Abstract
Anoxia induces a rapid elevation of the cytosolic Ca2+ concentration ([Ca2+]cyt) in maize (Zea mays L.) cells, which is caused by the release of the ion from intracellular stores. This anoxic Ca2+ release is important for gene activation and survival in O2-deprived maize seedlings and cells. In this study we examined the contribution of mitochondrial Ca2+ to the anoxic [Ca2+]cyt elevation in maize cells. Imaging of intramitochondrial Ca2+ levels showed that a majority of mitochondria released their Ca2+ in response to anoxia and took up Ca2+ upon reoxygenation. We also investigated whether the mitochondrial Ca2+ release contributed to the increase in [Ca2+]cyt under anoxia. Analysis of the spatial association between anoxic [Ca2+]cyt changes and the distribution of mitochondrial and other intracellular Ca2+ stores revealed that the largest [Ca2+]cyt increases occurred close to mitochondria and away from the tonoplast. In addition, carbonylcyanide p-trifluoromethoxyphenyl hydrazone treatment depolarized mitochondria and caused a mild elevation of [Ca2+]cyt under aerobic conditions but prevented a [Ca2+]cyt increase in response to a subsequent anoxic pulse. These results suggest that mitochondria play an important role in the anoxic elevation of [Ca2+]cyt and participate in the signaling of O2 deprivation.
O2 deprivation is the primary stress experienced by plants during flooding. Our previous work showed that [Ca2+]cyt elevation may mediate the rapid molecular and long-lasting physiological responses to O2 limitation (Subbaiah et al., 1994a, 1994b). Furthermore, the kinetics and magnitude of the anoxic [Ca2+]cyt increase were different from the patterns of [Ca2+]cyt changes induced by other stimuli in wheat aleurone cells (Bush, 1996) and Arabidopsis seedlings (Sedbrook et al., 1996). The [Ca2+]cyt elevation occurring in maize (Zea mays L.) cells under anoxia did not depend on extracellular Ca2+ but was prevented by ruthenium red, suggesting that the Ca2+ signal originated from one or more of the ruthenium-red-sensitive intracellular Ca2+ stores (Subbaiah et al., 1994a).
The origin and spatiotemporal patterns of the [Ca2+]cyt elevation are currently recognized as important elements of Ca2+ signaling, and the characteristic variations in these features appear to encode the qualitative and quantitative divergence of stimuli (Bush, 1995). Therefore, there has been a growing interest in the identification of the Ca2+ stores or channels responsible for the initiation and propagation of the [Ca2+]cyt changes in specific signaling pathways (for a recent example, see Franklin-Tong et al., 1996). In the present study we traced the origin of the Ca2+ signal as a part of our attempt to elucidate the nature and intracellular location of the O2 sensor. Being the primary site of O2 consumption and also an important target of ruthenium red action, the mitochondrion could serve as a Ca2+ store in response to anoxia in maize cells.
Mitochondria isolated from mung bean seedlings (Moore et al., 1986), rat liver (Nishida et al., 1989), and intact rat hepatocytes (Aw et al., 1987) were shown to release Ca2+ from their matrix immediately after O2 deprivation. However, these earlier analyses were carried out using organelles isolated out of the cell either before or after stimulation and thus may not represent real-time changes in an intact, living cell. In addition, the role of mitochondria in intracellular Ca2+ homeostasis had not been firmly established until recently (Rizzuto et al., 1994, and refs. therein). Only during the last few years has the interest in mitochondrial Ca2+ in the context of stimulus-response coupling been rekindled after a spurt of experimental observations (Martínez-Serrano and Satrústegui, 1992; Rizzuto et al., 1992, 1994; for review, see Gunter et al., 1994; Hajnoczky et al., 1995; Jouaville et al., 1995; Rutter et al., 1996; Babcock et al., 1997, and refs. therein). These reports indicate that mitochondria accumulate and release large quantities of Ca2+ and actively participate in cellular Ca2+ signaling.
Our knowledge of the role of mitochondria in intracellular Ca2+ homeostasis or cellular signaling in plant systems has been limited to only a few studies (Moore et al., 1986; Rugolo et al., 1990; Silva et al., 1992; Zottini and Zannoni, 1993; Aubert et al., 1996; Naton et al., 1996). Furthermore, there are other cellular compartments (and the plasma membrane) in plant cells in addition to mitochondria that have ruthenium-red-sensitive Ca2+ transporters (Brosnan and Sanders, 1993; Chason, 1994; Marshall et al., 1994; Allen et al., 1995); therefore, the scenario is more complicated than in animal cells. Recently, confocal microscopy or compartment-specific Ca2+ probes have been successfully used to address many long-standing questions about Ca2+ signaling, particularly regarding the role of subcellular compartments (Franklin-Tong et al., 1993, 1996; Rutter et al., 1996; Simpson and Russell, 1996; Babcock et al., 1997; for review, see Pozzan et al., 1994; Gilroy, 1997). In this study we combined the power of these two tools to investigate the relationship between mitochondrial and cytosolic Ca2+ changes in anoxic maize cells. The results indicate that mitochondria are involved in the Ca2+-mediated signaling of O2 deprivation in plants.
MATERIALS AND METHODS
Cell Culture
Maize (Zea mays L. P3377) cells were maintained and cultured as described previously (Subbaiah et al., 1994a).
Chemicals
Fluo-3, rhod-2 AM, MitoTracker Green FM, rhodamine B, DiOC6(3), and JC-1 were all purchased from Molecular Probes (Eugene, OR)3. All other chemicals were of the highest grade available and were obtained from Sigma or Calbiochem.
Dye Loading
Fluo-3 was loaded into maize cells, as described previously (Subbaiah et al., 1994a). Rhod-2 AM was loaded at a final concentration of 2 to 3 μm for 10 min. This dye possesses a delocalized, positive charge and is accumulated only by mitochondria because they possess the highest negative membrane potential in the cell. The ester form of the dye rapidly enters mitochondria and, after hydrolysis, is converted into the Ca2+-sensitive but less-mobile zwitterionic form. The dye gets trapped inside of the organelles, becoming insensitive to any subsequent changes in the mitochondrial membrane potential (Babcock et al., 1997). Rhod-2 AM has a dissociation constant (Kd) of 570 nm for Ca2+, which makes it very appropriate for measuring mitochondrial Ca2+. To improve its mitochondrial localization, rhod-2 AM was reduced using sodium borohydride immediately before incubation (Hajnoczky et al., 1995). The concentration of rhod-2 AM and a washing step following incubation of cells in the dye were critical to minimize the nonspecific labeling of the plasma membrane and cell wall.
To confirm the selective uptake of rhod-2 AM into mitochondria, cells were coloaded with rhod-2 AM and a mitochondria-specific fluorophore, MitoTracker Green FM (100 nm final concentration), and their distribution was monitored. MitoTracker Green FM loads specifically into mitochondria in a membrane-potential-independent manner. The two dyes showed no spectral overlap at the settings used (at the highest sensitivity, rhod-2 AM showed a negligible blur in the MitoTracker Green FM settings). JC-1 was used at 3 μm and the cells were incubated for 15 min in the dye. All dye incubations were at room temperature (26°C ± 2°C). At the end of the incubation, cells were washed with the perfusion buffer and were embedded in agarose in a perfusion plate, as described previously (Subbaiah et al., 1994a). The perfusion plate was connected to a buffer reservoir and perfused aerobically for 15 to 20 min before the imaging was started (Subbaiah et al., 1994a).
Confocal Microscopy
Fluorescence images were collected with the laser confocal system (MRC 1024, Bio-Rad) mounted on an inverted microscope (Diaphot, Nikon) and equipped with an argon-krypton laser. The ×60 water-immersion objective was used in the experiments reported here. Fluorescence of fluo-3 and DiOC6(3), excited at 488 nm, was collected through a 515-nm longpass barrier filter. Rhod-2 AM and rhodamine B were excited at 568 nm, and the fluorescence was collected through a 598 ± 20-nm long-pass barrier filter. The artifacts due to laser illumination, such as photobleaching of dyes (particularly with fluo-3) and cell damage, were minimized by keeping the excitation light to a minimum, acquiring only four to five representative optical sections, and allowing an interval of approximately 10 min between two successive acquisitions.
