Abstract
Neural and behavioral responses after peripheral immune challenge have been observed in numerous studies. The majority of these studies have utilized relatively high doses of lipopolysaccharide (LPS) as the immune stimulant. Little attention has been given to the effects of LPS dose ranges that simulate low grade-inflammation. The current studies were designed to characterize neural and behavioral responses following low-dose LPS stimulation. Results show burrowing and open field activity was significantly impaired following a single i.p. injection of 10, but not 1, µg/kg of LPS. In addition, following repeated 1 µg/kg LPS administration for 10 days, animals showed the progressive development of motor deficits over time. To correlate behavior with CNS activity, cFos activation was determined in the paraventricular nucleus, nucleus of the solitary tract, central amygdaloid nucleus, and ventrolateral medulla. Data revealed there was a dose-dependent activation in all brain areas examined, but only the PVN showed significant activation by low-dose LPS. Additionally, animals that received 1 µg/kg of LPS for 8 days had PVN cFos activation similar to animals that received a single 10 µg/kg LPS injection. These data demonstrate neural and behavior responses can be induced by low-grade inflammation and chronic exposure to sub-threshold levels of LPS can precipitate significantly heightened neural and behavioral responses.
Keywords: LPS, open field, burrowing, cFos, low-dose, sickness behavior
1. INTRODUCTION
Increasing evidence from clinical studies suggests a strong link between inflammation and the development of numerous psychological disorders, including major depression [1, 2], anxiety disorders [3], and autism [4]. To reveal how inflammation might cause behavioral abnormalities exhibited in these disorders, many studies have used bacterial endotoxin lipopolysaccharide (LPS) as the inflammatory stimulant to induce inflammation. It has been documented that a single intraperitoneal (i.p.) injection of a septic dose of LPS indeed causes depressive-like behaviors [5, 6], increased anxiety [7], and reduced social interaction [8] in experimental animals.
However, such use of septic doses of LPS to mimic the pathogenesis of psychological disorders may lack both face validity and construct validity. Human psychological disorders are not triggered by acute septic shock and levels of inflammatory cytokines reported in patients with psychological disorders are often higher than normal [9–11], but orders of magnitude lower than those induced by septic shock [12–16]. The levels of inflammatory cytokines in patients with various psychological disorders are actually more in line with those in patients with low-grade inflammation [17, 18]. Whether low-grade inflammation induces neural and behavioral responses relevant to the pathogenesis of psychological disorders has not been investigated.
In this study, we induced low-grade inflammation by injecting low doses of LPS intraperitoneally. The results demonstrate low-grade inflammation causes specific neuronal activation patterns in the brain that correspond to alterations in behavior.
2. METHODS
2.1. Subjects
Subjects were 8 to 13 week-old male inbreed FVB mice purchased from Charles River Laboratories (Wilmington, MA). Animals were allowed to acclimate in the animal facility for ~1 week prior to experimental procedures. Mice were group housed 5/cage in standard polycarbonate mouse cages and maintained on a 12 h light/dark cycle with lights being turned on at 0600 in an AAALAC (American Association of Accreditation of Laboratory Animal Care) facility. Food and water was available ad libitum unless experimental manipulations were being conducted. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and the experiments were carried out in accordance with a protocol approved by the Institutional Laboratory Animal Care and Use Committee (ILACUC) at The Ohio State University.
2.2. Specific Experimental Designs
Experiment 1: To assess CNS cFos activation patterns associated with low-dose endotoxin-induced sickness behavior, four groups of mice were given single i.p. injections of LPS (0, 1, 10, 500 µg/kg). Three hours following LPS injections, brains were removed, sectioned, and stained to determine the mean amount of cFos labeling in the paraventricular nucleus (PVN), nucleus of the solitary tract (NTS), central amygdaloid nucleus (CeA), and ventrolateral medulla (VLM). Three-to-four animals per group were used. See Fig. 1 for a graphical representation of data obtained.
Experiment 2: To determine if a single i.p. injection of 1 µg/kg of LPS was able to induce alterations in open field activity, two separate groups of animals were given either PBS (n=8) or 1 µg/kg of LPS (n=9) 2 hrs prior to evaluation in open field. See Fig. 2 A–C for a graphical representation of data obtained.
Experiment 3: To evaluate the effect of a higher dose of LPS, two separate groups of animals were given either a single i.p. injection of PBS (n=8) or 10 µg/kg of LPS (n=10) 2 hrs prior to evaluation in open field. See Fig. 2D–F for a graphical representation of data obtained.
Experiment 4: To examine the effects of low-dose LPS administration in additional behavioral testing paradigms, we used a burrowing test that has been shown to be extremely sensitive to low-dose LPS regimens. Two separate groups of animals were given either a single i.p. injection of PBS (n=5) or 1 µg/kg of LPS (n=5) 2 hrs prior to the evaluation of burrowing behavior. See Fig. 3A for a graphical representation of data obtained.
Experiment 5: To determine if 10 µg/kg of LPS was sufficient to cause a reduction in burrowing activity, animals were given a single i.p. injection of LPS and 2 hrs later tested in the burrowing apparatus. Additionally, we sought to evaluate if the hypothesized effect would be transient or would carry over till the 24 hrs post-injection assessment time-point. On day 1, two separate groups of animals were given either a single i.p. injection of PBS (n=7) or 10 µg/kg of LPS (n=9) 2 hrs prior to the evaluation of burrowing behavior. The same experimental animals were evaluated 24 hrs later without additional injections. See Fig. 3B for a graphical representation of data obtained.
Experiment 6: To evaluate the effects of repeated 1 µg/kg of LPS administration, animals were given a single dose of 1 µg/kg of LPS on day one then tested for burrowing behavior 2 hrs post-injection. The following day (i.e., 24 hrs later) all animals were given 1 µg/kg of LPS and tested for burrowing activity. This design consisted of 2 groups: one that received PBS (n=9) on day 1 and 1 µg/kg of LPS on day 2 (PBS-LPS), with the other group receiving 1 µg/kg of LPS (n=10) on both days 1 and 2 (LPS-LPS). See Fig. 4 for a graphical representation of data obtained.
Experiment 7: To determine the effects of repeated 1 µg/kg of LPS injections on open field activity, the same design used in Experiment 6 was used in Experiment 7. The number of animals used in the PBS-LPS and LPS-LPS groups were, n=9 and 11 respectively. See Fig. 5 for a graphical representation of data obtained.
