Abstract
The roof plate is a signalling centre positioned at the dorsal midline of the central nervous system and generates dorsalising morphogenic signals along the length of the neuraxis. Within cranial ventricles, the roof plate gives rise to choroid plexus, which regulates the internal environment of the developing and adult brain and spinal cord via the secretion of cerebrospinal fluid. Using the fourth ventricle as our model, we show that the organiser properties of the roof plate are determined by its boundaries with the adjacent neuroepithelium. Through a combination of in ovo transplantation, co-culture and electroporation techniques in chick embryos between embryonic days 3 and 6, we demonstrate that organiser properties are maintained by interactions between the non-neural roof plate and the neural rhombic lip. At the molecular level, this interaction is mediated by Delta-Notch signalling and upregulation of the chick homologue of Hes1: chairy2. Gain- and loss-of-function approaches reveal that cdelta1 is both necessary and sufficient for organiser function. Our results also demonstrate that while chairy2 is specifically required for the maintenance of the organiser, its ectopic expression is not sufficient to recapitulate organiser properties. Expression of atonal1 in the rhombic lip adjacent at the roof plate boundary is acutely dependent on both boundary cell interactions and Delta-Notch signalling. Correspondingly, the roof plate boundary organiser also signals to the roof plate itself to specify the expression of early choroid plexus markers. Thus, the roof plate boundary organiser signals bi-directionally to acutely coordinate the development of adjacent neural and non-neural tissues.
Keywords: Chick, Cath1, Atoh1, Atonal 1, Gdf7, Cyp26c1, Hes, Hairy, Transthyretin, Co-culture, Electroporation, GFP
INTRODUCTION
The development of the vertebrate central nervous system (CNS) involves the correct number and type of neurons arising at the correct positions within the neural tube at the correct time. This process is coordinated by groups of cells known as organisers, via the secretion of signalling molecules. One such organiser is the roof plate, which is present at the dorsal midline along the entire anteroposterior axis of the developing CNS (Chizhikov and Millen, 2005). The roof plate secretes bone morphogenetic proteins (BMPs) (Chesnutt et al., 2004; Chizhikov and Millen, 2004; Lee et al., 1998; Liem et al., 1997; Timmer et al., 2002) and wingless/Wnt protein signals (Alvarez-Medina et al., 2008; Muroyama et al., 2002) to pattern the development of the dorsal neural tube. In the spinal cord, ablation of the roof plate results in the lack of specification of the progenitors of the three dorsal-most groups of interneurons (dI1, dI2 and dI3, marked by the bHLH transcriptional regulators atonal1, ngn1 and mash1, respectively) (Lee et al., 2000). At the level of the hindbrain, only the dorsal-most group of atonal1- (math1/atoh1) expressing neural progenitor cells, which comprise the rhombic lip, are lost when the roof plate is ablated (Chizhikov et al., 2006). Expression of math1/atoh1 in vitro can be induced by and subsequently becomes dependent upon BMP signalling (Alder et al., 1996; Krizhanovsky and Ben-Arie, 2006).
In most regions, the roof plate comprises a narrow strip of cells; however, at certain points along the anteroposterior axis, known as the brain ventricles, the roof plate is expanded: paired lateral ventricles form in the telencephalon, the third ventricle in the diencephalon and the fourth ventricle in the hindbrain. Within these regions, the roof plate comprises a pseudostratified roof plate boundary (at the interface with the neuroepithelium) that borders a broadened single cell layer roof plate epithelium (Landsberg et al., 2005). As embryonic development progresses, this expanded single cell layer roof plate differentiates into a specialised epithelium, which establishes a close relationship with an ingrowing, dense and fenestrated vasculature (Hunter and Dymecki, 2007). The resulting choroid plexuses are a series of ventricular, secretory interfaces that form the blood-cerebrospinal fluid (CSF) barrier (Johansson et al., 2008). Thus, at ventricle regions of the brain, the roof plate is, as in other regions of the CNS, an early embryonic organiser of neuroepithelial development, but later develops into the epithelial component of the choroid plexus. As in the adult brain, the embryonic ventricle-CSF system serves several functions, including the distribution of nutrients, carriage of metabolites and the production of a fluid cushion for its physical protection (Redzic et al., 2005). Additionally, a growing body of evidence implicates the choroid plexus in signalling to the developing neural tube to stimulate proliferation or differentiation of neural progenitors. For example, the fourth ventricle choroid plexus has been shown to induce neurite outgrowth in cerebellar explants via its production of retinoic acid (Yamamoto et al., 1996). More recently it has been shown that CSF-borne Sonic Hedgehog (Shh) regulates the proliferation of cerebellar radial glial cells and production of progenitors of inhibitory neurons (Huang et al., 2010), whereas CSF-borne insulin-like growth factor 2 stimulates the proliferation of cortical neuronal progenitors (Lehtinen et al., 2011).
The regional adaptation of the roof plate into choroid plexus thus represents a significant developmental event, but one that is surprisingly poorly characterised. To understand this transformation, we looked at events surrounding the development of the roof plate of the fourth ventricle, which induces the formation of the adjacent rhombic lip (Alder et al., 1996). Our approach follows the important insight that the fourth ventricle roof plate is a developmental compartment: mouse transgenic fate maps reveal a sharp demarcation between a roof plate lineage that is characterised by expression of the BMP family member Gdf7 and the adjacent pool of Atoh1-positive rhombic lip neural derivatives (Currle et al., 2005; Hunter and Dymecki, 2007; Machold and Fishell, 2005; Wang et al., 2005). In recent years, it has become increasingly clear that the boundaries of such lineage restriction compartments function as bi-directional signalling centres (Kiecker and Lumsden, 2005). Thus, for example, the midbrain-hindbrain boundary (Langenberg and Brand, 2005; Zervas et al., 2004) and the zona limitans intrathalamica (ZLI) (Larsen et al., 2001) pattern adjacent neuroepithelium. In this study, we used the precedent of the experimental approaches taken by these studies to determine whether such a signalling centre is maintained at the boundary between the roof plate and the neuroepithelium.
Boundary-localised organisers show certain typical properties: they can be regenerated upon the experimental juxtaposition of compartment tissues (Guinazu et al., 2007; Irving and Mason, 1999); they display high and persistent levels of Hes gene expression, and require Hes genes for their maintenance (Baek et al., 2006; Hirata et al., 2001); their formation is regulated by Notch signalling (Tossell et al., 2011; Zeltser et al., 2001); and they signal bi-directionally to compartments on either side of a lineage restriction interface (Kiecker and Lumsden, 2004; Wassef and Joyner, 1997). We establish here that these criteria hold for the roof plate-neuroepithelial interface. Using gdf7 as a read-out of boundary-organiser activity, we show that a roof plate boundary organiser can be regenerated by tissue recombination in vivo and in vitro, is characterised by high level expression of chairy2 [a chick homologue of hes1 (Jouve et al., 2000)], and depends on chairy2 for its maintenance. Furthermore, we demonstrate that Delta-Notch signalling is both necessary and sufficient for maintenance of the roof plate boundary and the expression of atoh1 at the rhombic lip, and is also required for the patterning of choroid plexus epithelium. These observations indicate that the organiser properties of the fourth ventricle roof plate are invested in its boundaries with the neuroepithelium and that this roof plate boundary organiser acts to coordinately pattern adjacent neural and non-neural progenitors.
MATERIALS AND METHODS
Cloning of chairy2 expression vectors
The full-length and a C-terminally truncated chairy2 expression constructs were amplified by polymerase chain reaction from a full-length chick cDNA clone using the primers 5′-ATTGCGGCCGCATGCCTGCCGACCTGATGGAG-3′ and 5′-TGAATTCTCACCAGGGCCTCCAGACTG-3′, or 5′-TGAATTCTCAGACTGAGTCAGCGGTG-3′, and cloned into the NotI and EcoRI sites of the pCA-IRES-eGFPm5 expression construct (http://www.ncbi.nlm.nih.gov/pubmed/19602272). shRNA-expressing constructs (21 bp) were generated against eight different targets in the chairy2 cDNA, of which two are described in this study. The target sequences were 5′-AGGCCGACATCCTGGAGATGA (T7) and 5′-CT-GCCGACCTGATGGAGAAGA (T8). The following oligonucleotides were cloned into pBS/U6: 5′-GCCGACATCCTGGAGATGATTCAAGAGATCATCTCCAGGATGTCGGCCTTTTTTT-3′ and 5′-AAAAAAAGGCCGACATCCTGGAGATGATCTCTTGAATCATCTCCAGGATGTCGGCGGCC-3′ (T7) and 5′-GCCGACCTGATGGAGAAGATTCAAGAGATCTTCTCCATCAGGTCGGCAGTTTTTT-3′ and 5′-AAAAAACTGCCGACCTGATGGAGAAGATCTCTTGAATCTTCTCCATCAGGTCGGCGGCC-3′ (T8).
