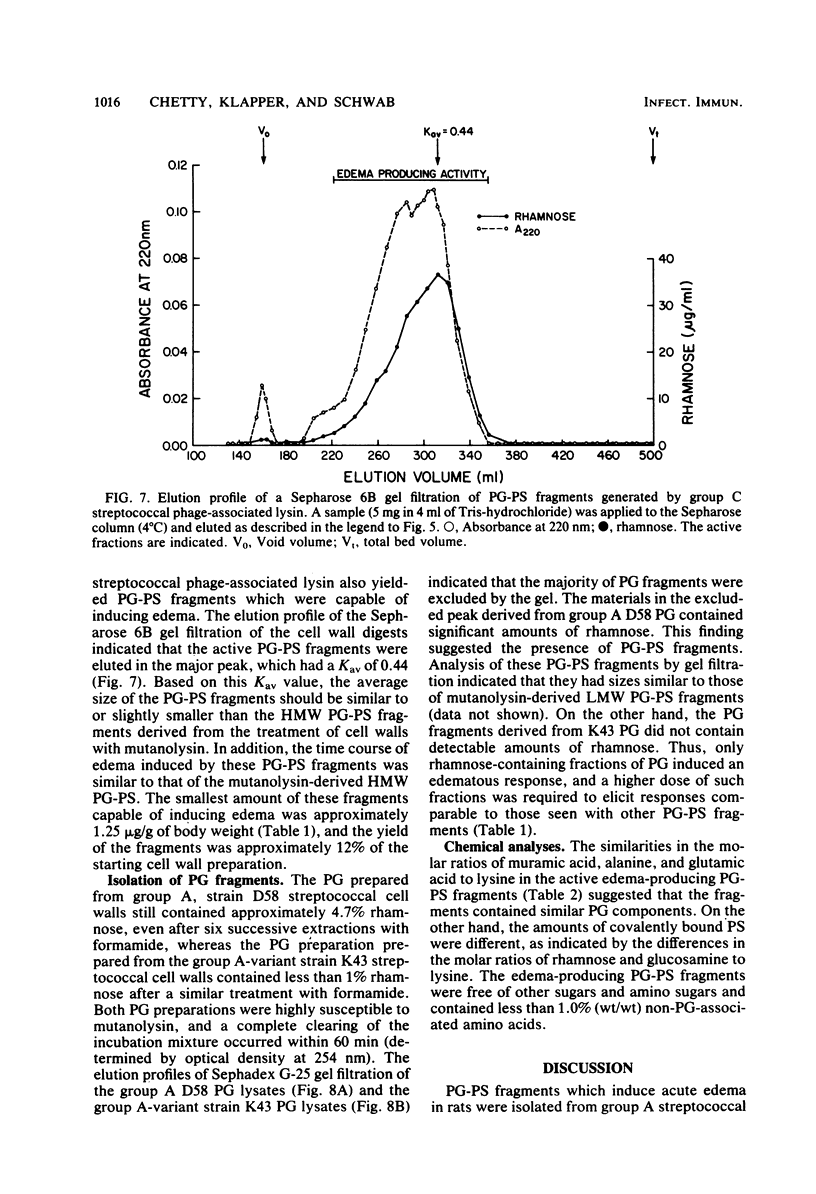
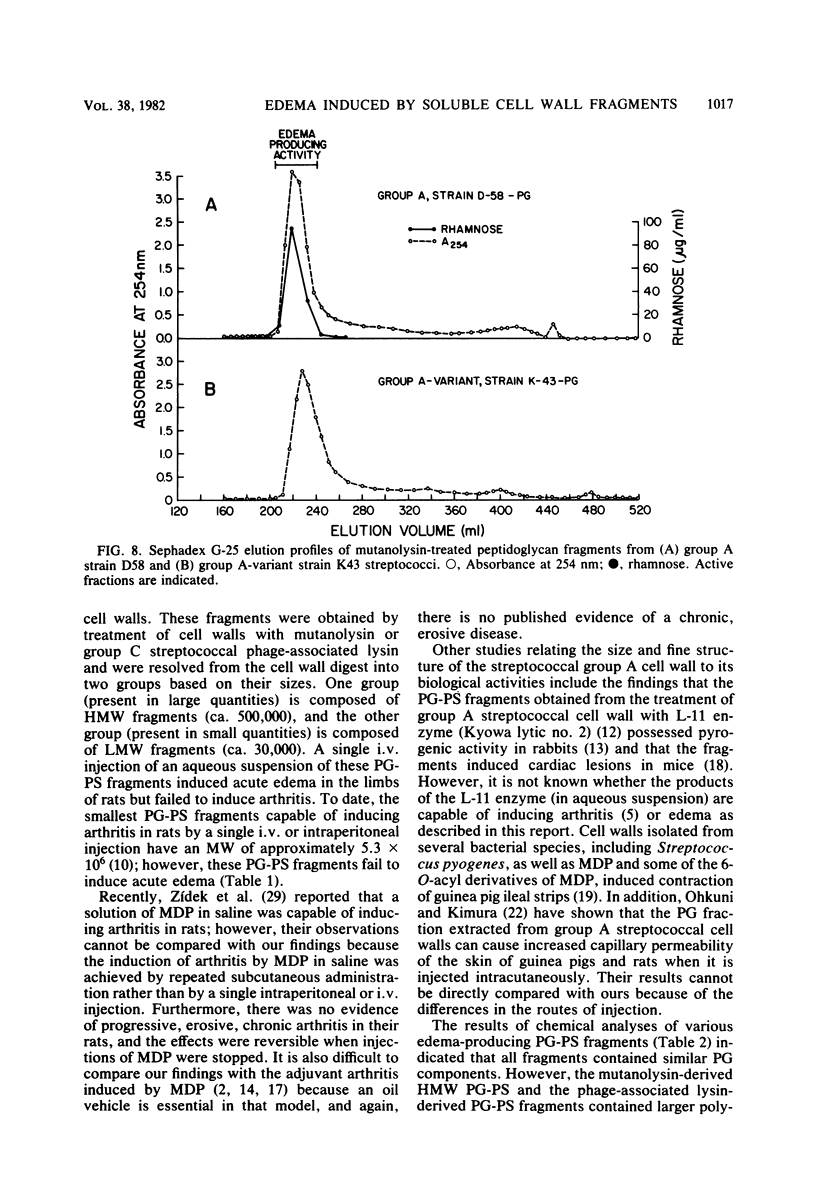
Peptidoglycan (PG)-polysaccharide (PS) polymers derived from group A streptococcal cell walls were solubilized by M-1 mutanolysin (endo-N-acetylmuramidase) and phage-associated lysin (N-acetylmuramyl-l-alanine amidase). Fragments were isolated by ultrafiltration and a series of gel filtrations and were injected intravenously into Sprague-Dawley rats. No fragments with a molecular weight of less than 5 × 106 were able to induce arthritis by systemic injection. However, the enzyme-derived fragments displayed a new biological activity. High-molecular-weight PG-PS fragments (≅500,000) derived from mutanolysin digests induced a severe edematous reaction in the front and hind limbs. The response started 5 to 10 min postinjection, reached maximum intensity in approximately 30 min, and disappeared by 10 h. The smallest dose capable of eliciting the response was 0.31 μg/g of body weight. Low-molecular-weight PG-PS (≅30,000) derived from the mutanolysin digests and the PG-PS fragments isolated from phage-associated lysin digests also induced edema; however, a higher dose was required to elicit the same response as that produced by high-molecular-weight PG-PS fragments. The active fragments contained rhamnose, glucosamine, muramic acid, alanine, glutamic acid, and lysine in various molar ratios. PG-PS fragments obtained by sonic degradation of cell walls (molecular weight ≥5.3 × 106), as well as enzyme-treated PG preparations and muramyl dipeptide, failed to elicit the response. These findings indicate that PG-PS fragments of sizes too small to be arthritogenic can affect the vascular endothelium to induce a rapidly developing edema. Fragments with this biological property could have a key role in the pathogenesis of experimental arthritis by influencing the tissue distribution of arthritogenic PG-PS.