Experiment 8: In order to determine the effects of chronic 1 µg/kg of LPS exposure on sickness behavior, all animals (n=5) were given i.p. injections of 1 µg/kg of LPS each day for 10 consecutive days and changes in behavior was compared across days. Two hrs following LPS injections on days 1, 2, 4, 7, and 10, animals were tested for sickness behavior in the open field. See Fig. 6 for a graphical representation of data obtained.
Experiment 9: To assess the effects of repeated 1 µg/kg of LPS injections on cFos expression in the PVN animals were given 8 consecutive days of either PBS (n=5) or 1 µg/kg of LPS (n=4). Three hrs following the last injections, animals brains were removed, sectioned, and stained to determine cFos expression in the PVN. See Fig. 4 for a graphical representation of data obtained.
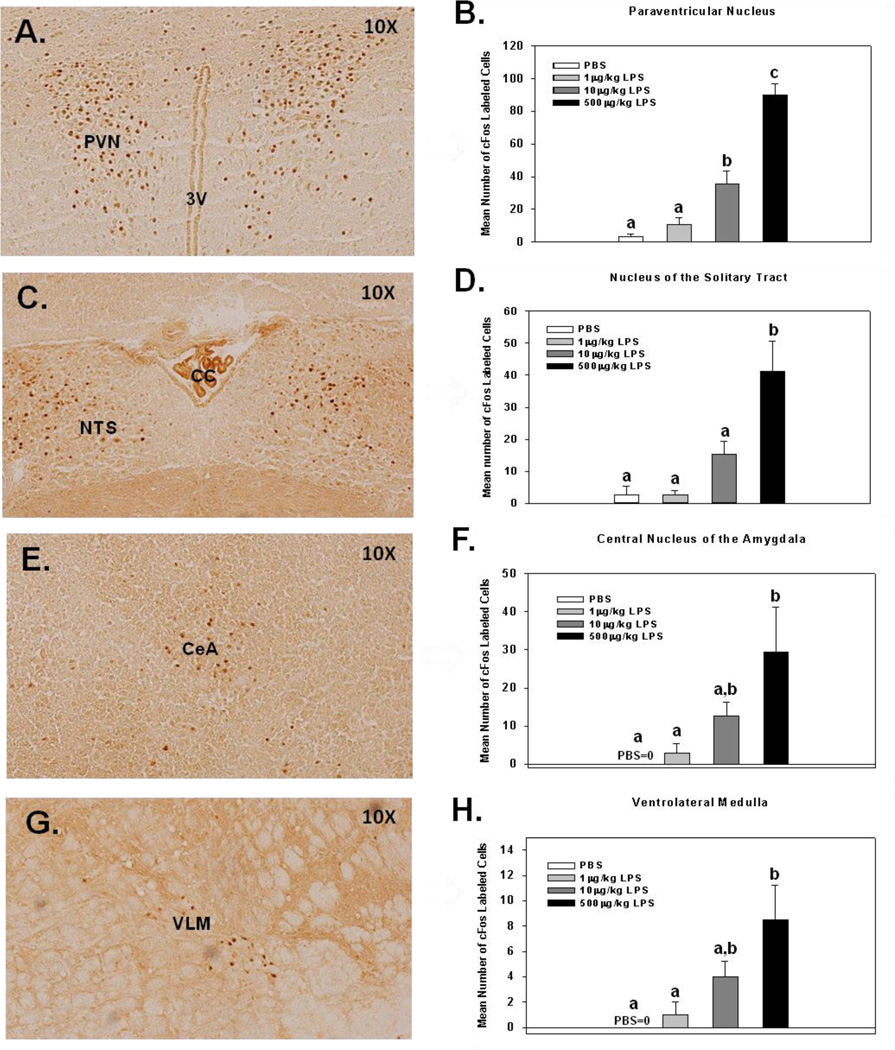
Fig. 1.
Representative pictures of cFos activation in the paraventricular nucleus (A), nucleus of the solitary tract (C), central nucleus of the amygdala (E), and ventrolateral medulla (G), 3 hrs following i.p. injection of 500 µg/kg of LPS and corresponding bar graphs for mean number of cFos labeled cells 3 hrs following PBS, 1, 10, or 500 µg/kg of LPS (B, D, F, and H). Different letters represent significant differences among groups (p’s≤0.05). Bars represent group means ± SEM.
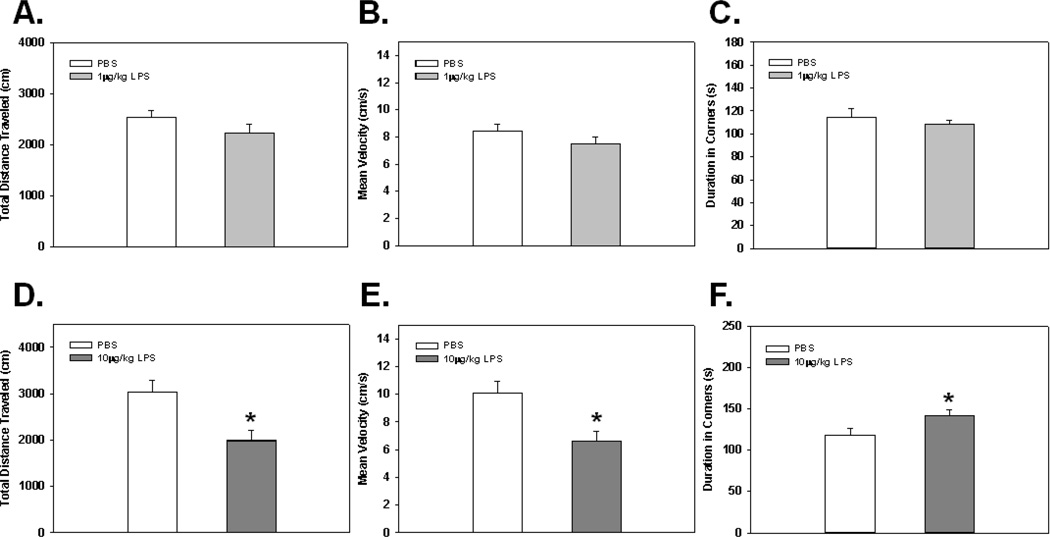
Fig. 2.
Total distance traveled, mean velocity, and duration spent in corners in the open field 2 hrs post-administration of 1 µg/kg (A Fig. 2C) and 10 µg/kg (D–E) of LPS. * Indicates a significant difference compared to control animals (p’s≤0.05). Bars represent group means ± SEM.