In vitro and in ovo manipulations
Fertilised green fluorescent protein transgenic (GFP-tg) eggs (McGrew et al., 2008) and wild-type eggs (Henry Stewart, UK) were incubated at 38°C for 3 to 6 days before windowing with sharp surgical scissors. A number of embryos at embryonic day (E)4-6 were removed from eggs and neural tubes dissected in Tyrode's solution. For explants, wild-type embryos were bisected along the dorsal midline and roof plate removed unilaterally with its boundary domain. Explants were placed, pial surface uppermost, on 0.4 μm inserts (Millicell-CM, Millipore) on sterile medium: Basal Medium Eagle (Gibco), 0.5% (w/v) D-(+)-glucose (Sigma), 1% I-1884 supplement (Sigma), 2 mM L-Glutamine (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml Fungizone (Gibco) in culture dishes.
Further wild-type and GFP-tg brains were dissected using flame-sharpened tungsten wire (0.1 mm) to isolate roof plate and neural tube tissue for co-cultures and grafts. For co-culture experiments, roof plate fragments retained the rhombic lip unilaterally to facilitate manipulation and provide an endogenous control for subsequent in situ labelling. GFP-tg and wild-type fragments were recombined on a culture insert (as above) to create precise, reconstituted roof plate/neuroepithelium interfaces. For in ovo grafting, yolk sacs of wild-type eggs (E3) were injected (for contrast) with India ink (Pelikan, 1:6 in Tyrode's solution) and GFP-tg fragments of roof plate (neuroepithelium completely removed) or dorsal neural tube transferred into size-matched excisions in host roof plate using tungsten wire.
For electroporation, the fourth ventricle of E3 or E4 wild-type eggs was injected with ~200 nl of 1-2 μg/μl DNA plasmids either singly or in combination: eGFPm5, chairy2:GFP, chairy2ΔWRPW:GFP, T7chairy2shRNA, T8chairy2shRNA, RCAS-cdelta1 (Henrique et al., 1997), RCAS-cdelta1(dn) (Henrique et al., 1997) and RCAS-RFP. Square-wave 10 V electrical pulses (3×50 ms) were passed between electrodes placed externally to the embryo.
Explants and co-cultures (37°C/6% CO2), graft-chimaeras and electroporated eggs (at 38°C, re-sealed with tape) were incubated for up to 48 hours.
Histology and photomicroscopy
Embryos were fixed in 4% (w/v) paraformaldehyde (in phosphate-buffered saline) and either dissected or processed for cryostat sectioning. Tissue was stained by in situ hybridisation (Myat et al., 1996) with digoxygenin- or fluorescein-labelled (Roche) riboprobes: gdf7 (Anthony Graham, King's College London, UK), atoh1 (Wilson and Wingate, 2006), cnotch1, cnotch2, cdelta1, cserrate1 (Henrique et al., 1995; le Roux et al., 2003; Myat et al., 1996), lfng (Zeltser et al., 2001), cyp26C1 (Reijntjes et al., 2004), ttr (Duan et al., 1991), and chairy1 and chairy2. Where appropriate, GFP signal was immunohistochemically enhanced with an anti-GFP antibody (IgG 1:150, Invitrogen). Overexpression construct function was confirmed by in situ hybridisation (supplementary material Fig. S1). For experiments, only embryos in which electroporation could be confirmed by GFP label in the correct territories were used for comparison. This exclusion criterion reduced the n values from a much larger sample of in situ and immunostained embryos in each experimental group. Some wholemounts were further processed for vibratome sectioning. Digital bright-field and fluorescence images were acquired on either stereo (Leica MZFLIII/QImaging Retiga Exi) or compound (Zeiss Axiophot/AxioCam) microscopes equipped with epifluorescence or by laser scanning confocal microscopy (Olympus AX70/Fluoview F500).
RESULTS
Tissue interactions recapitulate the pattern of gdf7 induction at the dorsal midline
In mouse, genetic fate-map studies have shown that tissues of the roof plate of the fourth ventricle and spinal cord are derived from progenitors that express Gdf7, which encodes a secreted BMP family member (Currle et al., 2005). In chick, the dorsal midline of the CNS expresses gdf7 along its axis. However, at sites of cranial ventricle expansions (Fig. 1A), mRNA transcripts are excluded from the roof plate epithelium and are localised at the boundaries of the roof plate (Louvi and Wassef, 2000). In the fourth ventricle (Fig. 1B,C), this observation implies that the site of gdf7 signalling lies at the interface with the atoh1-positive embryonic rhombic lip, consistent with a boundary organiser model. We investigated this hypothesis by testing whether interactions between roof plate epithelium and neuroepithelium are sufficient to induce gdf7. First, we assessed the effects of grafting ectopic hindbrain neuroepithelium or roof plate from E4 GFP-tg chick embryos into the roof plate of wild-type E3 chick hindbrain in ovo (n=12). Embryos were harvested 1 day later and one-third of neuroepithelium grafts displayed ectopic gdf7 around the interface with host roof plate (Fig. 1D,E). By contrast, gdf7 was not induced by grafting roof plate into roof plate (Fig. 1F,G; n=3). Next, various roof plate epithelium and neural tissue recombinations at E4-E6 (see Fig. 1A) were constructed in vitro and resulted in a robust induction of gdf7 after 48 hours in culture (E4, n=8/8; E5, n=11/15; E6, n=10/11). As with in vivo grafts, induction of gdf7 is confined to the interface between reconstituted roof plate and neuroepithelium (Fig. 1H,I) and cannot be induced by contact between roof plate and roof plate (Fig. 1J; n=10). Transverse sections of neuroepithelium/roof plate co-cultures suggest that ectopic gdf7 is induced exclusively in roof plate-derived explanted tissue (Fig. 1K). Neither in vivo nor in vitro manipulations revealed any regional differences in the competence of roof plate to express gdf7. Moreover, expression can result from interaction with neuroepithelium from any part of the dorsoventral axis of the hindbrain or from the spinal cord (supplementary material Fig. S2). Together, these results suggest that gdf7 is induced in roof plate cells by contact-mediated signals from the neural tissue.
Fig. 1.
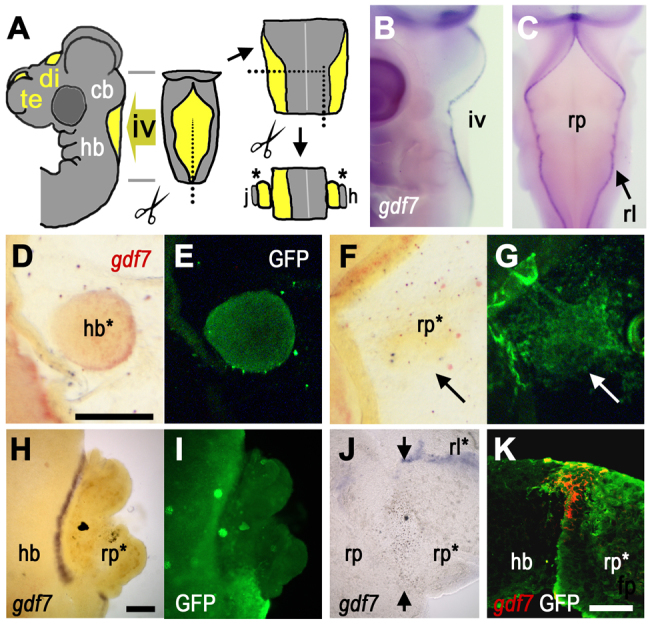
Induction of gdf7 by tissue interactions. (A) Schematic E4 chick head showing ventricles (yellow), which form bilaterally within the forebrain (fb) and at the dorsal midlines of both the diencephalon (di) (third ventricle) and cerebellum (cb)/hindbrain (hb) (fourth ventricle, iv). Tissue surrounding iv was dissected for grafts and co-cultures (j and h are references to J and H). (B,C) In lateral (B) and dorsal (C) views at E4, gdf7 (blue) is expressed at the boundary between hindbrain roof plate (rp) and rhombic lip (rl). (D,E) In ovo graft of GFP-tg hindbrain (hb*) into wild-type roof plate results in gdf7 induction (D, red) around the graft interface after 24 hours (GFP label in E). (F,G) gdf7 is not induced when E4 GFP-tg-roof plate (rp*, G) is grafted into E3 wild-type roof plate (arrow). For this homotypic graft, there is some evident mixing of donor and host cells. (H,I) In vitro roof plate/neuroepithelium co-cultures (E6) show robust gdf7 induction at the interface between GFP-tg hindbrain and wild-type roof plate (rp*, I). (J) Juxtaposition of E6 roof plate tissues (rp/rp*) in co-culture does not induce gdf7 (arrows indicate interface), while gdf7 is maintained in residual rhombic lip (rl*). (K) Confocal optical slice through a transverse section of a hindbrain/roof plate co-culture interface shows that gdf7 expression (red) is confined to GFP-tg-derived roof plate (rp*). Scale bars: 200 μm in D-G; 200 μm in H-J; 50 μm in K.