The microscope was adjusted at a particular z-position of the selected cells at the beginning of an experiment and was not manipulated again until the end of the experiment. In some experiments fluo-3-loaded cells were incubated with 1 μm rhodamine B or 170 nm DiOC6(3) for organelle localization while still on the stage. In these experiments fluo-3 contributed <1% to the total fluorescence from either rhodamine B or DiOC6(3). Anoxia was imposed or withdrawn by withholding or restoring perfusion, as described previously (Subbaiah et al., 1994a). We attempted to obtain the spatial information concerning [Ca2+]cyt from maize cells loaded with indo-1 or fura-1, the fluorescence indicators that respond to Ca2+ changes by spectral shifts. However, none of the ratiometric dyes or their ester forms was taken up sufficiently by the P3377 maize cells. The fluorescence from the dye-loaded cells was rarely quantifiable against the strong background of cells at the short wavelengths necessary to excite these dyes. Since confocal microscopy eliminates most of the intensity differences due to cell thickness, ratio imaging was not critical, particularly in the absence of ratiometric dyes that work in the visible range. Our choice to use nonratiometric Ca2+ dyes was based on careful preliminary analyses, as detailed in Results.
Calibration
At the end of a number of experiments, in vivo (in intact cells) calibration of fluo-3 fluorescence intensity versus Ca2+ concentration was performed as described previously (Subbaiah et al., 1994a). Calibration of rhod-2 AM fluorescence in vivo was also based on the fluorescence quenching by Mn. However, FMnSat/FCaSat for the K+ salt of rhod-2 AM determined in vitro (0.16; n = 2) was much smaller than the corresponding in vivo values (0.63 ± 0.07; n = 16). Therefore, the in vitro values of FMnSat/FCaSat (0.022; n = 2) and Ffree/FCaSat were used to calculate the minimum and maximum cellular values, as was also done by Babcock et al. (1997). For rhod-2 AM, these in vitro values were shown to be stable under a variety of physiological conditions (pH, ionic strength, viscosity, and the dielectric constant; Babcock et al., 1997). The deviation in the in vivo FMnSat/FCaSat of the dye could be due to fast Ca2+ buffering in the mitochondrial matrix or to incomplete saturation of the dye by Mn in intact cells. Because the in vivo calibrations are at best approximate, the actual fluorescence values are also presented for all of the calibrated Ca2+ changes.
No attempt was made to calibrate JC-1 fluorescence with actual membrane potential values. Because JC-1 is a ratiometric dye and our interest was only in comparing the mitochondrial potential in single cells, we simply present the ratio of dye emission at 588 and 522 nm. Therefore, the data provide only a qualitative comparison of the membrane potential.
Measurement of Mitochondrial Volume
Mitochondrial volume as a fraction of total cytosolic or cellular volume was obtained from fluo-3- and rhodamine-B-coloaded cells. The images of 1-μm z-sections covering the entire thickness of 15 cells were printed on graph paper and volumes were computed from the prints. The mitochondrial volume ranged from 20.9% to 41% (mean ± se, 32 ± 1.9%) of the total cytoplasmic volume or 6.9% to 15% (mean ± se, 10.4 ± 0.7%) of the total cell volume.
Identification of Stationary Mitochondria Using Three-Dimensional Reconstructions
Since organelle (particularly mitochondria) movement is a serious concern in live-cell imaging, we collected at least four or five serial optical sections at every point for the time-course studies. Based on sequential images collected, we chose for our analyses only those optical planes in which all of the major organelles were stationary throughout the experimental period. Furthermore, as bleaching was not a major concern with rhod-2 AM or rhodamine B (because of their brightness, generally a 10% laser was enough to excite the dyes), we were able to collect more optical planes without reducing the signal from the dyes. These were overlaid in three-dimensional reconstructs and mitochondrial positions were tracked throughout experiments. In maize cells only a small fraction of mitochondria, located very close to the plasma membrane, showed movement and their displacement was confined to a few micrometers. Thus, three-dimensional reconstructs of serial confocal sections enabled us to choose stationary mitochondria for analyses.
Analysis of Data
Image analyses were performed using the MetaMorph program (Universal Imaging, Westchester, PA) or the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). The fluorescence intensity was mapped into pseudocolor pixels, with red indicating the highest value and blue the lowest. The intensity is scaled linearly between 0 and 256 and is shown as a color bar in Figures 2–4. Before image analysis, all of the optical planes to be compared were aligned with MetaMorph using the major cellular organelles and the cell outlines as guides. Only those experiments in which the images could be aligned perfectly were used for analyses. Experiments in which there was a discernible organelle movement or in which planes were difficult to align were discarded. The problems from the use of single-wavelength dyes (such as uneven dye distribution and photobleaching) were minimized by expressing the data as percentage changes rather than absolute fluorescence differences.
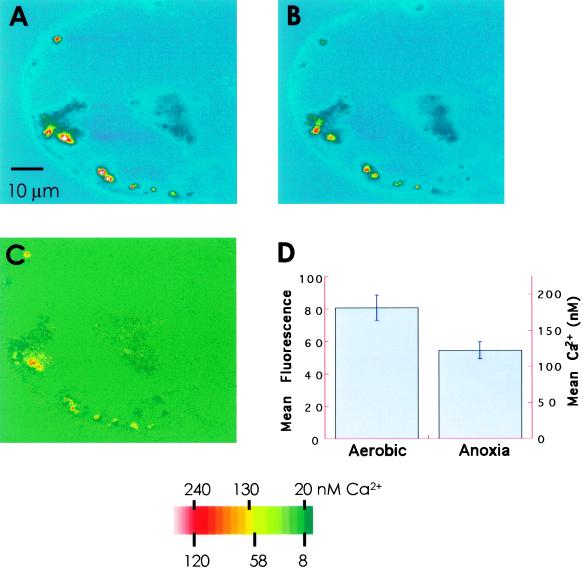
Figure 2.
Anoxia-induced changes in the mitochondrial Ca2+ of maize cells. The cells were loaded with rhod-2 AM and an optical section was collected where mitochondria showed no displacement. The bright spots in the images represent mitochondria. A, Cell under aerobic perfusion. B, The same cell after 10 min of anoxia. C, Anoxia-induced [Ca2+]m decrease. The optical sections were corrected for the background and the anoxic image shown in B was subtracted from the aerobic image in A. The derivative image is represented as a percentage of the aerobic image shown in A. D, Comparison of [Ca2+]m in aerobic and anoxic images. rhod-2 AM fluorescence was quantified from 16 discrete areas in each optical section (after correcting for the background) and is presented as the mean ± se. Calibrated Ca2+ values are given on the parallel axis. The coordinates and areas of the selected locations were the same for both of the images. The changes were statistically significant (P < 0.05). The color bar is applicable only to A and B.
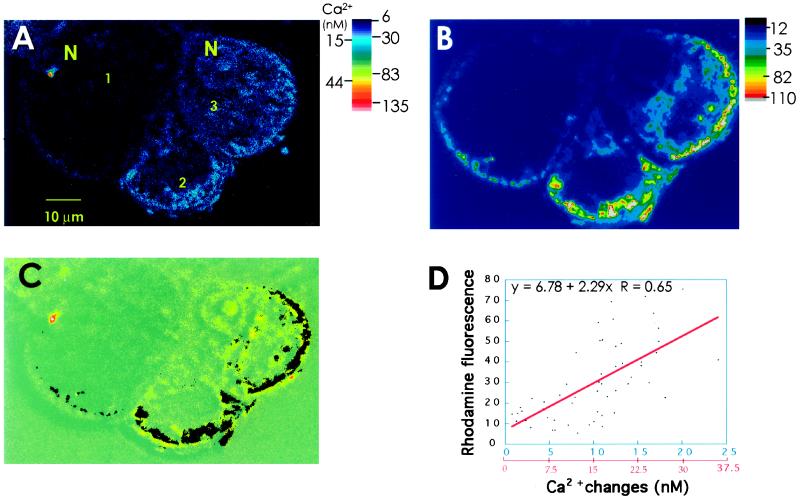
Figure 4.