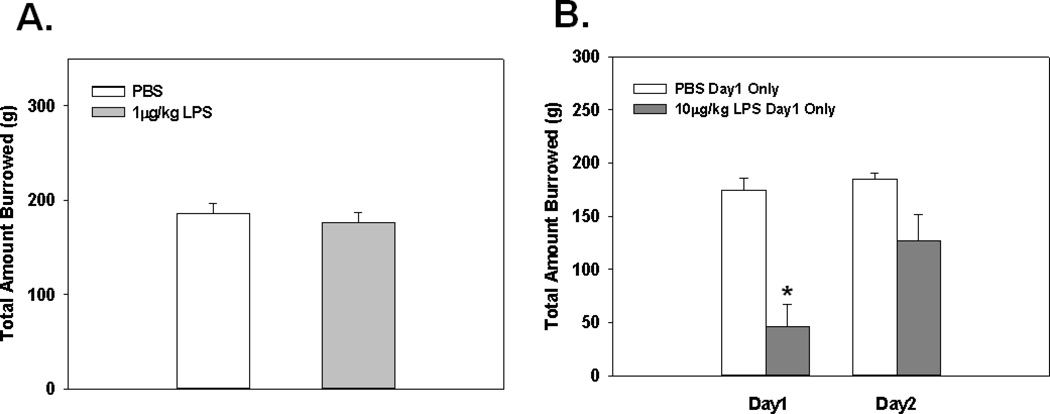
Fig. 3.
Mean total amount of food burrowed from tubes 2 hrs following a single 1µg/kg LPS administration (A). Mean total amount food burrowed 2 and 24 hrs following a single injection of 10 µg/kg of LPS on day1 only (B). * Indicates a significant difference compared to control animals (p≤0.0005). Bars represent group means ± SEM.
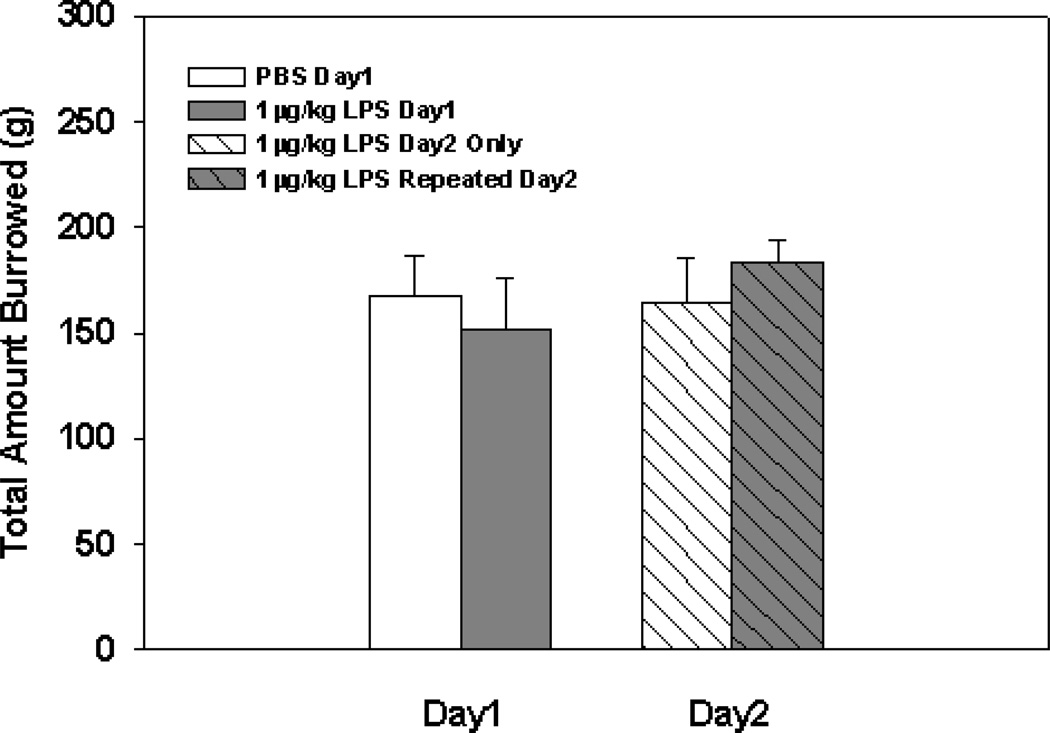
Fig. 4.
Mean total amount of food burrowed 2 hrs following injection with 1µg/kg of LPS or PBS on day1, followed by a second injection of 1 µg/kg of LPS to all groups 2 hrs before day2’s assessment. Bars represent group means ± SEM.
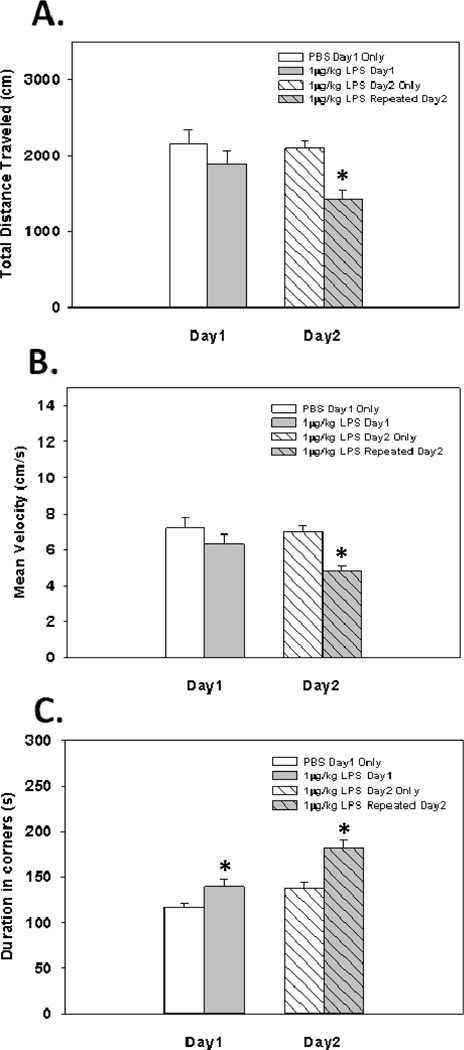
Fig. 5.
Total distance traveled, mean velocity, and duration spent in corners in the open field 2 hrs following injection with 1 µg/kg of LPS or PBS on day1, followed by a second injection of 1 µg/kg of LPS to all groups 2 hrs before day2’s assessment * Indicates a significant difference compared to control animals (p’s≤0.05). Bars represent group means ± SEM.