The roof plate boundary both spatially defines and maintains an atoh1-positive rhombic lip
The establishment and maintenance of the rhombic lip in mouse is known to be dependent on roof plate signalling, and subsequently on signals from the choroid plexus (Chizhikov et al., 2006; Krizhanovsky and Ben-Arie, 2006). In order to investigate whether an intact roof plate-neuroepithelium boundary is required for the maintenance of atoh1 in the rhombic lip of chick, we performed in vitro culture experiments of flat-mounted hindbrain and cerebellum at E4, E5 and E6 (Fig. 2A). First, we assessed the capacity of the rhombic lip to maintain atoh1 expression in the absence of the gdf7-positive domain. Within intact brain, gdf7 and atoh1 are expressed in adjacent domains at both 0 hours (Fig. 2B) and 48 hours (Fig. 2C). The expression of atoh1 at the rhombic lip is initially unaffected by removal of the gdf7-positive boundary tissue (Fig. 2D). However, atoh1 expression is lost by 48 hours (Fig. 2E). Downregulation is specific to the rhombic lip atoh1 expression domain, as the normal time course of the onset of expression of atoh1 in more ventrally derived respiratory nuclei (Rose et al., 2009) is preserved (data not shown). The loss of atoh1 at the rhombic lip can be rescued by co-culturing neuroepithelium with ectopic roof plate (rp*), which induces an ectopic gdf7-positive domain (Fig. 2F). This implies that the downregulation of atoh1 following removal of the gdf7-positive domain is due to the loss of a specific tissue interaction, rather than the consequences of experimental procedure. Furthermore, expression of atoh1 in co-culture is found only adjacent to a site of ectopically induced gdf7 (n=10/10), indicating that atoh1 expression is dependent on a gdf7-positive boundary.
Fig. 2.
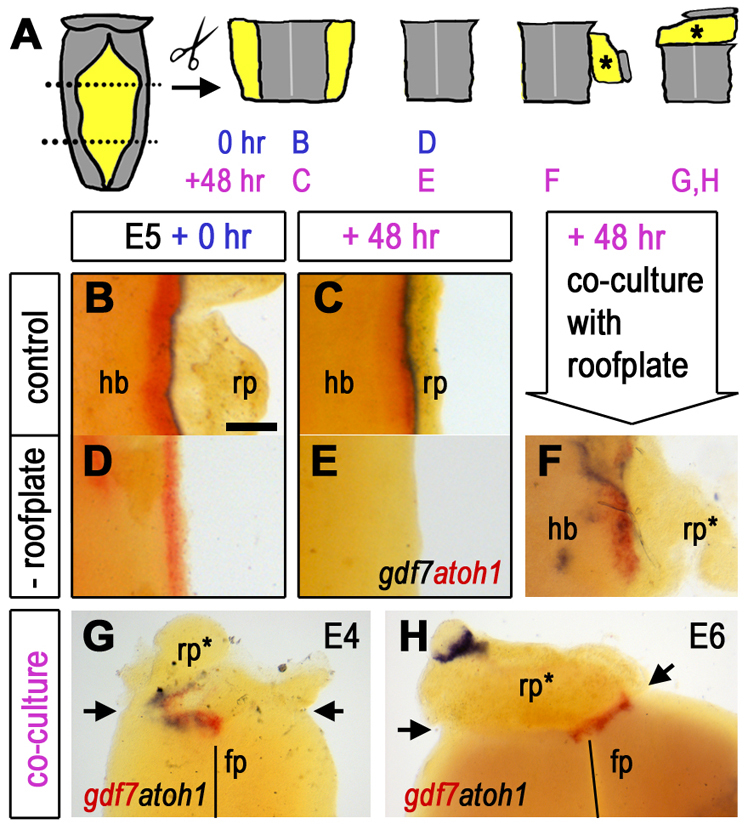
Rhombic lip identity is actively maintained by cell interactions across the neuroepithelial/roof plate boundary. (A) Tissue around the intact fourth ventricle was removed from embryos at E4, E5 and E6, sub-dissected and recombined in various orientations for co-culture. (B,C) Intact explants express gdf7 (blue) and atoh1 (red) at (B) 0 hours and at (C) 48 hours. (D,E) With the removal of the gdf7 (blue) domain, atoh1 (red) is initially maintained (D) but is downregulated by 48 hours (E). (F) This loss can be rescued by co-culturing roof plate-depleted rhombic lip with a donor roof plate (rp*). (G) E4 ventral hindbrain is competent to express atoh1 (floor plate, fp, indicated by solid line). Co-cultured roof plate epithelium (rp*) both expresses ectopic gdf7 (red) and induces localised ectopic atoh1 (blue) in adjacent neural tissue at the co-culture interface (arrows) after a 48-hour incubation. (H) At E6, induction of a gdf7-positive roof plate boundary (rp*) fails to induce ectopic atoh1 in adjacent ventral hindbrain at the co-culture interface (arrows) at 48 hours. Expression of atoh1 (blue) distal to the co-culture interface represents residual rhombic lip on the roof plate graft. Scale bar: 200 μm.
Given that atoh1 expression thus appears to rely on spatial proximity of the roof plate boundary, we explored whether competence to respond to roof plate signals also determines its expression domain at the rhombic lip. To investigate the competence of different dorsoventral regions of the neuroepithelium to respond to an induced gdf7-positive boundary with the expression of atoh1, we juxtaposed roof plate tissue with neuroepithelial tissue from a range of dorsoventral positions. We interpret the evident patchiness of induced gdf7 between tissues as reflecting the discontinuity of sufficient contact between tissues. Nevertheless, at E4 and E5, atoh1 expression could be induced at any dorsal or ventral coordinate in response to a recapitulated boundary marked by gdf7 expression (n=4/10) within ectopic roof plate (rp*, Fig. 2G). At E6, although the roof plate maintains competence to express gdf7 at a recapitulated boundary, atoh1 could not be induced in ventral hindbrain regions (Fig. 2H; n=8/8). Therefore, at E4 and E5 the spatial extent of atoh1 expression is more likely to be determined by the signalling properties of the roof plate, whereas at E6 an apparent restriction in competence in responding neuroepithelium also contributes to demarcating the rhombic lip.
Delta/Notch signalling regulates the experimental induction and in vivo maintenance of gdf7
In the preceding experiments, we showed that the induction of gdf7 in the roof plate correlates with the capacity to induce atoh1 in adjacent neural tube. The manner in which gdf7 can be induced outlines the characteristics of the inductive mechanism: an ectopic boundary can be induced in any part of the roof plate by any regional source of neuroepithelial cells. In terms of candidate proteins that mediate the induction of the boundary organiser, we thus anticipated a receptor that is uniformly expressed throughout the roof plate and a ligand that is expressed uniformly and exclusively throughout the neural tube. Based on these parameters and its conserved role in the formation and maintenance of other boundary-localised organisers (de Celis and García-Bellido, 1994; Pierfelice et al., 2011; Rulifson and Blair, 1995; Tossell et al., 2011; Zeltser et al., 2001), we examined the expression of Notch signalling pathway elements with respect to gdf7 as a marker of the boundary organiser. The gdf7-positive domain lies within a territory that is morphologically indistinguishable from the atoh1-expressing rhombic lip, but is distinct from the adjacent roof plate epithelium (Fig. 3A, arrow indicates roof plate boundary). As gdf7-expressing cells contribute exclusively to roof plate (Currle et al., 2005) and atoh1-positive cells to only the neural tube (Machold and Fishell, 2005; Wang et al., 2005), this boundary defines the spatial extent of the roof plate. Serial sectioning showed that genes encoding the Notch ligands cdelta1 (Fig. 3B) and cserrate1 (Fig. 3C) are excluded from this gdf7-positive roof plate margin. Similarly, lunatic fringe (lfng; Fig. 3D), a glucosyltransferase that differentially modulates the signalling efficiency of Notch ligands (Hicks et al., 2000), is expressed only in the neuroepithelium. Of the genes encoding receptors, cnotch1 (Fig. 3E) is expressed at a very low level within the gdf7-positive domain. By contrast, cnotch2 shows a widespread and low-level expression throughout both neural and roof plate epithelia, and is strongly upregulated in the gdf7 domain (Fig. 3F). Finally, chick homologues of hes1, chairy1 (Fig. 3G) and chairy2 (Jouve et al., 2000) (Fig. 3H) are expressed at high levels at the roof plate boundary, which is consistent with enhanced Notch activation at this interface (Ohtsuka et al., 1999).
Fig. 3.