Colocalization of anoxia-induced [Ca2+]cyt changes with the mitochondrial distribution in maize cells. A, Confocal imaging of anoxia-induced [Ca2+]cyt changes in fluo-3-loaded maize cells. The fluorescence image is the result of subtracting an optical section of aerobically perfused cells from a corresponding section of the same set of cells after 10 min of anoxia. There are three cells in this image, which are numbered counterclockwise. In cell 1, [Ca2+]cyt increased very little; in cell 2, the largest [Ca2+]cyt increases occurred at the periphery of the cell; and in cell 3, the changes are more uniform throughout the cytoplasm. N, Nucleus. B, Mitochondrial distribution in the cells shown in A. At the end of the experiment, the cells were perfused with 1 μm rhodamine B to stain mitochondria and the optical section was obtained from the same region of the cells shown in A. C, Overlay of A with B. For the sake of clarity, the image in B was binarized and inverted to convert mitochondria into dark particles. D, Linear-regression analysis of anoxic [Ca2+]cyt changes and mitochondria. The images in A and B were aligned using the MetaMorph program. Lines (n = 11) were randomly drawn across all of the representative areas of the cytoplasm (excluding vacuoles) in the three cells in the overlay shown in C. The Ca2+ and the mitochondrial pixel intensities that intercept these lines were measured. The values were plotted, and linear curve fitting was carried out. The fluorescence values are indicated in blue and the calibrated Ca2+ values are indicated in red on the x axis. The correlation was statistically significant (P < 0.05). The results shown here are representative of nine cells. This plane was chosen for illustration because it gave a wide range of [Ca2+]cyt changes and mitochondrial distribution.
RESULTS
[Ca2+]m Changes in Response to Anoxia
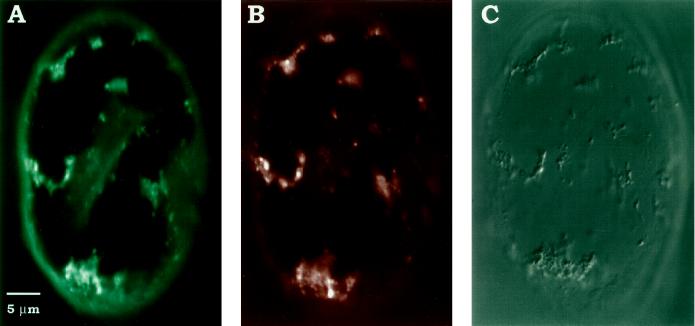
To understand the mitochondrial role in anoxic [Ca2+]cyt elevation, we measured the changes in [Ca2+]m using rhod-2 AM-loaded maize cells. This cationic Ca2+ fluor has recently been proven to be an appropriate indicator to follow changes in [Ca2+]m in many animal cell model systems (Babcock et al., 1997, and refs. therein). Figure 1A shows the localization of rhod-2 AM specifically in the mitochondria of maize cells, as indicated by the colocalization of its fluorescence with MitoTracker Green FM (Fig. 1B), which did not overlap with rhod-2 AM in its spectral properties. rhod-2 AM fluorescence also correlated with mitochondria, as seen in a Nomarski image of the same cell (Fig. 1C). Furthermore, a rapid reduction in the rhod-2 AM fluorescence upon the addition of FCCP (Table I) or a combination of oligomycin A and antimycin A (data not shown) provided pharmacological evidence that the dye was predominantly reporting mitochondrial Ca2+. We also observed that there was no photobleaching of intracellular rhod-2 AM fluorescence for at least 1 h of the experimental period. These analyses showed rhod-2 AM to be a useful probe with which to study mitochondrial Ca2+ in maize cells.
Figure 1.
Colocalization of rhod-2 AM and MitoTracker Green FM in maize cells incubated for 15 min before imaging by confocal microscopy. A, Intracellular distribution of rhod-2 AM in a single optical section of a maize cell. The cell was excited with a 543-nm He-Ne laser, and emission was collected at 575 nm. B, Localization of MitoTracker Green FM in the same cell shown in A. The image was collected at the same z-position as in A but with an excitation at 488 nm using an Ar ion laser and emission at 515 nm. C, Nomarski optics revealing mitochondria in the same cell. Scale bar = 5 μm.
Table I.
[Ca2+]m changes during anoxia-reoxygenation cycles and FCCP treatment
| Treatment | Change in rhod-2 AM Fluorescence | N |
|---|---|---|
| % | ||
| First anoxia | −19 ± 3 | 88 |
| Reoxygenation | +28 ± 10 | 82 |
| Second anoxiaa | −15 ± 9 | 48 |
| FCCPb | −48 ± 3 | 23 |
[Ca2+]m changes were measured as the percentage change in rhod-2 AM fluorescence. “−” indicates a decrease and “+” indicates an increase in [Ca2+]m from the preexisting value. The resting [Ca2+]m ranged from 180 to 240 nm, based on our in vivo calibrations. Values are ±se.
Second anoxic treatment was given only in three experiments.
FCCP was added to a 100 nm final concentration.
A majority of mitochondria, 80% of those sampled, showed a decrease in Ca2+ following anoxia and regained Ca2+ upon reoxygenation (Figs. 2 and 3B; Table I). The changes were statistically significant (P > 0.05, Fig. 2D) and reversible in subsequent anoxia-reoxygenation cycles (Table I). The data are from 16 cells in four different experiments. The reversibility of the fluorescence changes also indicated that the changes were not due to the loss of the dye but to changes in [Ca2+]m. Furthermore, there was a small but significant proportion of mitochondria (10%–25%) within single cells that did not show a fluorescence decrease in response to O2 deprivation. In a fraction of this subpopulation, fluorescence levels did not change significantly in response to the anoxia-reoxygenation treatments (Fig. 3A, green arrow), and in the rest the [Ca2+]m response was inverse to the typical response (i.e. fluorescence increased under anoxia and decreased upon reperfusion; Fig. 3A, red arrow). In spite of this heterogeneity, the average response was in the direction of a net [Ca2+]m release during anoxia and a net gain following reoxygenation (Table I). The pattern and magnitude of Ca2+ changes were similar in the motile mitochondria whose positions could be tracked within single optical slices. There was no distinct cell-to-cell variation in the responses of mitochondria to anoxia.
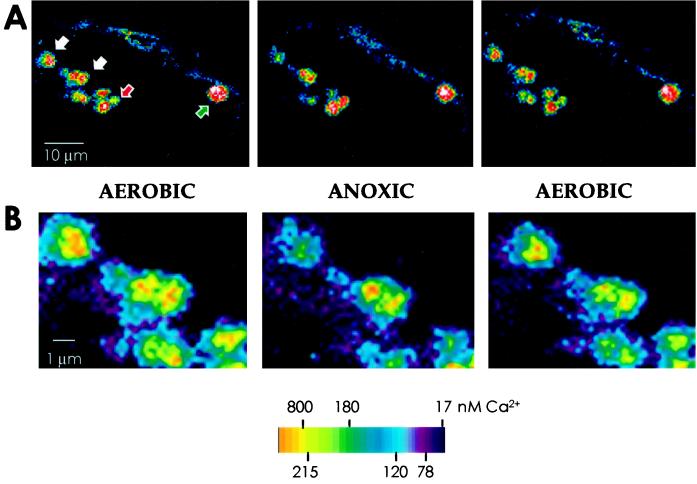
Figure 3.