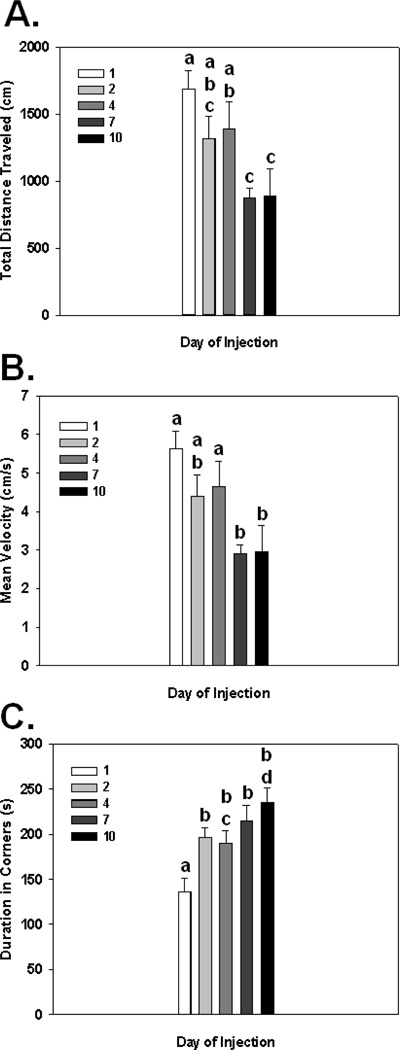
Fig. 6.
Total distance traveled (A), mean velocity (B), and duration spent in corners (C) in the open field following repeated injections of 1 µg/kg of LPS for 10 days. Different letters represent significant differences among groups (p’s≤0.05). Bars represent group means ± SEM.
2.3. Open Field
Open field apparatus were used to determine if low-dose LPS administration causes sickness/motor and/or anxiety-like behavioral deficits. The open field is a widely accepted behavioral test that requires no “learning” of novel tasks. We chose the open field as an appropriate test to study these effects because subtle changes that will be induced by very low doses of LPS might be overshadowed by sensitivity issues that could arise from other behavioral tests. Our open field apparatus consisted of a 40×40×25 cm Plexiglas® box that was divided into a 6×6 grid pattern. Subjects were given a single or repeated i.p. injection of LPS (Escherichia coli: 0111:B4; Sigma-Aldrich, St. Louis, MO) or sterile PBS depending on the experimental hypotheses. Two hours following LPS administration, subjects were tested in the open field for 5 min. Experimental subject activity was monitored using an automated digital video recording camera attached to computer containing a video tracking software template (Ethovision 8.0; Noldus, Leesburg, VA). Dependent measures included total distance traveled, mean velocity, duration spent in borders and center, and duration spent in corners (i.e., safest portion of the open field). For data analysis, the open field was divided into three primary zones: center, corners, and outer annulus (corners included).
2.4. Burrowing
To assess additional sickness/motivational behavioral deficits after low-dose LPS administration, an adapted burrowing protocol from Deacon et al. was used [19]. Burrowing is a species-typical task that does not require prior training. Additionally, burrowing is a behavioral test that is proven to be extremely sensitive to low-dose endotoxin exposure [19–21]. Given the low-dose regimens of the current experiments, burrowing is an ideal behavioral test to help reduce sensitivity issues commonly found in other apparatus. Current burrowing experiments consisted of three phases: 1 day of group facilitation, 3 days of individual baseline assessment, and 1 or 2 days of individual testing. On group facilitation days (allows for consistent burrowing among the animals), subjects were housed 5 animals per cage and burrowing tubes were placed in the animal’s home cage at 3 pm. Each burrowing apparatus consisted of a 20×7 cm plastic PVC pipe that had an open end raised 3 cm by machine screws. Additionally, burrowing tubes were filled with 200 g of standard rodent chow that acted as a familiar object that laboratory animals have been shown to burrow reliably [22]. The amount of food displaced from the burrowing tube is a reliable measure of sickness/motivational deficits since animals have to actively enter the tube and move the food to the surrounding area (i.e., area surrounding the apparatus). Following a 2 hr burrowing access time, burrowing tubes were removed and food hoppers returned to the cage. For the 3 individual baseline days, animals were placed individually into cages at 3 pm with burrowing tubes. Two hours later (5 pm), tubes were removed, the contents were weighed, and animals were placed back into their original home cage until the next burrowing assessment took place. Following baseline data acquisition, the mean amount burrowed was calculated from all 3 days, rank ordered, and animals were pseudorandomly assigned to treatment groups according to the mean baseline amount burrowed. This design was implemented to avoid animals that burrowed the most being assigned to the same group. Unreliable burrowers or non-burrowers, albeit uncommon, were removed from the experiments. Two hours prior (1 pm) to the first day of individual testing, subjects were given a single i.p. injection of LPS (1 or 10 µg/kg) or sterile PBS based on their baseline burrowing performance. At 3 pm, burrowing tubes were placed in cages with the individually housed animals. At 5 pm, food remaining in the burrowing tube was weighed and recorded. The amount of food remaining in the tube was then subtracted from 200g (i.e., starting weight of the food) to obtain a measurement of the amount of food displaced. For sensitization experiments, an additional injection of 1 µg/kg of LPS was given 2 hrs prior to the day two burrowing assessment. During testing, food (i.e., rodent chow used for burrowing assessment) was available ad libitum. The burrowing tubes were cleaned between each burrowing session with water to minimize odor cues. Of note, the amount of food consumed by experimental animals was not taken into consideration since food consumed by animals in their inactive phase is negligible.
2.5. Tissue Processing and Preparation
Separate experimental groups of animals were given a single i.p. dose of LPS (0, 1, 10, 500 µg/kg) 3 hrs prior to brain extraction (dose response cFos experiment), or repeated injections of 1 µg/kg of LPS for 8 days followed by brain extraction 3 hrs after the final LPS injection (repeated low-dose LPS experiment). Once brains were removed, they were immediately snap-frozen in ice-cold isopentane for 10 seconds, then stored in −80°C freezer until sectioning and processing. Frozen brains were sectioned on a cryostat to generate 20 µm thick sections for cFos immunohistological analyses of the paraventricular nucleus (PVN), nucleus of the solitary tract (NTS), central nucleus of the amygdale (CeA), and ventrolateral medulla (VLM). Areas of interest were identified using a standard mouse brain atlas and c-fos labeled cells were counted from all the sections collected throughout the entire interested structures. The results are expressed as mean number of cFos cells/section. Coordinates from bregma: PVN= −0.58 to −0.94 mm, VLM= −6.96 to −7.68 mm, CeA= −0.70 to −1.22 mm and NTS= −7.08 to −7.48 mm.