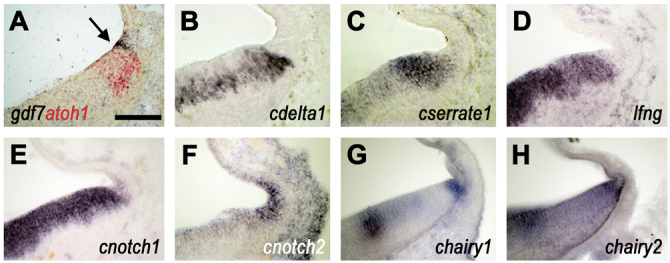
Delta/Notch pathway elements at the roof plate boundary. In situ hybridisation at the hindbrain/roof plate interface in transverse section at E5 (B-F are serial sections through the same brain) and at E4 (A,G,H). (A) gdf7 (blue) is expressed in roof plate cells at the boundary with the atoh1-positive (red) rhombic lip (arrow). (B) cdelta1 is expressed in neuroepithelial ventricular zone and excluded from the gdf7-positive domain. (C) cserrate1 is expressed strongly in the rhombic lip. (D) lfng is expressed uniformly and exclusively in neuroepithelial precursors. (E) cnotch1 is expressed in the ventricular zone throughout the hindbrain neuroepithelial ventricular zone and at a low level within the gdf7 domain. (F) cnotch2 is highly expressed in the gdf7-positive domain and uniformly at low levels within the roof plate epithelium and neuroepithelial ventricular zone. (G,H) The Hes1 homologues (G) chairy1 and (H) chairy2 are expressed in a complex regional pattern within the neuroepithelium, and are both highly expressed at the roof plate boundary. Scale bar: 100 μm.
These patterns of expression and evidence of increased levels of Notch signalling via Hes genes suggest that cdelta1 (expressed broadly in the hindbrain) and cnotch2 (present throughout the entire extent of the roof plate) are well placed to be upstream of gdf7 induction via either chairy1 or chairy2. To test this model, we first assessed whether the tissue recombination protocols that induce gdf7 in vitro (Figs 1, 2) could also induce chairy1 and chairy2. Co-culture of hindbrain and roof plate tissues never results in upregulation of chairy1 (n=14/14) at the interface of recombined tissues (Fig. 4A). However, chairy2 (E5, n=4/4; E6, n=3/4) was upregulated in roof plate epithelium at the co-cultured tissue interface with hindbrain (Fig. 4B). This suggests that the boundary between neuroepithelium and roof plate is a site of Delta-Notch signalling. From the expression patterns of potential ligands (Fig. 3), the most likely candidate Notch ligand to mediate boundary-organiser induction is cdelta1.
Fig. 4.
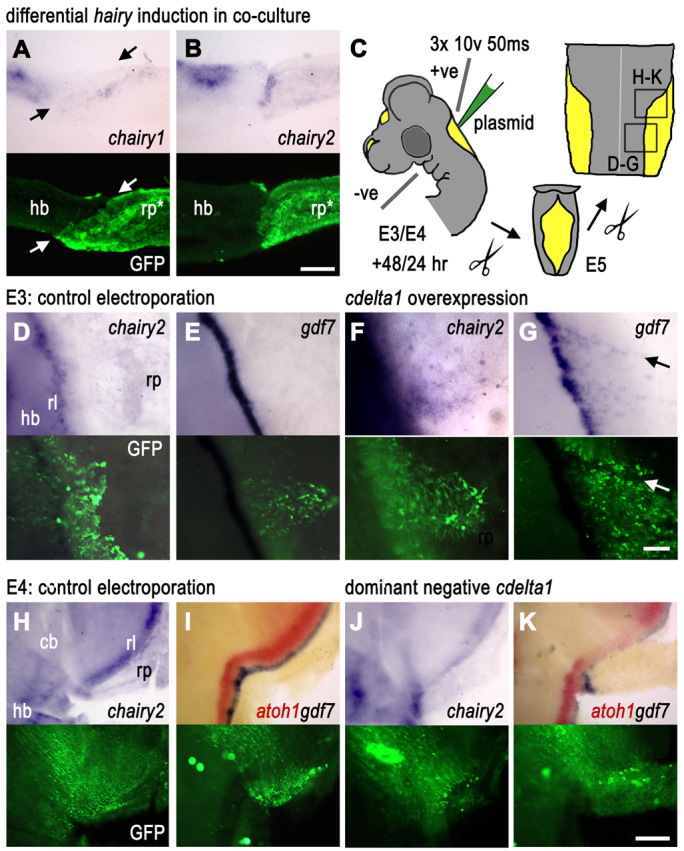
Dependence of roof plate boundary on Delta/Notch signalling. In all panels, in situ product is shown above and the fluorescent label in the same field of view, below. (A) Transverse sections of a 48-hour co-culture of E5 hindbrain neuroepithelium (hb) and GFP-tg roof plate (rp*) show that chairy1 is not induced within GFP-expressing roof plate epithelium at the boundary with neuroepithelium. chairy1 label distal to the recombined interface in rp* reflects endogenous expression in overlying mesenchyme (bright fluorescence). Arrows indicate boundary. (B) By contrast, chairy2 is locally upregulated in GFP-expressing roof plate epithelium adjacent to co-culture tissue interface. (C) Electroporation protocol for targeting rhombic lip (rl) and roof plate at E3 and E4. All subsequent images are views of flat-mounted hindbrain and cerebellum preparations 24 or 48 hours later (boxed regions indicate the field of view). (D,E) At E3, control co-electroporation of RCAS-RFP with pCAβ-eGFPm5 has no effect at E5 on (D) chairy2 or (E) gdf7. (F,G) Co-electroporation of RCAScdelta1 and pCAβ-eGFPm5 in the roof plate epithelium induces ectopic (F) chairy2 and (G) gdf7. Arrows indicate ectopic expression. (H,I) At E4, control co-electroporation of RCAS-RFP with pCAβ-eGFPm5 has no effect at E5 on the expression of (H) chairy2 or (I) atoh1 (red) or gdf7 (blue). (J) Overexpression of the dominant-negative cdelta1 [co-electroporation of RCAS-cdelta1(dn) and pCAβ-eGFPm5] downregulates chairy2 in the rhombic lip interface with roof plate. (K) Dominant-negative cdelta1 expression results in a loss of both gdf7 in the roof plate and atoh1 within the adjacent rhombic lip. Scale bars: 100 μm in A,B; 100 μm in D-G; 200 μm in H-K.
To confirm the role of cdelta1, we used in ovo electroporation to overexpress RCAS (avian retrovirus) constructs encoding either a full-length cdelta1 or a dominant-negative cdelta1 within the roof plate of E3 and E4 chick embryos, respectively (Fig. 4C). Constructs were co-electroporated with a pCAβ-eGFPm5 plasmid to enable identification of transfected cells. In a separate set of electroporations, we used an RCAS-RFP plasmid co-electroporated with the pCAβ-eGFPm5 plasmid to control for non-specific effects of either electroporation or viral transfection. At E3, control electroporations show no effect on either chairy2 (Fig. 4D) or gdf7 expression (Fig. 4E), However, overexpression of cdelta1 (see also supplementary material Fig. S1) induces chairy2 in GFP-labelled roof plate cells (Fig. 4F; n=6/13) and induces ectopic gdf7expression in E5 roof plate (arrow in Fig. 4G; n=6/9). Control electroporations at E4 have no effect on chairy2 (Fig. 4H), gdf7 or atoh1 (Fig. 4I). However, in contrast to full-length cdelta1, electroporation of a dominant-negative version of cdelta1 [RCAS-cdelta1(dn)] into the rhombic lip at E4 results in a subsequent downregulation after 24 hours of chairy2 (asterisk, Fig. 4J; n=3/7), gdf7 in the roof plate and atoh1 in the adjacent rhombic lip (Fig. 4K; n=2/6). We attribute the low penetrance of this effect to the heterogeneity in tissue expression of this viral construct. In combination, this series of results suggests that Delta-Notch signalling is necessary and sufficient for chairy2 and gdf7 expression. Although we cannot discount that cdelta1 may also be cell-autonomously required for atoh1 expression, a more parsimonious explanation for the downregulation of atoh1 expression is that it is secondary to the loss of boundary signals (recapitulating the results of physical removal of roof plate in Fig. 2E). This would imply that the boundary-organiser maintained by Delta activation of the Notch receptor in the roof plate interface in turn maintains atoh1 at the rhombic lip.
The correct level of chairy2 expression is required for the maintenance of the roof plate boundary
As HES genes have been shown to be required for the maintenance of the boundary-localised organisers, such as the midbrain-hindbrain boundary organiser and the ZLI (Baek et al., 2006; Hirata et al., 2001) and chairy2 was specifically upregulated at a reconstituted hindbrain roof plate/neuroepithelium interface (Fig. 4B), we investigated whether chairy2 is functionally required for maintaining gene expression at the boundary organiser. We constructed IRES-GFP plasmids encoding either a full-length chairy2 or a putative dominant-negative chairy2, in which the C-terminal Groucho/TLE co-repressor binding site has been deleted. These were electroporated into E4 neural tube and expression of gdf7 and atoh1 examined 24 hours later (Fig. 4C). Control electroporation of the pCAβ-eGFPm5 plasmid had no effect on either gdf7 (blue) or atoh1 (red) (Fig. 5A). By contrast, overexpression of either full-length chairy2 (Fig. 5B; n=5/11) or dominant-negative chairy2ΔWRPW plasmids (Fig. 5C; n=2/9) results in the downregulation of both gdf7 in the roof plate and atoh1 in the adjacent rhombic lip (see also supplementary material Fig. S1). Although the knock down of Hes gene function might be expected to attenuate Delta-Notch signalling, the duplication of this phenotype through chairy2 overexpression was surprising. We reasoned that the effects of upregulated full-length chairy2 might indirectly reflect the suppression of cdelta1 in neuroepithelium, thus phenocopying the effects of the cdelta1 dominant negative (Fig. 4K). Accordingly, overexpression of chairy2 (n=6/7) leads to a robust downregulation of cdelta1 expression (Fig. 5D).