Heterogeneity in the response of mitochondria to anoxia-reoxygenation treatments in maize cells. A, Imaging of [Ca2+]m carried out as described for Figure 2. Mitochondria indicated by white arrows (an enlargement of this region is shown in B) followed the typical pattern of response, i.e. anoxic release of Ca2+ and reperfusion-induced uptake of the ion, with an average fluorescence decrease of 28% upon deaeration and a mean increase of 19.5% upon reoxygenation. The mitochondrion, indicated by the green arrow, showed only a small (<10%) decrease in fluorescence in response to anoxia and no change after reaeration. In the group marked by the red arrow, mitochondrial fluorescence increased upon anoxia (by 20%) and was restored upon reoxygenation (a 25% decrease). B, Part of A enclosing mitochondria marked by white arrows shown at a greater enlargement, highlighting the mitochondria that showed the typical anoxic response.
Do [Ca2+]m Changes Contribute to Changes in the [Ca2+]cyt in Anoxic Maize Cells?: Colocalization of Anoxia-Induced [Ca2+]cyt Changes with Mitochondria
The significance of mitochondrial Ca2+ release to the anoxic [Ca2+]cyt elevation was investigated by colocalization studies. We constructed a fine map of the cytosolic microdomains where Ca2+ was initially elevated in response to O2 deprivation and compared the distribution of mitochondria in these locations with the rest of the cytoplasm. Our previous studies using conventional fluorescence microscopy showed that the anoxia-induced changes in [Ca2+]cyt begin as areas localized generally around the nucleus or adjacent to the plasma membrane (Subbaiah et al., 1994a), regions that are known to be rich in mitochondria (Liu et al., 1987; Rutter et al., 1996). However, the resolution in our previous studies was not sufficient to identify the organelles around the initiation points of the Ca2+ signal (Subbaiah et al., 1994a).
In the present study the [Ca2+]cyt changes were imaged in 1- to 2-μm-thick optical sections of fluo-3-loaded cells using confocal microscopy. Under our loading conditions fluo-3 generally remained in the cytosol and nucleus of P3377 maize cells for at least 2 h, with very little sequestration into mitochondria or other cellular compartments (Subbaiah et al., 1994a). Furthermore, a number of related dyes that stain the cytoplasm but do not report Ca2+ (e.g. fluorescein diacetate and CellTracker Orange) showed no spatial redistribution within the cytosol following anoxia. In addition, there was no discernible change in the structure of the cytoplasm or organelles at least for 1 h of continued anoxia, and our O2-deprivation treatments were generally terminated within 30 min. These observations validated our use of the nonratiometric Ca2+ indicator fluo-3 to report spatial changes in [Ca2+]cyt and correlate them with the distribution of mitochondria and other potential Ca2+ stores.
From the confocal analysis it was evident that the [Ca2+]cyt changes were localized only to distinct areas in the cytosol even after 10 to 20 min of anoxia (Fig. 4A). Figure 4B shows the costaining of cells with the mitochondrial dye rhodamine B. An overlay of these two images (Fig. 4C) indicates that a considerable spatial association of the domains of [Ca2+]cyt increases with the mitochondrial populations. We quantified the association of the [Ca2+]cyt changes with mitochondria by measuring and correlating the cytosolic distribution of Ca2+ increases and mitochondrial densities as shown in Figure 4D. The Ca2+ increases were found to be correlated significantly with the mitochondria in a majority of the measured areas (P > 0.05, Fig. 4D). In the experiment shown in Figure 4, Ca2+ increases greater than 15 nm (or 10 fluorescence units) showed a stronger linear correlation with mitochondrial distribution than smaller Ca2+ increases. Such a relationship indicates that the largest [Ca2+]cyt increases occurred around mitochondria. We hypothesize that it was because of the release of Ca2+ from these organelles. The smaller Ca2+ increases may have been due to a slow diffusion of the ion away from mitochondrial locations, which is probably why they were seen at locations other than around mitochondria.
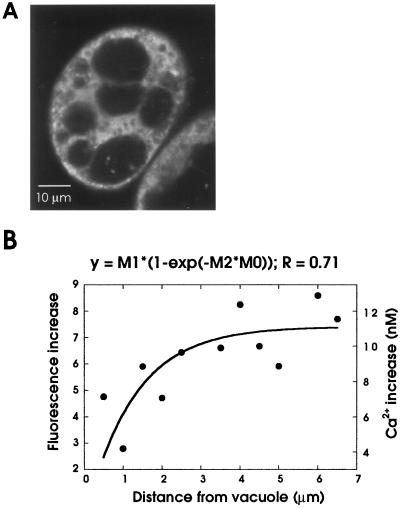
A spatial comparison of [Ca2+]cyt changes was also carried out with the other potential Ca2+ stores using DiOC6(3) staining (Fig. 5A). The dye stained the ER as a diffuse, network-like fluorescence and, being potentiometric, also marked mitochondria as bright, particulate fluorescence (Fig. 5A; Liu et al., 1987). DiOC6(3) also identified vacuoles by negative staining (Fig. 5A). Because the ER was evenly distributed throughout the cytoplasm, the anoxic Ca2+ changes aligned with some parts of the ER network but were more spatially discrete than the ER (data not shown). However, the possibility of a heterogeneous distribution of Ca2+ channels on the ER (as reported for some animal cells; Rooney and Meldolesi, 1996) does not allow us to rule out the ER as a potential Ca2+ store under anoxia. Measurement of [Ca2+]cyt changes around the vacuole revealed a significant ionic gradient, which increased away (at least up to 4 μm) from the tonoplast under anoxia (Fig. 5B), indicating that this organelle might act predominantly as a sink rather than a source for the anoxic Ca2+ signal.
Figure 5.
Spatial comparison of anoxia-induced [Ca2+]cyt changes with the distribution of ER and vacuoles in maize cells. A, Localization of ER and vacuoles in maize cells. Cells were perfused in 170 nm DiOC6(3) and the confocal image was collected. The fluorescent regions include the ER and mitochondria, and spaces that were not stained represent the vacuoles. The brightest spots represent mitochondria, and the diffuse network-like fluorescence is the ER. B, [Ca2+]cyt gradients around the tonoplast. In subtraction images from fluo-3 experiments that depict anoxic [Ca2+]cyt changes (such as the one shown in Fig. 4A), radial lines were randomly drawn from the tonoplast to the cell periphery. The fluorescence increases that intercept the lines were measured and averaged from 22 replicates (comprising six cells). The mean fluorescence intensities and the calibrated Ca2+ values were plotted against the distance from the vacuole, using an empirical fit derived from an exponential equation, shown on the top.
We previously showed that caffeine, an agonist of the ryanodine channel located on the tonoplast and ER (Sanders et al., 1995; Muir and Sanders, 1996), induced an aerobic elevation of Ca2+ but failed to block a subsequent anoxia-induced [Ca2+]cyt increase (Subbaiah et al., 1994a). We also found that TMB-8, an antagonist of the inositol trisphosphate-receptor (Schumaker and Sze, 1987); neomycin sulfate, an inhibitor of phospholipase C (Franklin-Tong et al., 1996); and ryanodine, an inhibitor of ryanodine channel when used at micromolar concentrations (Allen et al., 1995), had no effect on the anoxic [Ca2+]cyt increase (data not shown). Although the spatial correlation studies do not completely rule out the ER as a possible Ca2+ source, these results indicated that the vacuole did not significantly contribute to the [Ca2+]cyt elevation under O2 deprivation. At the same time, the inverse relationship between mitochondrial and cytosolic Ca2+ changes in response to anoxia, the spatially discrete nature of the anoxic Ca2+ signal, and a significant coincidence between the [Ca2+]cyt increases and the mitochondrial distribution strongly implied that Ca2+ entered the cytosol from mitochondria.
Although no detailed studies were made, we consistently noticed rapid dye accumulation around the nuclei of fluo-3-loaded maize cells in our previous studies (Subbaiah et al., 1994a). Our current observations using confocal microscopy showed unambiguously that fluo-3 entered the nuclei of maize cells and that the dye can be used to report nuclear Ca2+ changes (Fig. 4). Large fluorescence increases in the nuclear-localized fluo-3 accompanied the [Ca2+]cyt increases under anoxia (Fig. 4, cells 1 and 3). However, the actual magnitude of these changes needs to be carefully determined, since a separate calibration may be necessary owing to altered properties of the dye localized in the nucleus (Burnier et al., 1994). Nuclear Ca2+ changes under anoxia will be an important area of focus of our future studies.