2.6. Immunohistochemistry: cFos
On day 1 of cFos staining, slides containing brain areas of interest were dried for 10 min and then fixed using 4% paraformaldehide for 10 min, rinsed in TBS-T, quenched in 0.3% H2O2 for 15 min, followed by another TBS-T wash. Brain slices were then blocked in 10% sheep serum for 60 min, and incubated in rabbit anti-cFos primary antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. On day 2, brain sections were rinsed in TBST, incubated in goat anti-rabbit secondary antibody (1:500; Vector Labs, Burlingame, CA) for 1 hr, washed in TBS-T, and then incubated for 1 hr in ABC solution (Vector Labs, Burlingame, CA). Following avidin/biotin conjugation, sections were rinsed in 0.1% phosphate buffer and stained with DAB mixture (Vector Labs, Burlingame, CA) for 2–5 min until desired color was achieved. Slides containing tissue sections were then dehydrated with an ascending series of ETOH, cleared, and cover-slipped. Total cFos labeled cells in all dose groups were counted manually on a Nikon e800 (Melville, NY) microscope under 10× magnification.
2.7. Statistical Procedures
Data obtained from behavioral and cFos assessments, from a single time-point, were analyzed by standard one-way ANOVAs, with LPS Treatment as the between subject variable. Experiments involving multiple days or time-points, repeated measures ANOVAs were used where LPS Treatment and Day was used as the between and within subjects variables, respectively. Significant interactions among dependent variables were subjected to Fisher’s PLSD post hoc analyses. An alpha level of p<0.05 was the criterion for rejection of the null hypothesis. Results are reported as treatment means ± SEM.
3. RESULTS
3.1. Central cFos activation following low and high-dose peripheral LPS
Activation of cFos in the PVN, NTS, CeA, and VLM 3 hrs after i.p. injection of varying doses of LPS was evaluated. There were significant main effects of LPS treatment on the mean number of cFos labeled cells in the PVN, NTS, CeA and VLM (F(3,10)=34.64; p<0.0001; F(3,9)=13.06; p<0.001; F(3,9)=4.22; p<0.05; F(3,9)=3.72; p<0.05, respectively; see Fig. 1B, D, F & H). Fisher’s PLSD post hoc analyses for the PVN revealed that a single 10 µg/kg i.p. injection of LPS was sufficient to cause a significant increase in the mean number of cFos labeled cells compared to PBS (p<0.01) and 1 µg/kg LPS (p<0.05) groups. Furthermore, animals receiving a single 500 µg/kg LPS injection had a significant increase in cFos labeled cells compared to all other groups (p's<0.0001). Lastly, there were no significant increases in the mean number of cFos labeled cells following a single 1 µg/kg LPS injection compared to PBS treated animals (p=0.52). When examining group differences in the NTS, post hoc analyses revealed the only group with a statistically significant increase in cFos activation compared to all other groups were animals receiving 500 µg/kg of LPS (p<0.01). Although the 10 µg/kg LPS group did not differ from the 1 µg/kg LPS or PBS groups, there was a trend for an increase (p=0.09; p=0.12, respectively). As for cFos activation in the CeA, post hoc analyses showed that animals receiving 500 µg/kg of LPS had a statistically significant increase in cFos labeled cells compared to animals receiving 1 µg/kg of LPS (p<0.05) or PBS (p<0.05). A trend for a difference between the 500 µg/kg LPS group and the 10 µg/kg LPS group was also apparent (p=0.08). Fisher's post hoc analyses for cFos labeled cells in the VLM showed data similar to that of the CeA where animals receiving 500 µg/kg of LPS had a statistically significant increase in cFos labeled cells compared to animals receiving 1 µg/kg of LPS (p<0.05) or PBS (p<0.05), and a trend for a difference compared to animals receiving 10 µg/kg of LPS (p<0.22). Overall, there was a dose dependent increase in cFos labeling for all brain areas examined. Graphical representations of all post hoc analyses are shown in Figs. 1B, D, F and H.
3.2. Burrowing and open field behavioral effects following a single low-dose LPS administration
Relatively few studies have thoroughly examined the effects of low-level peripheral inflammation on sickness/motivational behaviors. Therefore, initial experiments were designed to validate, and extend the current literature by examining behavioral effects of low-dose LPS administration in two well established, sensitive tests of sickness behavior. Results show that 1 µg/kg of LPS did not alter performance in the open field for the total distance traveled, mean velocity, or duration spent in the corners (see Fig. 2A–C), or in burrowing behavior as measured for the total amount burrowed (see Fig. 3A). Additionally, measures for anxiety-like behavior such as duration spent in the border and the center of the open field were not different among groups (data not shown). However, burrowing behavior and open field activity were significantly reduced in both tests when animals received 10 µg/kg of LPS. These data were reflected by a significant main effect of LPS Treatment (F(1,14)=14.95; p<0.005; see Fig. 3B) and a significant LPS Treatment × Day interaction (F(1,14)=7.80; p<0.05; see Fig. 3B) for the total amount burrowed. Post hoc analyses indicated animals receiving a single 10 µg/kg LPS injection had a significant reduction in the total amount burrowed on day 1 when compared to control animals (p<0.0005), and the significance of this effect was reduced (p=0.06) by day 2 of assessment (i.e., closer to PBS treated animals). When examining open field activity, analyses revealed significant main effects of LPS Treatment for the total distance traveled (F(1,16)=10.09; p<0.01; see Fig. 2D), mean velocity (F(1,16)=10.09; p<0.01; see Fig. 2E), and duration spent in corners (F(1,16)=4.40; p<0.05; see Fig. 2F), indicating animals receiving 10 µg/kg of LPS showed signs of sickness behavior. Measures of anxiety-like behavior showed that LPS-treated animals did not significantly differ from controls for the duration spent in the border or duration spent in the center (F(1,16)=2.73; F(1,16)=2.00; ns; respectively). Taken together, a single 10 µg/kg LPS dose was required to cause sickness behavior alterations in the open field and burrowing tasks, while not causing anxiety-like behavior.