Fig. 5.
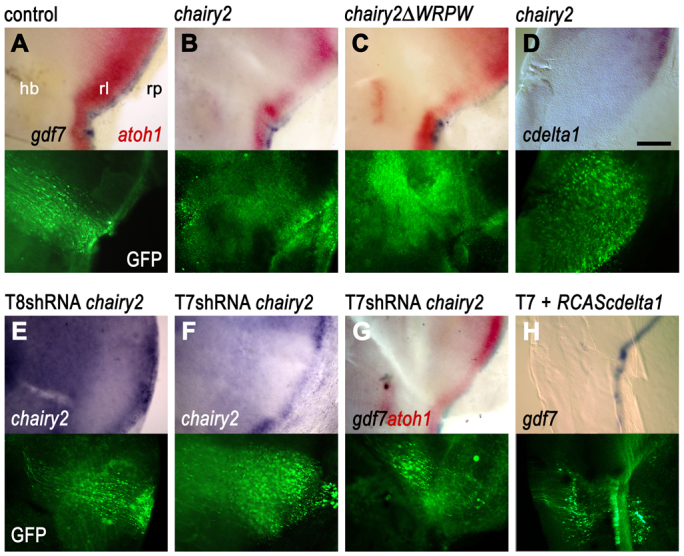
Requirement for the correct level of chairy2 expression for roof plate boundary maintenance. For each panel, in situ hybridisation label (above) is shown alongside GFP label in the same field of view (below). (A) Electroporation of a control pCAβ-eGFPm5 plasmid into the roof plate/neuroepithelium interface at E4 has no effect on the expression of gdf7 or adjacent atoh1 in GFP-labelled cells at E5. (B,C) By contrast, overexpression of either (B) a full-length chairy2-IRESGFP or (C) a putative dominant-negative chairy2ΔWRPW-IRES-GFP construct at E4 results at E5 in a downregulation of gdf7 and adjacent atoh1 within the electroporated domains. (D) chairy2-IRES-GFP overexpression causes an autonomous downregulation of cdelta1 at the rhombic lip (rl) and neuroepithelium. Two different shRNA chairy2-GFP constructs were used to validate the effects of chairy2 functional downregulation. (E) T8 chairy2 shRNA electroporated at E4 has no effect on chairy2 expression at E5. (F,G) T7 chairy2 shRNA electroporation at E4 downregulates expression of chairy2 at E5 (F) in addition to inducing loss of gdf7 and a reduction in adjacent atoh1 (G). (H) T7 chairy2 shRNA prevents the induction of ectopic gdf7 by RCAS-cdelta1 within the roof plate and leads to a patchy expression of endogenous gdf7 at the roof plate boundary. Scale bar: 200 μm.
To confirm that the effects of the putative dominant-negative chairy2 reflect loss of function, we constructed shRNA constructs against chairy2. Constructs against a number of target sequences have no effect on chairy2 expression and demonstrate that there are no non-specific effects of shRNA overexpression on chairy2 (e.g. T8, Fig. 5E; n=5/5). By comparison, a single target sequence variant (T7) induced a robust downregulation of chairy2 mRNA transcripts (Fig. 5F; n=5/8). Expression of the T7 chairy2 shRNA construct also downregulates gdf7 and adjacent atoh1 expression (Fig. 5G; n=2/9) in a similar manner to chairy2ΔWRPW. Co-electroporation of RCAS-cdelta1 with T7 chairy2 shRNA fails to rescue fully the patchy expression of gdf7 (Fig. 5H) and could not induce ectopic gdf7 (n=7). This suggests that, in normal circumstances, the putative function of Delta in maintaining gdf7 expression depends on the maintenance of appropriate levels of chairy2 at the rhombic lip.
The roof plate boundary signals to the presumptive choroid plexus
Our observations demonstrate that maintenance of gene expression in the rhombic lip depends on the integrity of the neighbouring roof plate/neuroepithelial boundary. This implies that the dorsalising properties of roof plate are localised precisely at the boundary itself, as opposed to more medial roof plate epithelium. Such a boundary-localised organiser would have the potential to signal bi-directionally to both neural tube and roof plate. To test this possibility, we examined the expression of two genes, cyp26C1 and transthyretin (ttr), which are involved in retinoid and steroid hormone processing, respectively, in the presumptive choroid plexus. The retinoic acid catabolising enzyme, cyp26C1, is expressed robustly at the roof plate boundary and at a lower level within the roof plate epithelium (Wilson et al., 2007). It is excluded from the central diamond of roof plate tissue [which, in mouse, is not derived from Gdf7-positive cells (Hunter and Dymecki, 2007)]. Targeted electroporation of the upper rhombic lip and roof plate boundary at E4 with a control GFP plasmid (Fig. 6A) labels neural derivatives in the neural tube but, viewed at higher magnification, has no effect on roof plate expression of cyp26C1 (Fig. 6B). Overexpression of either full-length chairy2 (Fig. 6C,D; n=7/10) or the putative dominant-negative chairy2ΔWRPW (Fig. 6E,F; n=6/12) results in a loss of cyp26C1, both at the roof plate boundary and non-autonomously in the adjacent roof plate epithelium, mirroring the loss of atoh1 in the hindbrain neuroepithelium. Loss of cyp26C1 within the roof plate epithelium, in both cases, extends beyond the site of GFP expression, suggesting that expression is regulated by signals from the roof plate boundary. Overexpression of cdelta1 within the roof plate epithelium (which induces ectopic gdf7, Fig. 4G) upregulates roof plate cyp26C1 localised within the site of GFP expression (Fig. 6G,H), suggesting that the high level of cyp26C1 expression, characteristic of the roof plate boundary, is maintained by Delta-Notch interactions in the same manner as gdf7 expression.
Fig. 6.
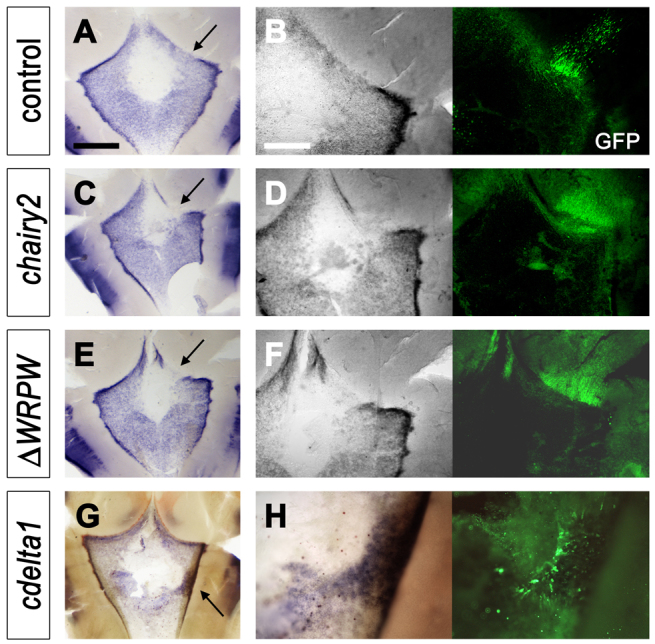
Boundary signals regulate roof plate cyp26C1 expression. cyp26C1 in low magnification views of intact E5 hindbrain roof plates (left) and higher magnification views of regions electroporated (arrows) at E4 (A-F) or E3 (G,H) with matching GFP expression (right). (A,B) Control GFP electroporation does not disrupt the normal distribution of cyp26C1, which is excluded from the most medial roof plate epithelium and is highest at the boundary with the rhombic lip. (C,D) Both chairy2-IRES GFP construct (C,D) and chairy2ΔWRPW-IRES GFP deletion constructs (E,F) induce a non-autonomous downregulation of cyp26c1. (G,H) Overexpression of cdelta1 results in a regionally autonomous upregulation of cyp26C1. Scale bars: 600 μm in A,C,E,G; 300 μm in B,D,F,H.