Is Anoxic [Ca2+]cyt Elevation Dependent on Mitochondrial Ca2+ Status?
The contribution of mitochondrial Ca2+ to the anoxic [Ca2+]cyt increase was further assessed by experimentally depleting Ca2+ from mitochondria using FCCP. This compound is a protonophore that strongly depolarizes the mitochondrial inner membrane and releases mitochondrial Ca2+ (Silva et al., 1992; Babcock et al., 1997, and refs. therein). To validate the effects of FCCP (which is not organelle specific), we also tested the effects of mitochondria-specific inhibitors, a combination of oligomycin A and antimycin A. Mitochondrial electron-transport inhibitors such as antimycin A by themselves do not depolarize mitochondria, because an inhibition of electron transport induces the ATP synthase to act as an ATPase, resulting in the maintenance of the membrane potential. However, coincubation of oligomycin A with an electron-transport inhibitor would block the ability of ATP synthase to act in the reverse mode. Therefore, oligomycin A and antimycin A have often been used in combination to dissipate the membrane potential and release mitochondrial Ca2+ in many animal cell systems (Budd and Nicholls, 1996; White and Reynolds, 1996; Babcock et al., 1997).
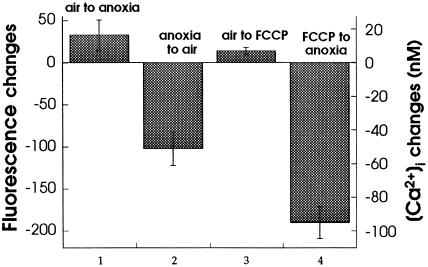
In rhod-2 AM-loaded cells, treatment with FCCP induced a rapid (within the first 5 min) loss of the mitochondrial signal (Table I). Such a loss in rhod-2 AM fluorescence was previously shown to be mainly due to a release ofmitochondrial Ca2+ and not to dye leakage (Hajnoczky et al., 1995; Babcock et al., 1997). These inhibitors also induce mitochondrial Ca2+ release in cells expressing mitochondrial aequorin, the mitochondrial localization of which is insensitive to membrane potential changes (Rizzuto et al., 1992). This suggests that the rhod-2 AM fluorescence loss in inhibitor-treated maize cells was indeed due to a loss of mitochondrial Ca2+. To complement the observations on [Ca2+]m, the effects of these inhibitors on [Ca2+]cyt were also studied in fluo-3-loaded cells. Maize cells showed a normal anoxic elevation and reoxygenation-induced recovery of [Ca2+]cyt prior to treatment with FCCP (Fig. 6). Perfusion with FCCP induced a small, transient increase in the [Ca2+]cyt of aerobic cells, but subsequently the inhibitor-treated cells failed to respond to anoxia by an increase in [Ca2+]cyt (Fig. 6). Together, these results suggest that the [Ca2+]cyt elevation in response to anoxia is dependent on the availability of Ca2+ in mitochondria.
Figure 6.
Effect of FCCP pretreatment on [Ca2+]cyt changes in maize cells. Cells loaded in fluo-3 were given an anoxia-reoxygenation cycle. Later they were perfused with 100 nm FCCP for 10 min and anoxia was imposed on the cells. Anoxia was extended for 20 min to detect any delayed Ca2+ elevation. The [Ca2+]cyt changes were monitored by confocal imaging and quantified using the MetaMorph program. The differences in fluorescence intensity and the calibrated Ca2+ changes (means ± se) at the end of each of the indicated treatments were plotted. Oligomycin A/antimycin A treatment (both at 2 μm) had similar effects on [Ca2+]cyt (data not shown).
Prolonged FCCP treatment under anoxia (to detect any delayed [Ca2+]cyt increase) also led to a severe loss of cytoplasmic Ca2+ signal (Fig. 6). This could have been due to the additional effects of FCCP on other metabolic processes, particularly on cellular ATP levels (Budd and Nicholls, 1996; Jou et al., 1996, and refs. therein); therefore, the results need to be carefully interpreted. However, in view of the [Ca2+]m-releasing effect of FCCP from intact cells (Table I) and from isolated maize mitochondria (Silva et al., 1992), the failure of FCCP-treated cells to respond to anoxia may be a consequence of nonavailability of Ca2+ in mitochondria. This is further supported by the effects of the mitochondria-specific inhibitors oligomycin A and antimycin A, which also induced a loss in mitochondrial Ca2+ (data not shown). Recent studies of mammalian cells also indicate mitochondria to be the sole or predominant FCCP-sensitive cellular Ca2+ pool (Fulceri et al., 1991; Drummond and Fay, 1996; Babcock et al., 1997, and refs. therein).
Attempts were also made to test the effects of ruthenium red on the mitochondrial Ca2+ changes, because ruthenium red is a potent blocker of the anoxic [Ca2+]cyt increase in maize cells (Subbaiah et al., 1994a). Within minutes of adding ruthenium red to rhod-2 AM-loaded cells, the mitochondrial fluorescence decreased (data not shown). In vitro studies showed that ruthenium red quenches the fluorescence of rhod-2 AM and that this dye-drug combination could not be used for Ca2+ imaging in mitochondria (data not shown). However, the rapid quenching of rhod-2 AM fluorescence observed in intact cells suggests that soon after its entry into the cell ruthenium red localizes into the mitochondria and may therefore act on the mitochondrial Ca2+ fluxes in maize cells.
Mitochondrial Potential Changes in Relation to the Kinetics of [Ca2+]m Release under Anoxia
An efflux of Ca2+ can occur from mitochondria either by electroneutral exchanges (with Na+ or H+) or through a reversal of the uniporter caused by a collapse of the inner membrane potential (Gunter et al., 1994). To verify the mechanism of anoxic mobilization of [Ca2+]m, we measured anoxia-induced changes in mitochondrial membrane potential in maize cells using the potentiometric fluoroprobe JC-1. This dye selectively labels mitochondria and is maximally excited at 490 nm, with two emission maxima at 522 and 585 nm. The fluorescence intensity of the dye does not change at 522 nm but linearly increases at 585 nm as a function of mitochondrial potential (Reers et al., 1995). Therefore, the emission ratio of the dye at 585/522 nm has been extensively used as a reliable measure of the mitochondrial membrane potential in animal cells (Smiley et al., 1991; Ankarcrona et al., 1995; Di Lisa et al., 1995).
JC-1 entered the maize cells immediately after incubation and localized into mitochondria, as observed by the punctate fluorescence (data not shown), which was very similar to rhod-2 AM or rhodamine B staining (Figs. 1A and 4B). The difference in the emission ratio at 585/522 nm of different mitochondria in the same cell and among cells ranged from 1.1 to 4.8 (mean = 3.5), signifying a great intracellular and intercellular heterogeneity in the membrane potential of maize mitochondria. Furthermore, the rapid decrease in the 585/522 nm ratio after FCCP treatment and the increase in the ratio following nigericin (a K+/H+ ionophore that abolishes a pH gradient but induces a compensatory increase in membrane potential) treatment (Table II) indicated that JC-1 responds to the changes in mitochondrial potential in plants cells as well. The effect of oligomycin A/antimycin A treatment on the emission ratio of JC-1 was ambiguous. There were no measurable changes in the mitochondrial potential during the first 20 min of anoxia (Table II). Any significant decrease in the membrane potential required at least 30 min or more of O2 deprivation. However, [Ca2+]m decreased substantially in these cells by even shorter periods of anoxia (Fig. 2C; Table I), indicating that the anoxic efflux of Ca2+ from mitochondria occurred predominantly by membrane-potential-independent pathways.
Table II.