3.3. Burrowing and open field behavioral effects following a 2 day repeated low-dose LPS regimen
Evidence for sustained peripheral inflammation as an underlying mechanism for many behavioral deficits in affective disorders has been noted. To date, studies examining the mechanisms by which affective disorders are induced often use high-dose LPS (e.g., 500 µg/kg) to generate inflammation in rodents, which can lead to exaggerated behavioral alterations. Additionally, using high-dose LPS administration may not be the appropriate model to study sustained inflammatory related disorders, since reported elevations in circulating cytokines in patients are on the picogram scale. Therefore, we sought to determine if repeated low-dose LPS injection over 2 days would have a greater detrimental effect on burrowing and open field behavior compared to single dose LPS administration.
Results show that there were no significant main or interaction effects on the total amount burrowed following repeated 1 µg/kg LPS injections (see Fig. 4). However, upon behavioral examination of animals receiving repeated 1 µg/kg LPS injections in the open field, data revealed main effects of LPS Treatment on the total distance traveled (F(1,18)=6.04; p<0.05; see Fig. 5A), mean velocity (F(1,18)=6.06; p<0.05; see Fig. 5B), and the duration spent in the corners of the open field (F(1,18)=15.52; p<0.001; see Fig. 5C). Furthermore, significant LPS Treatment × Day interaction effects were evident for the total distance traveled and mean velocity (F(1,18)=4.96; p<0.05; F(1,18)=5.00; p<0.05; see Fig. 5A&B, respectively), but not for duration spent in corners (F(1,18)=2.47; p=0.13; see Fig. 5C). Fisher's post hoc analyses showed animals receiving repeated 1 µg/kg LPS injections on day 1 and 2 had a reduction in the total distance traveled, compared to animals that received 1 µg/kg of LPS on day 2 only (p<0.0005), and compared to their day 1 performance after receiving only one 1 µg/kg LPS injection (p<0.05). Additionally, post hoc analyses showed animals that received repeated 1 µg/kg LPS injections on day 1 and 2 had a reduced mean velocity compared to animals that received 1 µg/kg of LPS on day 2 only (p<0.0005), and compared to their day 1 performance after receiving only one 1 µg/kg LPS injection (p<0.05). Lastly, post hoc analyses revealed animals that received repeated 1 µg/kg LPS injections on day 1 and 2 had an increase in the duration spent in corners compared to animals that received 1 µg/kg LPS on day 2 only (p<0.001), and compared to their day 1 performance after receiving only one 1 µg/kg LPS injection (p<0.005). Examination of the effects of repeated low-dose LPS on anxiety-like behavior in the open field revealed no significant main or interaction effects of LPS Treatment on the duration spent in the border (F(1,18)=2.89; (F(1,18)=1.85; ns; respectively). Additionally, there were no main or interaction effects of LPS Treatment on the duration spent in the center (F(1,18)=5.76; (F(1,18)=0.03; ns; respectively)
3.4. Effects of chronic low-dose administration on open field activity and PVN cFos activation
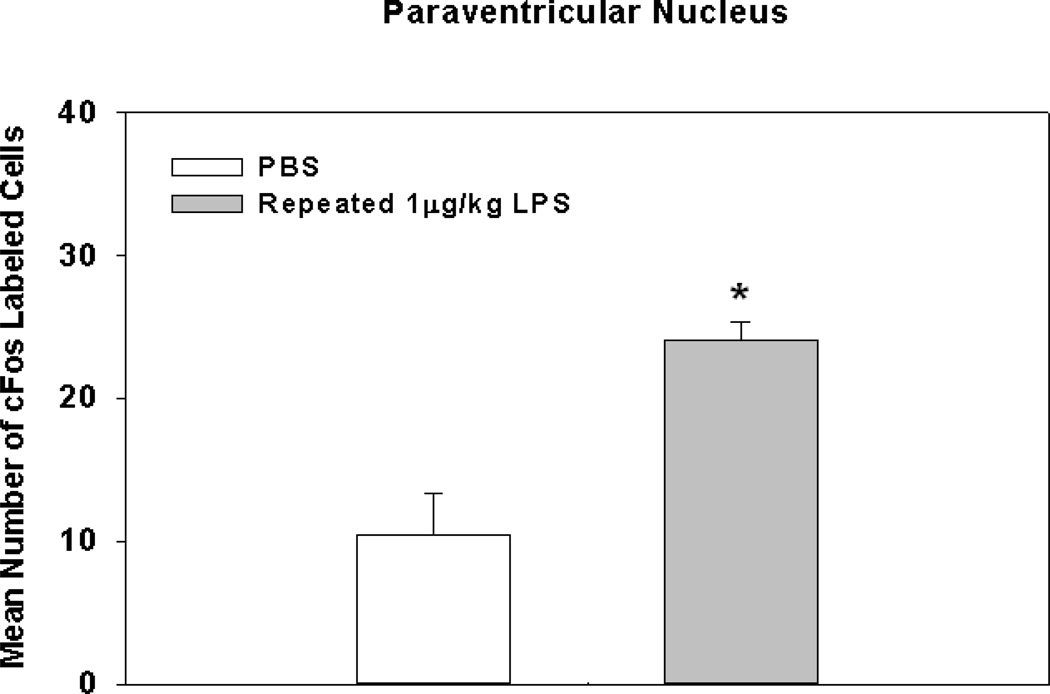
Knowing that there was a behavioral sensitization effect following 2 injections of 1 µg/kg LPS on open field activity, we examined the behavioral effect of repeated low-dose LPS administration over a 10 day period. Additionally, even though a single 1 µg/kg dose of LPS did not cause increased cFos activation, we sought to determine if cFos activation in the PVN corresponded to behavioral deficits seen following repeated exposure to low-grade peripheral inflammation. Results indicated that there were main effects of repeated LPS treatment on the total distance moved, mean velocity, and duration spent in corners (F(4,20)=4.62; p<0.01; F(4,20)=4.62; p<0.01; F(4,20)=6.21; p=0.005, respectively; see Fig. 6A–C). Fisher's post hoc analyses revealed the total distance moved after receiving 7 and/or 10 consecutive injections of 1 µg/kg of LPS was reduced significantly from the same animals after receiving a single injection (p's<0.005) or 4 injections (p's<0.05). When examining group differences for mean velocity post hoc analyses indicated the same pattern, and significance between groups as it was for the total distance traveled (see directly above). As for group differences for duration spent in corners, post hoc tests showed that animals receiving a single dose of 1 µg/kg of LPS on day 1 only had a statistically significant reduction compared to the same group assessed following 2, 4, 7, and 10 days of 1 µg/kg of LPS (p’s<0.01). In addition to sickness behavior measurements, anxiety-like behavioral measurements were assessed. Data showed a significant increase in the duration spent in the border (F(4,20)=3.36; p<0.05;) and a decrease in the duration spent in the center (F(4,20)=3.42; p<0.05) of the open field. However, with the progressive worsening of the sickness behavior (reduced movement velocity and total distance traveled), these data could represent a false positive for anxiety-like behavior. When examining correlates between open field activity following repeated LPS injections and cFos activation, data showed that animals following 8 consecutive days of 1 µg/kg LPS injections had a statistically significant increase in cFos activation in the PVN compared to controls (F(1,7)=15.54; p<0.01; see Fig. 7).