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr M. E., Jr, Shen L. L., Hermans J. Mass-length ratio of fibrin fibers from gel permeation and light scattering. Biopolymers. 1977 Jan;16(1):1–15. doi: 10.1002/bip.1977.360160102. [DOI] [PubMed] [Google Scholar]
- Chang Y. H., Pearson C. M., Chedid L. Adjuvant polyarthritis. V. Induction by N-acetylmuramyl-L-alanyl-D-isoglutamine, the smallest peptide subunit of bacterial peptidoglycan. J Exp Med. 1981 Apr 1;153(4):1021–1026. doi: 10.1084/jem.153.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G. Rheumatic-like cardiac lesions in mice. Science. 1966 Oct 14;154(3746):285–287. doi: 10.1126/science.154.3746.285. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. C., Wicher K., McCarty M. Immunochemical analysis of streptococcal group A, B, and C carbohydrates, with emphasis on group A. Infect Immun. 1982 Jul;37(1):209–215. doi: 10.1128/iai.37.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Kotani S., Kato K. Studies on cell walls of group A Streptococcus pyogenes, type 12. 1. Mechanism of lysis by the L-11 enzyme and possible structure of a peptide moiety of the peptidoglycan. Biken J. 1968 Dec;11(4):293–311. [PubMed] [Google Scholar]
- Hamada S., Narita T., Kotani S., Kato K. Studies on cell walls of group A Streptococcus pyogenes, type 12. II. Pyrogenic and related biological activities of the higher molecular weight fraction of an enzymatic digest of the cell walls. Biken J. 1971 Sep;14(3):217–231. [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on bacteriophages of hemolytic streptococci. I. Factors influencing the interaction of phage and susceptible host cell. J Exp Med. 1957 Sep 1;106(3):365–384. doi: 10.1084/jem.106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Tanaka A., Kotani S., Shiba T., Kusumoto S., Yokogawa K., Kawata S., Ozawa A. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980 Jul;29(1):70–75. doi: 10.1128/iai.29.1.70-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Hamada S., Kotani S., Hasegawa K., Ishida T. Studies on cell walls of group A Streptococcus pyogenes, type 12. 3. Induction of cardiac lesions in mice by a higher molecular weight fraction from a digest of cell walls with the L-11 enzyme. Biken J. 1971 Sep;14(3):233–245. [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Tsujimoto M., Kusumoto S., Shiba T., Kawata S., Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982 Feb;35(2):612–619. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H., Cromartie W. J. Relation of rheumatic-like cardiac lesions of the mouse to localization of group A streptococcal cell walls. J Exp Med. 1969 Jan 1;129(1):37–49. doi: 10.1084/jem.129.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni H., Kimura Y. Increased capillary permeability in guinea pigs and rats by peptidoglycan fraction extracted from Group A streptococcal cell walls. Exp Cell Biol. 1976;44(2):83–94. doi: 10.1159/000163102. [DOI] [PubMed] [Google Scholar]
- SCHWAB J. H., CROMARTIE W. J. Studies on a toxic cellular component of group A streptococci. J Bacteriol. 1957 Nov;74(5):673–679. doi: 10.1128/jb.74.5.673-679.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zídek Z., Maśek K., Jiricka Z. Arthritogenic activity of a synthetic immunoadjuvant, muramyl dipeptide. Infect Immun. 1982 Feb;35(2):674–679. doi: 10.1128/iai.35.2.674-679.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]