Effects of ionophores, respiratory inhibitors, and anoxia on the mitochondrial membrane potential in JC-1-loaded maize cells
| Treatment | JC-1 Fluorescence Ratio |
|---|---|
| Aerobic to 20 min of anoxia | 3.60 ± 1.20 to 3.10 ± 0.80 (n = 26) |
| Aerobic to 10 min of FCCPa | 1.14 ± 0.17 to 0.18 ± 0.04 (n = 19) |
| Aerobic to 10 min of nigericina | 2.40 ± 0.04 to 2.48 ± 0.05 (n = 25) |
Membrane potential was measured in terms of the ratio of fluorescence at 585/522 nm. An increase in the ratio indicates an increase in the negativity of the membrane potential (i.e. hyperpolarization of mitochondria), whereas a decrease in the ratio denotes a decrease in the membrane potential or depolarization of mitochondria.
FCCP and nigericin were added at a final concentration of 2 μm to the perfusion buffer.
DISCUSSION
The long-term goal of our studies is to elucidate the pathway of O2 sensing in plants. Our previous work in maize seedlings and cultured cells indicated that mobilization of Ca2+ from the intracellular pool(s) leads to an increase in the [Ca2+]cyt and triggers the responses to anoxia (Subbaiah et al., 1994a, 1994b). In this report we sought the identity of the Ca2+ source that generates the anoxic Ca2+ signal. The identification of the store that releases Ca2+ in response to anoxia may indicate the nature of upstream events that lead to Ca2+ mobilization, and possibly the character of the O2 sensor, aside from the downstream events that translate the ionic signal into molecular responses. Our analysis indicates that mitochondria act as a significant Ca2+ store contributing to anoxic [Ca2+]cyt elevation.
Evidence for the Mitochondrial Origin of the Anoxic Ca2+ Signal
Our evidence for a significant role of mitochondria in the anoxic [Ca2+]cyt increase is 3-fold: demonstration of a reversible, O2-dependent Ca2+ release pathway in mitochondria, colocalization of [Ca2+]cyt increases with mitochondria, and the dependence of anoxic [Ca2+]cyt elevation on the presence of Ca2+ in mitochondria.
Until recently, the direct measurement of [Ca2+]m within living cells has been problematic. The highly specific Ca2+ indicators of the fura-2 family enabled the measurement of changes in mitochondrial Ca2+ with some success but only after the fluorescence of the cytosolic dye was quenched with cytotoxic heavy metal ions (Miyata et al., 1991). A direct and unequivocal measurement of changes in [Ca2+]m became possible with the development of chimeric constructs that express the Ca2+ indicator protein aequorin specifically in mitochondria (Rizzuto et al., 1992, 1994; Brini, 1997). These studies yielded an averaged response of [Ca2+]m from several thousands of cells, although not in single cells or organelles (Rutter et al., 1996). In a more recent study, Rutter et al. (1996) measured [Ca2+]m changes successfully at the single-cell level, and this analysis revealed Ca2+ dynamics of two major groups of mitochondria. However, mitochondrial-targeted aequorin has not yet been tested in plants.
In the present study a combination of confocal microscopy and a compartment-specific probe (rhod-2 AM) enabled us to measure real-time Ca2+ fluxes in a single mitochondrion or in small groups of mitochondria within single, intact, living cells. Our measurements showed that Ca2+ in individual mitochondria rapidly and reversibly changed in response to changes in O2 availability (Figs. 2 and 3). Furthermore, the [Ca2+]m changes were inversely related to the changes in [Ca2+]cyt, suggesting that mitochondria indeed contributed to the elevation of [Ca2+]cyt under anoxia and also to its restoration upon reoxygenation.
The inference that the mitochondria acted as a potential store of releasable Ca2+ for the anoxic [Ca2+]cyt elevation was further validated by a predominant (although not complete) colocalization of [Ca2+]cyt changes with mitochondria (Fig. 4). Confocal imaging technology offered sufficient spatial resolution of the Ca2+ increase such that we could compare the distribution of intracellular Ca2+ stores in the vicinity of the ionic changes. These spatial correlation studies indicated that the anoxia-induced changes in [Ca2+]cyt occurred close to mitochondria (Fig. 4C) and away from the vacuole (Fig. 5B). A greater spatial coincidence of the largest [Ca2+]cyt increases with mitochondria (Fig. 4D) also suggests that mitochondria could act as a primary source of the signal. The lack of a perfect correlation between the Ca2+ changes and mitochondria suggests that Ca2+ might enter the cytosol from other sources, such as by an influx of extracellular Ca2+ or from intracellular stores other than mitochondria. Our spatial correlation analysis with the ER did not completely rule out this organelle as a source of the anoxic [Ca2+]cyt increase. However, the vacuole, which is the major intracellular Ca2+ pool in plant cells, appears to play no role in the anoxic [Ca2+]cyt elevation (Fig. 5B), as was also indicated by the failure of pharmacological agents that are targeted to this store (and the ER as well) to inhibit the anoxic [Ca2+]cyt increase (Subbaiah et al., 1994a; data not shown).
A partial correlation between Ca2+ changes and mitochondria also could be due to one or more of the following: a slow diffusion of the ion away from mitochondrial locations, a lack of response from some mitochondria (Fig. 3A), and the movement of some mitochondria out of the field during the later part of the imaging period. Since the intense fluorescence of rhodamine B showed a considerable overlap with the fluo-3-measurement channel, we could not stain the cells with rhodamine B either before or during a fluo-3 experiment. However, for our spatial localization analysis, only those optical planes in which the mitochondrial movement was minimum were selected. This was based on bright-field observations before the start of the fluo-3 measurements and on the serial optical sections of rhodamine B staining at the end of experiments. The spatial association of anoxic [Ca2+]cyt increases with mitochondria (Fig. 4) reinforces the view that the [Ca2+]cyt increase is related to the anoxia-induced decrease in [Ca2+]m (Fig. 2). In addition, the emptying of [Ca2+]m either by the protonophores or by the electron-transport inhibitors prevented the [Ca2+]cyt elevation to a subsequent anoxic treatment (Fig. 6). Taken together, evidence supports the proposal that mitochondria play a significant role in the initiation of the anoxic Ca2+ signal.
Can Mitochondria Act as a Store of Mobilizable Ca2+?
Our estimate of the [Ca2+]m in maize cells (about 180–240 nm) suggests that it is only approximately 2 times the resting concentration of [Ca2+]cyt. This is consistent with the previous reports of free Ca2+ levels in plant or animal mitochondria (Zottini and Zannoni, 1993; Rizzuto et al., 1994). However, mitochondria appear to contribute to a significant increase in [Ca2+]cyt under anoxia, even though there is only a small gradient of free Ca2+ across the resting mitochondrial membrane. For example, isolated mung bean mitochondria released >5 μm Ca2+ within 5 min of O2 deprivation (Moore et al., 1986). The [Ca2+]cyt in the P3377 maize cell line was about 80 to 100 nm and increased under anoxia by 2.5- to 3-fold (Subbaiah et al., 1994a; present study). Considering mitochondrial volume as 30% of the total cytosolic volume (see Methods for details), anoxic mitochondria should release an amount of Ca2+ at least equivalent to 7- to 8-fold the aerobic levels of [Ca2+]cyt.
Although resting Ca2+ values in mitochondria do not exceed 1 μm, the organelles appear to accumulate large pools (15–20 μm) of Ca2+ in actively metabolizing cells (Rutter et al., 1996; Brini et al., 1997). Even if maize mitochondria do not attain such large quantities of the ionic Ca2+, the capacity of mitochondria for reversible Ca2+ binding would sufficiently account for the [Ca2+]cyt elevation under anoxia. Freshly isolated mitochondria contain a total matrix Ca2+ level of 5 to 30 nmol/mg and can rapidly take up Ca2+ to levels of 60 to 80 nmol/mg in maize and other species (Rugolo et al., 1990; Silva et al., 1991). With a reversible Ca2+-binding capacity estimated to be 40 times that of the cytoplasm (equivalent to >200 mm matrix Ca2+; Budd and Nicholls, 1996; Babcock et al., 1997), mitochondria can potentially contribute to all of the anoxic Ca2+ increase. For example, in hepatocytes 50% of the mitochondrial matrix Ca2+ was released during the first 30 min of anoxia (Aw et al., 1987).