Fig. 7.
Mean number of cFos labeled cells in the paraventricular nucleus of the hypothalamus following repeated 1 µg/kg LPS injections for 8 days. * Indicates a significant difference compared to control animals (p’s≤0.05). Bars represent group means ± SEM.
4. Discussion
Many affective disorders have been attributed to underlying inflammatory dysfunction. Clinical observations showed patients with mood disorders such as depression, anxiety, and autism have increased basal levels of circulating proinflammatory cytokines. In addition, experimental evidence from animal studies showed that a single injection of high-dose bacterial mimics (i.e., LPS) induce both peripheral inflammation and behavioral deficits similar to those seen in affective disorders. These findings have ushered in the inflammatory theory of affective disorders–inflammation can be a pathogenic factor of affective disorders.
However, the use of acute high-dose LPS to simulate inflammatory stimulation lacks face validity in the context of the pathogenesis of affective disorders for the following reasons: 1) affective disorders are not known to be a consequence of acute septic inflammation; 2) high-dose LPS, which mimics septic shock conditions, causes damage to multiple organs including kidneys, liver, and lungs and systemic effects such as loss of vascular integrity and hypotension none of which is a symptom of patients with affective disorders; and 3) human studies report circulating inflammatory cytokine levels are indeed higher in patients with affective disorders, but orders of magnitude lower than those reported in high-dose LPS animal studies. Therefore, acute injection of high-dose LPS does not produce the type of inflammation resembling affective disorders.
What clinical observations do suggest is that patients with affective disorders may bear chronic low-grade inflammation because small increases in basal circulating inflammatory cytokine levels, typically less than 20 pg/ml, have often been detected in these patients [10, 23]. It is important to note that such low-grade inflammation might affect the central nervous system (CNS) very differently from that induced by an injection of high-dose LPS. High-dose LPS induces the expression of large amounts of inflammatory cytokines in the blood, typically over 100 pg/ml [24, 25]. Further, high-dose LPS administration is known to activate the CNS by stimulating brain endothelial cells and the circumventricular organs, or by transporting inflammatory cytokines into the brain across the blood brain barrier, resulting in neuroinflammation, or by stimulating vagal afferents [26, 27]. On the other hand, with low-grade inflammation or localized inflammation, behavioral changes can be induced independent of circulating cytokines and vagal afferents [21, 28]. Therefore, studies on how low-grade inflammation causes behavioral deficits could truly mimic the conditions of affective disorders and reveal the relevant neuro-immune pathways involved in the pathogenesis of these affective disorders.
Only a few studies have examined behavioral consequences following an injection of low-dose LPS, which causes low-grade inflammation. One initial study showed LPS levels as low as 5 µg/kg was able to significantly reduce social exploration in mice [20]. In another study, Teeling et al. showed that i.p. injection of LPS as low as 0.5 µg/kg was sufficient to cause a reduction in burrowing activity assessed 4 hrs following LPS injection [21]. This effect had a substantial recovery by the second day of assessment whereas animals that received high doses of LPS (50, and 100 µg/kg) did not show significant recovery by the second day. In addition, they showed injection of 1 µg/kg of LPS reduced open field activity. Therefore, the lowest dose of LPS that induced observable behavioral changes was ~0.5–1 µg/kg in that study. In the current study, a single injection of 10 µg/kg LPS was able to cause decreased burrowing behavior on day 1 and this effect was abrogated by day 2’s assessment (although a trend was apparent; see Fig. 3B). Additionally, a single injection of 10 µg/kg of LPS caused a decrease in total distance traveled, mean velocity, and duration spent in corners of the open field apparatus. We were not able to detect behavioral changes from a single injection of 1 µg/kg of LPS. Thus, the threshold dose of LPS for the induction of behavioral changes is higher than that reported by Teeling et. al. This could be a result of the differences in the LPS serotype, gender, and/or strains of mice used in these studies. Nonetheless, our effective dose of LPS, 10 µg/kg, was at least 25 times lower than those used in most of the high-dose LPS (higher than 250 µg/kg) studies and the behavioral consequences of this low-dose LPS injection display the same characteristics induced by low-grade inflammation from previous studies: animals show reduced activity in various tests followed by complete recovery on the second day after the LPS challenge.
Neural response to low-grade inflammation has not been examined. The present results show an LPS-induced, dose-dependent increase in cFos expression in the PVN, NTS, CeA, and the VLM. Activation of these brain areas have been associated with sickness behavior following peripheral high-dose LPS in previous studies. Interestingly, low-dose LPS (10 µg/kg) induced significant cFos activation only in the PVN, not in NTS and VLM. Prior reports showed that the NTS and VLM are the major relay stations by which high-dose LPS activates PVN [29, 30]. In addition, i.p. high-dose LPS activates PVN primarily via vagal afferents which stimulates VLM first before activating PVN [30]. It is possible that our sampling time, 3 hrs after the LPS administration, is too late to observe cFos expression in the VLM. An alternative explanation is that low-dose LPS might directly stimulate PVN, bypassing the NTS and VLM. This is similar to our previous report that indicated localized inflammation induces location-specific response only in the PVN, not in the NTS and VLM [31]. Although vagal afferents are critical for transmitting sensory signals from the peritoneal cavity, recent studies showed that spinal afferents from the peritoneal cavity can also be activated by sensory inputs [32]. It is possible the direct spinohyothalamic pathway [33] can mediate CNS signaling by low-grade inflammation.