We next examined whether boundary signalling affects the mRNA expression of transthyretin (ttr), which encodes a thyroid hormone binding protein. ttr is expressed in islands of non-neural roof plate and, like cyp26C1, is excluded from the central domain of the roof plate (Fig. 7A). However, unlike cyp26C1, ttr is absent from the roof plate margins adjacent to the gdf7-positive boundaries and from the boundaries themselves. Control targeted electroporation of the roof plate/neuroepithelium boundary with either pCAβ-eGFPm5 (Fig. 7B) or RCAS-RFP plasmid (not shown) has no effect on ttr expression. However, overexpression of full-length chairy2 (Fig. 7C,D; n=3/5) or chairy2WRPW (Fig. 7E,F; n=3/4) results in a non-autonomous loss of ttr that is mostly accompanied by a downregulation of gdf7 (Fig. 7D,F). By contrast, overexpression of cdelta1 results in a localised downregulation of ttr (Fig. 7G,H; n=8/11), which is consistent with the cell-autonomous suppression of a putative choroid plexus fate. We cannot exclude that the concomitant induction of an ectopic gdf7-positive domain may also contribute to the suppression of ttr.
Fig. 7.
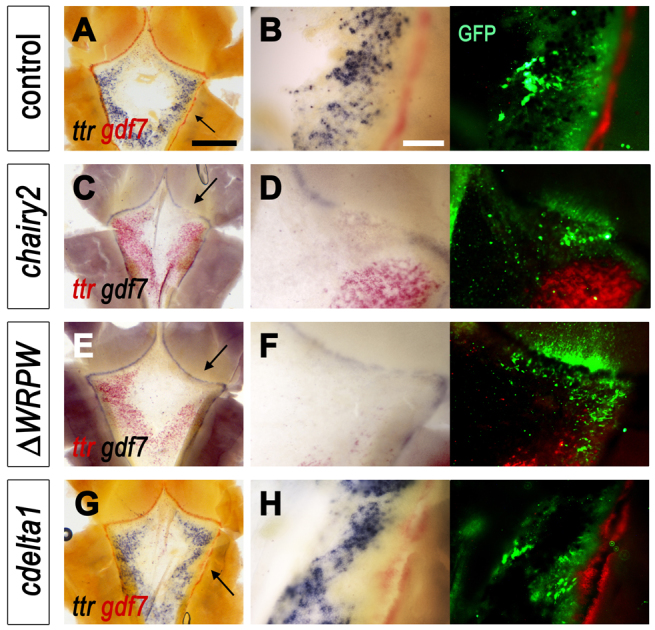
Boundary signals regulate roof plate transthyretin (ttr) expression. (A-H) ttr and gdf7 in low magnification views of the intact fourth ventricle roof plate at E5 (left) with higher magnification of regions electroporated (arrows) at E4 (C-F) or E3 (A,B,G,H). Matching fluorescence images (right) show GFP and red fluorescent in situ label for either ttr (D,F) or gdf7 (B,H). (A,B) Normal ttr expression at the margins of the roof plate and distant to the gdf7-positive boundary is unaffected by control overexpression of GFP (B). (C-F) Both the full-length chairy2 (chairy2-IRES GFP) construct (C,D) and dominant-negative chairy2ΔWRPW-IRES-GFP deletion construct (E,F) cause a non-autonomous downregulation of ttr. (G) Overexpressing cdelta1 at E3 results in a local downregulation of ttr at E5. (H) At higher magnification, the suppression of ttr corresponds to the induction of ectopic gdf7 (red) in the roof plate. Scale bars: 600 μm in A,C,E,G; 300 μm in B,D,F,H.
DISCUSSION
In this study, we show that the signalling properties of the hindbrain roof plate are invested in the boundary between the roof plate compartment and the neuroepithelium. This boundary organiser is maintained by upregulated chairy2 expression, which is downstream of a Delta-Notch interaction. Signals from the roof plate/neuroepithelial boundary both dynamically maintain an atoh1-positive rhombic lip and pattern the choroid plexus (Fig. 8). This study re-evaluates the nature of the CNS roof plate organiser and reveals its potential to coordinately regulate the development of the adjacent neural and non-neural epithelia. Our observations offer a model for the synchronised development of neural and non-neural tissues in ventricular regions of the CNS.
Fig. 8.
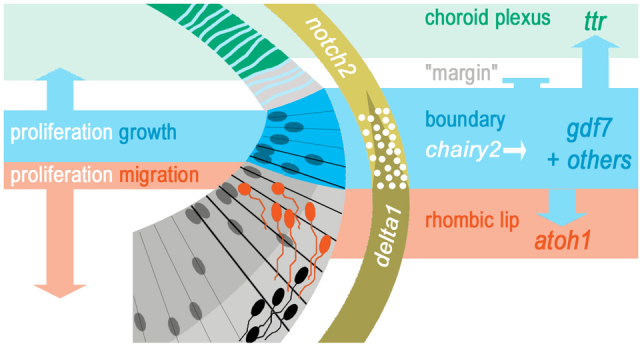
Dynamic interactions at the roof plate boundary organiser. Proposed signalling relationships between the gdf7-positive roof plate boundary (blue), the presumptive ttr-positive choroid plexus (green) and the atoh1-positive rhombic lip (red).
A roof plate boundary/localised organiser signals to both the hindbrain neuroepithelium and the roof plate epithelium
Previous studies of the development of the choroid plexus have pointed to the importance of lineage in determining its cellular fate (Currle et al., 2005; Hunter and Dymecki, 2007) and self-organisation, via endogenous Shh production, in regulating its expansion (Huang et al., 2009) and vascularisation (Nielsen and Dymecki, 2010). These results suggest that choroid plexus specification and growth are largely autonomous of events within the neural tube. This corresponds with observations that a choroid plexus lineage is established early in the regional specification of the neuraxis (Thomas and Dziadek, 1993) and that this establishment is independent of influences from head mesenchyme (Wilting and Christ, 1989). Our results show that these regional and lineage-related mechanisms of choroid plexus specification are mediated by local interactions between neural tissue and roof plate.
Signals from the roof plate boundary are required for the expression of choroid plexus genes and regulate the precision of their boundaries of expression. Focal loss of boundary organiser activity by electroporation of chairy2 constructs leads to acute, non-autonomous changes in roof plate epithelial ttr and cyp26C1 expression. Conversely, induction of ectopic roof plate boundary via cdelta1 expression results in cell-autonomous upregulation of cyp26C1 and a downregulation of ttr expression, reflective of the endogenous pattern of expression of these genes at the roof plate boundary and its margin. In particular, boundary organiser activity reserves a ttr-negative roof plate ‘margin’ (Fig. 8) that may be significant for the establishment of a secondary, lateral progenitor zone within the roof plate later in development. Proliferation in this marginal region drives late growth of the choroid plexus and is regulated by Shh produced by the more medial choroid plexus epithelium (Huang et al., 2009).
In addition to organising gene expression and expression boundaries, the boundary organiser may play an important role in the growth of the roof plate. The observation that Notch activation can drive proliferation in all roof plate epithelial cells (Hunter and Dymecki, 2007) prompts a model where the roof plate/neuroepithelium boundary, which we show here to be the site of Delta-Notch interactions, might be the predominant, if not exclusive, source of choroid plexus epithelial cells. Correspondingly, molecular fate maps have shown that all but a small medial domain in the fourth ventricle roof plate is derived from cells that have either expressed gdf7 or are derived from gdf7-positive precursors (Hunter and Dymecki, 2007). However, although, by inference, this establishes the spatial dynamics of cells that contribute fourth ventricle choroid plexus epithelium, the role of Gdf7 signalling per se in regulating this growth is unclear. Indeed, in the mouse telencephalon, Gdf7 ‘lineage’ is not predictive of choroid plexus epithelium fate, as the large posterior region of the choroid plexus epithelium does not contain cells of Gdf7 lineage. Many other diffusible factors are expressed at the roof plate/neuroepithelium boundary at these stages, including proteins that may pattern blood vessel differentiation and ingrowth (L. Wilson, D. Chambers and R.J.T.W., unpublished) that are important components of choroid plexus development (Dohrmann and Herdson, 1970). Identification of these signalling molecules will be an important goal for future studies. However, regardless of putative signals, the attenuated development of choroid plexus epithelium downstream of disrupted Notch signalling in zebrafish (Bill et al., 2008; García-Lecea et al., 2008) points towards the existence of a conserved, Notch-regulated mechanism as the central motif in a bi-directionally signalling roof plate boundary organiser.
Tissue interactions, Notch signalling and chairy2 maintain the roof plate boundary organiser in dynamic equilibrium
The roof plate boundary organiser is maintained by both Notch activation and chairy2 upregulation in the roof plate. Previous studies of the maintenance of boundary-localised organisers in mouse and zebrafish have demonstrated a role for Hes genes (Baek et al., 2006; Geling et al., 2003; Geling et al., 2004; Hirata et al., 2001; Ninkovic et al., 2005); however, this has generally been presumed to be independent of signalling through Notch (Geling et al., 2004; Kageyama et al., 2007). By contrast, we show that the expression of cdelta1 is both necessary and sufficient for chairy2 expression at the interface between neural and non-neural epithelia, and for the function of the organiser. Thus, when cdelta1 is overexpressed in the roof plate, both ectopic chairy2 and gdf7 are induced. Blocking Delta signalling using a dominant-negative ligand disrupts boundary maintenance and downregulates chairy2 expression. This effect is duplicated by expressing both chairy2 shRNA and dominant-negative chairy2 constructs, while the induction of an organiser by ectopic cdelta1 is suppressed in the absence of chairy2.