Heterogeneity in the Mitochondrial Anoxic Response
Although the data in Table I represent a good estimate of the changes in [Ca2+]m in response to anoxia-reoxygenation cycles at the whole-cell level, there was a heterogeneity in the response of an individual mitochondrion within a cell (Fig. 3A). This heterogeneity may be a reflection of differences in the metabolic status of an individual mitochondrion, which is known to influence the ability for Ca2+ retention or uptake. However, we have not investigated the possible reasons for these differences. Measurable differences in the Ca2+-uptake capacities of the perinuclear and subplasmalemmal subsets of mitochondria were also observed in ATP-stimulated endothelial cells (Lawrie et al., 1996; Rutter et al., 1996). In these cells subplasmalemmal populations of mitochondria, in contrast to the more interior organelles, preferentially took up Ca2+ from the extracellular medium (Lawrie et al., 1996). Neither the intracellular location of mitochondria in relation to the plasma membrane nor the availability of Ca2+ in the extracellular medium was related to the heterogeneity in the mitochondrial response of maize cells (data not shown).
Structural and functional variations among mitochondria within a single cell have been extensively reported. In particular, heart and skeletal muscle cells possess two distinct populations of mitochondria, intermyofibrillar and subsarcolemmal fractions, which are localized in specialized cellular compartments. Aside from the composition of membrane lipids and ions, of enzyme and respiratory activities, and of protein-import properties, etc. (Takahashi and Wood, 1996, and refs. therein), the intermyofibrillar and subsarcolemmal mitochondria also differ in their Ca2+-uptake/release properties (Pinsky et al., 1981, and refs. therein) and in their tolerance to anoxia-reperfusion injury (Duan and Karmazyn, 1989). In addition, plant mitochondria within single cells differ in their genome size, structure, and contents (for review, see Wolstenholme and Fauron, 1995).
Mitochondrial Ca2+ Release under Anoxia Is Independent of Transmembrane Depolarization
The uptake and retention of Ca2+ in mitochondria is known to be mostly a membrane-potential-dependent process (Fiskum and Lehninger, 1980; Rugolo et al., 1990; McCormack and Denton, 1993), although membrane-potential-independent mechanisms have also been reported (Silva et al., 1992; Pastorino et al., 1995; Rutter et al., 1996). Mitochondria in maize cells showed both intercellular and intracellular variation in their membrane potential. It is not clear whether this heterogeneity in the membrane potential was associated with the variation observed in the Ca2+ response (Fig. 3). Smiley et al. (1991) reported that the membrane potential varied not only across mitochondria in a living cell but also within a single, long, contiguous mitochondrion. The authors attributed the heterogeneity in the membrane potential to, among other things, an uneven distribution of Ca2+ within the mitochondrion (Reers et al., 1995).
The overlapping optical properties of rhod-2 AM and JC-1 did not allow us to investigate the relationship between the membrane potential of individual mitochondria and their ability to release Ca2+. Nevertheless, we were able to examine whether a loss in [Ca2+]m was always preceded by mitochondrial depolarization. A complete collapse of the potential brought about by FCCP treatment was correlated with a large loss in mitochondrial Ca2+ (Table I; Fig. 6). However, under O2 deprivation Ca2+ release occurred prior to the loss of the membrane potential. In rat hepatocytes the mitochondrial membrane potential decreased by less than 20% from the aerobic values, whereas 50% of the matrix Ca2+ was released during the first 30 min of anoxia (Aw et al., 1987). Nishida et al. (1989), who studied the sequence of anoxia-induced events in isolated mitochondria, also observed that Ca2+ release preceded the loss in membrane potential. In fact, isolated maize mitochondria were shown to have a Ca2+-release pathway that was not dependent on a decrease in the membrane potential (Silva et al., 1992). In addition, the ability of ruthenium red to block both the electroneutral Ca2+ efflux from mitochondria (Gunter et al., 1994) and the anoxia-induced [Ca2+]cyt elevation (Subbaiah et al., 1994a) also suggest that the Ca2+ release in anoxic maize cells might occur by membrane-potential-independent mechanisms.
Significance of Mitochondrial Ca2+ Release in Anoxia Signaling
From recent studies the role of mitochondria in physiological and pathological contexts has assumed a great significance in both plant (Aubert et al., 1996; Naton et al., 1996) and animal systems (for review, see Gunter et al., 1994; Ankarcrona et al., 1995). In animal cells the dynamics of [Ca2+]m, particularly in relation to anoxia-reoxygenation cycles, have been extensively studied. For example, in hepatocytes anoxia induced a biphasic increase in [Ca2+]cyt (Gasbarrini et al., 1992) and a release of mitochondrial Ca2+ (Aw et al., 1987; Nishida et al., 1989). The depletion of mitochondrial matrix Ca2+ was proposed to be important for the postanoxic survival of the cells (Gunter et al., 1994; Pastorino et al., 1995). Thus, intramitochondrial Ca2+ fluxes appear to be tightly regulated and critical for the survival of mammalian cells under O2 deprivation. However, the role of mitochondria as a source of mobilizable Ca2+ in the perception of anoxia has rarely been investigated even in animal systems.
Our results argue that the [Ca2+]cyt-mediated signaling of anoxia involves the release of Ca2+ from mitochondria rather than mere inhibition of energy-coupled removal of the ion from the cytosol. The identification of [Ca2+]m as the source of the anoxic Ca2+ signal would allow us to focus on the mitochondria-related events or components in our search for the O2 sensor. The elucidation of how O2 deprivation initiates Ca2+ release from mitochondria may indicate exactly where the changes in O2 levels are sensed in the cell. A potential candidate for the O2 sensor is the mitochondrial electron-transport chain. However, in view of the sensitivity of gene-expression changes to even mild alterations in O2 availability (Paul and Ferl, 1991), such that the genes are induced at much higher concentrations than the Km (O2) of Cyt a3, a low-affinity system could be a more appropriate sensor (such as a component of the plasma membrane redox system). The Ca2+ released from mitochondria may communicate the metabolic changes occurring in mitochondria under O2 deprivation to the cytoplasm and the nucleus. Consistent with this, our preliminary observations indicate that anoxia induces large changes in the nuclear-localized fluo-3 fluorescence (e.g. cells 1 and 3, Fig. 4; also see fig. 4 in Subbaiah et al., 1994a). Furthermore, our earlier results revealed that an elevation of this ion in maize cells is indeed a requisite step in initiating rapid, compensatory metabolic and gene-expression responses to anoxia (Subbaiah et al., 1994a, 1994b).
ACKNOWLEDGMENTS
We thank Prof. Peter Hepler (University of Massachusetts) and Dr. Imad N. Saab (University of Illinois) for critically reading the manuscript and the Beckman Institute Optical Visualization Facility (University of Illinois) for the use of their confocal microscope for the analyses shown in Figure 1. We are grateful to Dr. Ed Bonder and Mr. M. Rodriguez (Department of Cell Biology, Rutgers University, Newark, NJ), for facilities and help with the data analysis.
Abbreviations:
- [Ca2+]cyt and [Ca2+]m
cytosolic and mitochondrial free Ca2+ concentrations, respectively
- DiOC6(3)
3,3′-dihexyloxacarbocyanine iodide
- FCCP
carbonylcyanide p-trifluoromethoxyphenyl hydrazone
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazylcarbocyanine iodide
Footnotes
This work was supported by a grant from the National Research Initiative Competitive Grants Program, U.S. Department of Agriculture (no. 96-35100-3143 to M.M.S. and C.C.S.) and by a National Science Foundation grant (no. DCB-9206692 to D.S.B.).