The present study is the first to examine the neural and behavioral responses after repeated administration of low-dose LPS to simulate chronic low-grade inflammation. In the burrowing task, injection of 1 µg/kg of LPS did not induce behavioral alterations. Neither did a subsequent injection of 1 µg/kg of LPS on the second day. In the open field test, however, although injection of 1 µg/kg of LPS did not induce behavioral changes on day one, this injection repeated on day 2 significantly reduced total distance traveled and mean velocity, and increased duration spent in the corners of the open field. This suggests that the presence of low-level LPS on day one potentiated the effect of the low-level LPS on day 2. Thus, open field, not burrowing, is the more sensitive test to track chronic low-grade LPS-induced behavioral changes. When we extended 1 µg/kg LPS injections over 10 days, sickness behavior (i.e., decreased total distance traveled, decreased mean velocity, and increased duration spent in corners) became progressively worse. In addition, cFos activation in the PVN was increased significantly following 8 days of repeated 1 µg/kg LPS injections compared to control animals. Interestingly, the number of cFos labeled cells approached levels found following a single injection of 10 µg/kg of LPS (see Fig. 1B), which was able to cause significant behavioral deficits in the burrowing and open field tasks (see Figs. 3B and 2D–F). The kinetic effects of low-dose LPS are consistent with the observation by West. et al. who showed exposure to high levels of LPS causes LPS tolerance on subsequent LPS exposure, but exposure to low levels of LPS augments the effects of subsequent LPS exposure [34]. Although, the acquisition of endotoxin tolerance after repeated high-dose LPS injections has been well established, recent reports have shown that certain aspects of immunity do not follow this current dogma [35] Overall, these results demonstrate for the first time that low-level LPS, even at levels insufficient to cause acute behavioral alterations, can precipitate significant sickness behavior and neural responses over time.
Acknowledgements
This work was supported in full by the NIH grants: RO1 MH-046801-JFS, T32 DE-014320-JFS, F32 DE-022230-AJT, and RO1 AI-076926-NQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Reference List
- 1.Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48–54. doi: 10.3899/jrheum.110903. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usmani ZA, Carson KV, Cheng JN, Esterman AJ, Smith BJ. Pharmacological interventions for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;11:CD008483. doi: 10.1002/14651858.CD008483.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassi GS, Kanashiro A, Santin FM, de Souza GE, Nobre MJ, Coimbra NC. Lipopolysaccharide-Induced Sickness Behaviour Evaluated in Different Models of Anxiety and Innate Fear in Rats. Basic Clin Pharmacol Toxicol. 2012;110:359–369. doi: 10.1111/j.1742-7843.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 9.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19:397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- 11.Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, et al. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J Affect Disord. 2005;87:305–311. doi: 10.1016/j.jad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Hillenbrand A, Knippschild U, Weiss M, Schrezenmeier H, Henne-Bruns D, Huber-Lang M, et al. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. BMC Surg. 2010;10:26. doi: 10.1186/1471-2482-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghani RA, Zainudin S, Ctkong N, Rahman AF, Wafa SR, Mohamad M, et al. Serum IL-6 and IL-1-ra with sequential organ failure assessment scores in septic patients receiving high-volume haemofiltration and continuous venovenous haemofiltration. Nephrology (Carlton) 2006;11:386–393. doi: 10.1111/j.1440-1797.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- 14.Kremer JP, Jarrar D, Steckholzer U, Ertel W. Interleukin-1, -6 and tumor necrosis factor-alpha release is down-regulated in whole blood from septic patients. Acta Haematol. 1996;95:268–273. doi: 10.1159/000203895. [DOI] [PubMed] [Google Scholar]
- 15.Gardlund B, Sjolin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 16.Cannon JG, Tompkins RG, Gelfand JA, Michie HR, Stanford GG, van der Meer JW, et al. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 17.Maes M, Twisk FN, Kubera M, Ringel K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-alpha, PMN-elastase, lysozyme and neopterin. J Affect Disord. 2012;136:933–939. doi: 10.1016/j.jad.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Syrenicz A, Garanty-Bogacka B, Syrenicz M, Gebala A, Walczak M. Low-grade systemic inflammation and the risk of type 2 diabetes in obese children and adolescents. Neuro Endocrinol Lett. 2006;27:453–458. [PubMed] [Google Scholar]
- 19.Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- 20.Fishkin RJ, Winslow JT. Endotoxin-induced reduction of social investigation by mice: interaction with amphetamine and anti-inflammatory drugs. Psychopharmacology (Berl) 1997;132:335–341. doi: 10.1007/s002130050353. [DOI] [PubMed] [Google Scholar]
- 21.Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Subpyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Maes M, Bosmans E, De JR, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 24.Datta SC, Opp MR. Lipopolysaccharide-induced increases in cytokines in discrete mouse brain regions are detectable using Luminex xMAP technology. J Neurosci Methods. 2008;175:119–124. doi: 10.1016/j.jneumeth.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 27.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Ching S, Chen Q, Li Q, An Y, Quan N. Localized inflammation in peripheral tissue signals the CNS for sickness response in the absence of interleukin-1 and cyclooxygenase-2 in the blood and brain. Neuroscience. 2008;157:895–907. doi: 10.1016/j.neuroscience.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ek M, Arias C, Sawchenko P, Ericsson-Dahlstrand A. Distribution of the EP3 prostaglandin E(2) receptor subtype in the rat brain: relationship to sites of interleukin-1-induced cellular responsiveness. J Comp Neurol. 2000;428:5–20. doi: 10.1002/1096-9861(20001204)428:1<5::aid-cne2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Gaykema RP, Chen CC, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130:130–145. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 31.Belevych N, Buchanan K, Chen Q, Bailey M, Quan N. Location-specific activation of the paraventricular nucleus of the hypothalamus by localized inflammation. Brain Behav Immun. 2010;24:1137–1147. doi: 10.1016/j.bbi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peeters PJ, Aerssens J, de HR, Stanisz A, Gohlmann HW, Hillsley K, et al. Molecular profiling of murine sensory neurons in the nodose and dorsal root ganglia labeled from the peritoneal cavity. Physiol Genomics. 2006;24:252–263. doi: 10.1152/physiolgenomics.00169.2005. [DOI] [PubMed] [Google Scholar]
- 33.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West MA, Koons A. Endotoxin tolerance in sepsis: concentration-dependent augmentation or inhibition of LPS-stimulated macrophage TNF secretion by LPS pretreatment. J Trauma. 2008;65:893–898. doi: 10.1097/TA.0b013e3181877fde. [DOI] [PubMed] [Google Scholar]
- 35.Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun. 2011;25:1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]