Although these results suggest that organiser function is downstream of Hes proteins, overexpression of chairy2 is not sufficient to induce an ectopic organiser. Conversely, our full-length construct acts as a dominant-negative, downregulating both gdf7 and atoh1. This is likely to be an indirect consequence of the demonstrable loss of cdelta1 in neural epithelium, consistent with evidence that high levels of Hes1 lead to a cell-autonomous reduction in delta expression in neural precursors (Cui, 2005) by disrupting the intracellular dynamics of protein concentration (Shimojo et al., 2008). Direct or indirect experimental downregulation of cdelta1 in neural cells thus leads to a loss of Notch activation in the roof plate and hence a lack of maintenance of the boundary organiser. Although chairy2 is evidently permissive for Notch signalling and organiser induction, it does not induce ectopic gdf7 expression in the roof plate epithelium. This raises the possibility that the induction of organiser gene expression depends on either one of a number of Hes-independent Notch pathways (Pierfelice et al., 2011) or a Notch-independent cell-cell interaction.
Our results demonstrate such a role of Delta-Notch signalling in maintenance of the roof plate boundary organiser but leave unresolved the issue of how this boundary is established. As with other boundary organisers, the asymmetric distribution of delta is of crucial importance for determining which roof plate cells express gdf7 and undergo enhanced Notch-dependent proliferation (Hunter and Dymecki, 2007). Hes signalling may also enforce a lineage segregation through cell fate allocation, as demonstrated in the Hes1/Hes3/Hes5 triple knockout mouse (Imayoshi et al., 2008). However, the maintenance of a prominent, if reduced, fourth ventricle roof plate in this mutant (Baek et al., 2006) also suggests the initial establishment of a delta1-free midline compartment does not, unlike other boundary-organisers, depend on Hes function. Thus, although elevated Hes expression is characteristic of all CNS organisers, this may disguise a greater heterogeneity in the mechanisms that establish delta-free compartments at boundaries.
The significance of the roof plate boundary-organiser
The identification of Delta/Notch signalling across a compartment boundary as a central organising principle of fourth ventricle roof plate patterning extends the more general model of vertebrate neural organising centres as systems that rely explicitly on activated Notch signalling for their maintenance (Tossell et al., 2011; Zeltser et al., 2001), in a manner analogous to the Drosophila wing imaginal disc (Diaz-Benjumea and Cohen, 1995; Doherty et al., 1996; Rulifson and Blair, 1995). Taken together, the demarcation of all CNS organisers by high and persistent Hes1 expression (Baek et al., 2006) suggests a striking conservation of the use of Notch signalling to maintain boundary-localised organisers (de Celis and García-Bellido, 1994; Tossell et al., 2011), through either organising cell fate allocation (Imayoshi et al., 2008), suppressing cell differentiation (Kageyama et al., 2008), inducing boundary properties (this study) or a combination of all three. For the dorsal midline, Notch activation seems, in addition, to induce a proliferative response in roof plate cells (Hunter and Dymecki, 2007), but only at sites of ventricle formation. If, indeed, the same boundary mechanisms operate throughout the narrow, non-proliferative roof plate compartment of spinal cord (supplementary material Fig. S2) and midbrain, then the modulation of a mitogenic response to Notch activation may be the key to understanding the as yet undetermined regional factors controlling ventricle formation (Thomas and Dziadek, 1993).
The dynamic maintenance of the roof plate organiser corresponds with our observations that an atoh1-positive rhombic lip is acutely dependent on the integrity of the roof plate boundary. This is consistent with the idea that the rhombic lip is not a precursor pool allocated by dorsoventral coordinates (Briscoe and Ericson, 2001), but a zone of active induction of migratory neural derivatives through local tissue interactions. This corresponds with fate-mapping data that suggests the production of rhombic lip derivatives are generated by neuroepithelial cells in contact with the roof plate (Wingate and Hatten, 1999) and are continuously replenished (Machold and Fishell, 2005). This exquisite equilibrium between signalling, proliferation, specification and migration at the roof plate boundary may account for apparent sensitivity of dorsal hindbrain to environmental insult during human foetal development (Lammer and Armstrong, 1992). Most significantly, this equilibrium also ensures that the establishment of the choroid plexus, which is increasingly recognised as an important organiser of brain development (Huang et al., 2010; Lehtinen et al., 2011), is intimately linked to the dorsal neural tube through a shared boundary organiser.
Supplementary Material
Acknowledgements
We thank Domingos Henrique and David Ish-Horowitz for expression constructs, and Alessio Delogu for his help with cloning strategies.
Footnotes
Funding
This work was supported by an MRC studentship to E.R.B., by a grant from the Wellcome Trust, and by grants from the BBSRC to R.J.T.W. and to Helen Sang (Roslin Institute/GFP-tg eggs). Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.082255/-/DC1
References
- Alder J., Cho N. K., Hatten M. E. (1996). Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17, 389-399 [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R., Cayuso J., Okubo T., Takada S., Martí E. (2008). Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development 135, 237-247 [DOI] [PubMed] [Google Scholar]
- Baek J. H., Hatakeyama J., Sakamoto S., Ohtsuka T., Kageyama R. (2006). Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development 133, 2467-2476 [DOI] [PubMed] [Google Scholar]
- Bill B. R., Balciunas D., McCarra J. A., Young E. D., Xiong T., Spahn A. M., Garcia-Lecea M., Korzh V., Ekker S. C., Schimmenti L. A. (2008). Development and Notch signaling requirements of the zebrafish choroid plexus. PLoS ONE 3, e3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Ericson J. (2001). Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 11, 43-49 [DOI] [PubMed] [Google Scholar]
- Chesnutt C., Burrus L. W., Brown A. M., Niswander L. (2004). Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. 274, 334-347 [DOI] [PubMed] [Google Scholar]
- Chizhikov V. V., Millen K. J. (2004). Mechanisms of roof plate formation in the vertebrate CNS. Nat. Rev. Neurosci. 5, 808-812 [DOI] [PubMed] [Google Scholar]
- Chizhikov V. V., Millen K. J. (2005). Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev. Biol. 277, 287-295 [DOI] [PubMed] [Google Scholar]
- Chizhikov V. V., Lindgren A. G., Currle D. S., Rose M. F., Monuki E. S., Millen K. J. (2006). The roof plate regulates cerebellar cell-type specification and proliferation. Development 133, 2793-2804 [DOI] [PubMed] [Google Scholar]
- Cui Y. (2005). Hairy is a cell context signal controlling Notch activity. Dev. Growth Differ. 47, 609-625 [DOI] [PubMed] [Google Scholar]
- Currle D. S., Cheng X., Hsu C. M., Monuki E. S. (2005). Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development 132, 3549-3559 [DOI] [PubMed] [Google Scholar]
- de Celis J. F., García-Bellido A. (1994). Roles of the Notch gene in Drosophila wing morphogenesis. Mech. Dev. 46, 109-122 [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M. (1995). Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121, 4215-4225 [DOI] [PubMed] [Google Scholar]
- Doherty D., Feger G., Younger-Shepherd S., Jan L. Y., Jan Y. N. (1996). Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10, 421-434 [DOI] [PubMed] [Google Scholar]
- Dohrmann G. J., Herdson P. B. (1970). The choroid plexus of the mouse: a macroscopic, microscopic and fine structural study. Z. Mikrosk. Anat. Forsch. 82, 508-522 [PubMed] [Google Scholar]
- Duan W., Achen M. G., Richardson S. J., Lawrence M. C., Wettenhall R. E., Jaworowski A., Schreiber G. (1991). Isolation, characterization, cDNA cloning and gene expression of an avian transthyretin. Implications for the evolution of structure and function of transthyretin in vertebrates. Eur. J. Biochem. 200, 679-687 [DOI] [PubMed] [Google Scholar]
- García-Lecea M., Kondrychyn I., Fong S. H., Ye Z. R., Korzh V. (2008). In vivo analysis of choroid plexus morphogenesis in zebrafish. PLoS ONE 3, e3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling A., Itoh M., Tallafuss A., Chapouton P., Tannhäuser B., Kuwada J. Y., Chitnis A. B., Bally-Cuif L. (2003). bHLH transcription factor Her5 links patterning to regional inhibition of neurogenesis at the midbrain-hindbrain boundary. Development 130, 1591-1604 [DOI] [PubMed] [Google Scholar]
- Geling A., Plessy C., Rastegar S., Strähle U., Bally-Cuif L. (2004). Her5 acts as a prepattern factor that blocks neurogenin1 and coe2 expression upstream of Notch to inhibit neurogenesis at the midbrain-hindbrain boundary. Development 131, 1993-2006 [DOI] [PubMed] [Google Scholar]