Names are necessary to report factually on available data; however, the U.S. Department of Agriculture neither guarantees nor warrants the standard of the product, and the use of the name by the U.S. Department of Agriculture implies no approval of the product to the exclusion of others that may also be suitable.
LITERATURE CITED
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty M, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw TY, Anderson BS, Jones DP. Suppression of mitochondrial respiratory function after short-term anoxia. Am J Physiol. 1987;252:C362–C368. doi: 10.1152/ajpcell.1987.252.4.C362. [DOI] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, De Giorgi F, Marsault R, Massimino ML, Cantini M, Rizzuto R, Pozzan T. Subcellular analysis of Ca2+ homeostasis in primary cultures of skeletal muscle myotubes. Mol Biol Cell. 1997;8:129–143. doi: 10.1091/mbc.8.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JM, Sanders D. Identification and characterization of high-affinity binding sites for inositol triphosphate in red beet. Plant Cell. 1993;5:931–940. doi: 10.1105/tpc.5.8.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd SA, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- Burnier M, Centeno G, Burki E, Brunner HR. Confocal microscopy to analyze cytosolic and nuclear calcium in cultured vascular cells. Am J Physiol. 1994;35:C1118–C1127. doi: 10.1152/ajpcell.1994.266.4.C1118. [DOI] [PubMed] [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Bush DS. Effects of gibberellic acid and environmental factors on cytosolic calcium in wheat aleurone cells. Planta. 1996;199:89–99. [Google Scholar]
- Chason A. Characterization of the tonoplast Ca2+/H+ antiport system from maize roots. Plant Physiol Biochem. 1994;32:341–346. [Google Scholar]
- Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486:1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond RM, Fay FS. Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflugers Arch Eur J Physiol. 1996;431:473–482. doi: 10.1007/BF02191893. [DOI] [PubMed] [Google Scholar]
- Duan J, Karmazyn M. Acute effects of hypoxia and phosphate on two populations of heart mitochondria. Mol Cell Biochem. 1989;90:47–56. doi: 10.1007/BF00225220. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Lehninger AL. The mechanisms and regulation of mitochondrial Ca2+ transport. Fed Proc. 1980;39:2432–2436. [PubMed] [Google Scholar]
- Franklin-Tong VE, Drfbak BK, Allan AC, Watkins PAC, Trewavas AJ. Growth of pollen tubes of Papaver rhoeas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-trisphosphate. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE, Ride JP, Read ND, Trewavas AJ, Franklin FCH. The self-incompatibility response in Papaver rhoeas is mediated by cytosolic free calcium. Plant J. 1993;4:163–177. [Google Scholar]
- Fulceri R, Bellomo G, Mirabelli F, Gamberucci A, Beneditti A. Measurement of mitochondrial and non-mitochondrial Ca2+ in isolated intact hepatocytes: a critical re-evaluation of the use of mitochondrial inhibitors. Cell Calcium. 1991;12:431–439. doi: 10.1016/0143-4160(91)90069-q. [DOI] [PubMed] [Google Scholar]
- Gasbarrini A, Borle AB, Farghali H, Bender C, Francavilla A, Van Thiel D. Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem. 1992;267:6654–6663. [PubMed] [Google Scholar]
- Gilroy S. Fluorescent probes of plant cell function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:165–190. doi: 10.1146/annurev.arplant.48.1.165. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu S-S, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Jou M-J, Peng T-I, Sheu S-S. Histamine reduces oscillations of free mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J Physiol. 1996;497:299–308. doi: 10.1113/jphysiol.1996.sp021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- Lawrie AM, Rizzuto R, Pozzan T, Simpson AWM. A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J Biol Chem. 1996;271:10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bushnell WR, Brambl R. Potentiometric cyanine dyes are sensitive probes for mitochondria in intact plant cells. Plant Physiol. 1987;84:1385–1390. doi: 10.1104/pp.84.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Corzo A, Leigh RA, Sanders D. Membrane-potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994;5:683–694. [Google Scholar]
- Martínez-Serrano A, Satrústegui J. Regulation of cytosolic free calcium concentration by intrasynaptic mitochondria. Mol Biol Cell. 1992;3:235–248. doi: 10.1091/mbc.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. The role of intramitochondrial Ca2+ in the regulation of oxidative phosphorylation in mammalian tissue. Biochem Soc Trans. 1993;21:793–799. doi: 10.1042/bst0210793. [DOI] [PubMed] [Google Scholar]
- Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Moore AL, Proudlove MO, Ackerman KEO. The role of intracellular organelles in the regulation of cytosolic calcium levels. NATO ASI Ser A Life Sci. 1986;104:277–283. [Google Scholar]
- Muir SR, Sanders D. Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- Naton B, Hahlbrock K, Schmelzer E. Correlation of rapid cell death with metabolic changes in fungus-infected, cultured parsley cells. Plant Physiol. 1996;112:433–444. doi: 10.1104/pp.112.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Inoue T, Kamiike W, Kawashima Y, Tagawa K. Involvement of Ca2+ release and activation of phospholipase A2 in mitochondrial dysfunction during anoxia. J Biochem. 1989;106:533–538. doi: 10.1093/oxfordjournals.jbchem.a122887. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Snyder JW, Hoek JB, Farber JL. Ca2+ depletion prevents anoxic death of hepatocytes by inhibiting mitochondrial permeability transition. Am J Physiol. 1995;268:C676–C685. doi: 10.1152/ajpcell.1995.268.3.C676. [DOI] [PubMed] [Google Scholar]
- Paul A-L, Ferl RJ. Adh1 and Adh2 regulation. Maydica. 1991;36:129–134. [Google Scholar]
- Pinsky WW, Lewis RM, McMillin-Wood J, Hara H, Hartley CJ, Gillette PC, Entman ML. Myocardial protection from ischemic arrest: potassium and verapamil cardioplegia. Am J Physiol. 1981;240:H326–H335. doi: 10.1152/ajpheart.1981.240.3.H326. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldoelsi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Bastiannuto C, Brini M, Murgia M, Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AWM, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rooney E, Medlolesi J. The endoplasmic reticulum in PC12 cells: evidence for a mosaic of domains differently specialized in Ca2+ handling. J Biol Chem. 1996;46:29304–29311. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- Rugolo M, Pistocchi R, Zannoni D. Calcium ion transport in higher plant mitochondria (Helianthus tuberosus) Physiol Plant. 1990;79:297–302. [Google Scholar]
- Rutter GA, Burnett P, Rizzuto R, Brini M, Murgia M, Pozzan T, Tavare JM, Denton RM. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc Natl Acad Sci USA. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Muir SR, Allen GJ. Ligand- and voltage-gated calcium release channels at the vacuolar membrane. Biochem Soc Trans. 1995;23:856–861. doi: 10.1042/bst0230856. [DOI] [PubMed] [Google Scholar]
- Schumaker K, Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987;262:3944–3946. [PubMed] [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH. Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996;111:243–257. doi: 10.1104/pp.111.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MAP, Carnieri EGS, Vercesi AE. Calcium transport by corn mitochondria. Evaluation of the role of phosphate. Plant Physiol. 1992;98:452–457. doi: 10.1104/pp.98.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Mitochondria support inositol 1,4,5-triphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J Biol Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- Smiley ST, Reers M, Mottola-Hartshorn C, Chen A, Lin M, Chen LB, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension cultured cells. Plant Cell. 1994a;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994b;105:369–376. doi: 10.1104/pp.105.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Wood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. J Biol Chem. 1996;271:27285–27291. doi: 10.1074/jbc.271.44.27285. [DOI] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme DR, Fauron CM-R (1995) Mitochondrial genome organization. In CS Levings III, IK Vasil, eds, The Molecular Biology of Plant Mitochondria. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–59
- Zottini M, Zannoni D. The use of Fura-2 fluorescence to monitor the movement of free calcium ions into the matrix of plant mitochondria (Pisum sativum and Helianthus tuberosus) Plant Physiol. 1993;102:573–578. doi: 10.1104/pp.102.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]