- Guinazu M. F., Chambers D., Lumsden A., Kiecker C. (2007). Tissue interactions in the developing chick diencephalon. Neural Dev. 2, 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D., Adam J., Myat A., Chitnis A., Lewis J., Ish-Horowicz D. (1995). Expression of a Delta homologue in prospective neurons in the chick. Nature 375, 787-790 [DOI] [PubMed] [Google Scholar]
- Henrique D., Hirsinger E., Adam J., Le Roux I., Pourquié O., Ish-Horowicz D., Lewis J. (1997). Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661-670 [DOI] [PubMed] [Google Scholar]
- Hicks C., Johnston S. H., diSibio G., Collazo A., Vogt T. F., Weinmaster G. (2000). Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat. Cell Biol. 2, 515-520 [DOI] [PubMed] [Google Scholar]
- Hirata H., Tomita K., Bessho Y., Kageyama R. (2001). Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain. EMBO J. 20, 4454-4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Ketova T., Fleming J. T., Wang H., Dey S. K., Litingtung Y., Chiang C. (2009). Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 136, 2535-2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Liu J., Ketova T., Fleming J. T., Grover V. K., Cooper M. K., Litingtung Y., Chiang C. (2010). Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl. Acad. Sci. USA 107, 8422-8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. L., Dymecki S. M. (2007). Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development 134, 3449-3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Shimogori T., Ohtsuka T., Kageyama R. (2008). Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development 135, 2531-2541 [DOI] [PubMed] [Google Scholar]
- Irving C., Mason I. (1999). Regeneration of isthmic tissue is the result of a specific and direct interaction between rhombomere 1 and midbrain. Development 126, 3981-3989 [DOI] [PubMed] [Google Scholar]
- Johansson P. A., Dziegielewska K. M., Liddelow S. A., Saunders N. R. (2008). The blood-CSF barrier explained: when development is not immaturity. Bioessays 30, 237-248 [DOI] [PubMed] [Google Scholar]
- Jouve C., Palmeirim I., Henrique D., Beckers J., Gossler A., Ish-Horowicz D., Pourquié O. (2000). Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development 127, 1421-1429 [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohtsuka T., Kobayashi T. (2007). The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development 134, 1243-1251 [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohtsuka T., Shimojo H., Imayoshi I. (2008). Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 11, 1247-1251 [DOI] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. (2004). Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 7, 1242-1249 [DOI] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. (2005). Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 6, 553-564 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V., Ben-Arie N. (2006). A novel role for the choroid plexus in BMP-mediated inhibition of differentiation of cerebellar neural progenitors. Mech. Dev. 123, 67-75 [DOI] [PubMed] [Google Scholar]
- Lammer E., Armstrong D. (1992). Malformations of hindbrain structures among humans exposed to isoretinin (13-cis-retinoic acid) during early pregnancy. In Retinoids in Normal Development and Teratogenesis (ed. Morriss-Kay G.), pp. 281-295 Oxford: Oxford University Press; [Google Scholar]
- Landsberg R. L., Awatramani R. B., Hunter N. L., Farago A. F., DiPietrantonio H. J., Rodriguez C. I., Dymecki S. M. (2005). Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron 48, 933-947 [DOI] [PubMed] [Google Scholar]
- Langenberg T., Brand M. (2005). Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain-hindbrain boundary. Development 132, 3209-3216 [DOI] [PubMed] [Google Scholar]
- Larsen C. W., Zeltser L. M., Lumsden A. (2001). Boundary formation and compartition in the avian diencephalon. J. Neurosci. 21, 4699-4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux I., Lewis J., Ish-Horowicz D. (2003). Notch activity is required to maintain floorplate identity and to control neurogenesis in the chick hindbrain and spinal cord. Int. J. Dev. Biol. 47, 263-272 [PubMed] [Google Scholar]
- Lee K. J., Mendelsohn M., Jessell T. M. (1998). Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 12, 3394-3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J., Dietrich P., Jessell T. M. (2000). Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature 403, 734-740 [DOI] [PubMed] [Google Scholar]
- Lehtinen M. K., Zappaterra M. W., Chen X., Yang Y. J., Hill A. D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P., et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K. F., Jr, Tremml G., Jessell T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127-138 [DOI] [PubMed] [Google Scholar]
- Louvi A., Wassef M. (2000). Ectopic engrailed 1 expression in the dorsal midline causes cell death, abnormal differentiation of circumventricular organs and errors in axonal pathfinding. Development 127, 4061-4071 [DOI] [PubMed] [Google Scholar]
- Machold R., Fishell G. (2005). Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48, 17-24 [DOI] [PubMed] [Google Scholar]
- McGrew M. J., Sherman A., Lillico S. G., Ellard F. M., Radcliffe P. A., Gilhooley H. J., Mitrophanous K. A., Cambray N., Wilson V., Sang H. (2008). Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development 135, 2289-2299 [DOI] [PubMed] [Google Scholar]
- Muroyama Y., Fujihara M., Ikeya M., Kondoh H., Takada S. (2002). Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat A., Henrique D., Ish-Horowicz D., Lewis J. (1996). A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev. Biol. 174, 233-247 [DOI] [PubMed] [Google Scholar]
- Nielsen C. M., Dymecki S. M. (2010). Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev. Biol. 340, 430-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J., Tallafuss A., Leucht C., Topczewski J., Tannhäuser B., Solnica-Krezel L., Bally-Cuif L. (2005). Inhibition of neurogenesis at the zebrafish midbrain-hindbrain boundary by the combined and dose-dependent activity of a new hairy/E(spl) gene pair. Development 132, 75-88 [DOI] [PubMed] [Google Scholar]
- Ohtsuka T., Ishibashi M., Gradwohl G., Nakanishi S., Guillemot F., Kageyama R. (1999). Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 18, 2196-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierfelice T., Alberi L., Gaiano N. (2011). Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840-855 [DOI] [PubMed] [Google Scholar]
- Redzic Z. B., Preston J. E., Duncan J. A., Chodobski A., Szmydynger-Chodobska J. (2005). The choroid plexus-cerebrospinal fluid system: from development to aging. Curr. Top. Dev. Biol. 71, 1-52 [DOI] [PubMed] [Google Scholar]
- Reijntjes S., Gale E., Maden M. (2004). Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev. Dyn. 230, 509-517 [DOI] [PubMed] [Google Scholar]
- Rose M. F., Ren J., Ahmad K. A., Chao H. T., Klisch T. J., Flora A., Greer J. J., Zoghbi H. Y. (2009). Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron 64, 341-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson E. J., Blair S. S. (1995). Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121, 2813-2824 [DOI] [PubMed] [Google Scholar]
- Shimojo H., Ohtsuka T., Kageyama R. (2008). Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52-64 [DOI] [PubMed] [Google Scholar]
- Thomas T., Dziadek M. (1993). Capacity to form choroid plexus-like cells in vitro is restricted to specific regions of the mouse neural ectoderm. Development 117, 253-262 [DOI] [PubMed] [Google Scholar]
- Timmer J. R., Wang C., Niswander L. (2002). BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459-2472 [DOI] [PubMed] [Google Scholar]
- Tossell K., Kiecker C., Wizenmann A., Lang E., Irving C. (2011). Notch signalling stabilises boundary formation at the midbrain-hindbrain organiser. Development 138, 3745-3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang V. Y., Rose M. F., Zoghbi H. Y. (2005). Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48, 31-43 [DOI] [PubMed] [Google Scholar]
- Wassef M., Joyner A. L. (1997). Early mesencephalon/metencephalon patterning and development of the cerebellum. Perspect. Dev. Neurobiol. 5, 3-16 [PubMed] [Google Scholar]
- Wilson L. J., Wingate R. J. (2006). Temporal identity transition in the avian cerebellar rhombic lip. Dev. Biol. 297, 508-521 [DOI] [PubMed] [Google Scholar]
- Wilson L. J., Myat A., Sharma A., Maden M., Wingate R. J. (2007). Retinoic acid is a potential dorsalising signal in the late embryonic chick hindbrain. BMC Dev. Biol. 7, 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting J., Christ B. (1989). An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 255, 487-494 [DOI] [PubMed] [Google Scholar]
- Wingate R. J. T., Hatten M. E. (1999). The role of the rhombic lip in avian cerebellum development. Development 126, 4395-4404 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., McCaffery P., Dräger U. C. (1996). Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Brain Res. Dev. Brain Res. 93, 182-190 [DOI] [PubMed] [Google Scholar]
- Zeltser L. M., Larsen C. W., Lumsden A. (2001). A new developmental compartment in the forebrain regulated by Lunatic fringe. Nat. Neurosci. 4, 683-684 [DOI] [PubMed] [Google Scholar]
- Zervas M., Millet S., Ahn S., Joyner A. L. (2004). Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43, 345-357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


